Abstracts
Temporal and spatial variations in species composition and vertical distribution of macroalgal communities growing on mangrove trees were analyzed bimonthly in the Ilha do Cardoso State Park, São Paulo state (25°03'S and 47°55'W), Southeastern Brazil. The macroalgal communities from mangroves of Perequê and Sítio Grande rivers comprised 10 and 18 taxa respectively. Bostrychia radicans (Mont.) Mont. and B. calliptera (Mont.) Mont. were the predominant taxa, present almost throughout the year and in all the sites studied. The species composition of macroalgal communities from both mangroves presented temporal and spatial variations related to environmental factors. The highest number of taxa was observed during colder, drier months, coinciding with the highest means of high water neap and short periods of continuous emersion (April to August). Some mangrove algae such as B. calliptera, Rhizoclonium spp., Caloglossa spp., and Boodleopsis pusilla (Collins) W. Taylor, Joly et Bernatowicz showed a high degree of tolerance to desiccation, being able to tolerate continuous emersion up to six days. The spatial variations in species composition were related to light, as observed in Catenella caespitosa (Withering) L. Irvine, which occurred in well-lit sites. No pattern of vertical zonation was observed, since Rhizoclonium spp., B. radicans, and B. calliptera occur over the entire vertical range. Variations in the range of vertical distribution of macroalgae of Perequê mangrove were mainly related to the variations in the tidal levels (mean high water neap and/or mean high water spring) while those observed in Sítio Grande mangrove were related to salinity variations, except for B. calliptera and Caloglossa spp. related to tidal levels and high irradiance, respectively.
Ilha do Cardoso; mangroves; macroalgae; vertical distribution
A composição de espécies e a distribuição vertical das comunidades de macroalgas associadas às espécies arbóreas de manguezais do Parque Estadual da Ilha do Cardoso, estado de São Paulo (25°03'S e 47°55'W), Brasil, foram analisadas bimestralmente. As comunidades de macroalgas dos manguezais dos rios Perequê e Sítio Grande apresentaram 10 e 18 táxons, respectivamente. Bostrychia radicans (Mont.) Mont. e B. calliptera (Mont.) Mont. foram os táxons predominantes, presentes durante quase todo o ano e em todos os locais estudados. A composição em espécies das comunidades de macroalgas dos dois manguezais apresentaram variações temporal e espacial relacionadas aos fatores ambientais. O maior número de táxons foi observado durante os meses mais frios e secos, coincidentes com as maiores médias das marés de quadratura e curtos períodos de emersão (abril a agosto). Algumas macroalgas, tais como B. calliptera, Rhizoclonium spp., Caloglossa spp., e Boodleopsis pusilla (Collins) W. Taylor, Joly et Bernatowicz apresentaram um alto grau de tolerância à dessecação, com períodos de emersão contínua de até seis dias. As variações espaciais na composição em espécies foram relacionadas à luz, como observadas em Catenella caespitosa (Withering) L. Irvine, encontrada em locais muito iluminados. Não foi observado um padrão de zonação vertical, uma vez que Rhizoclonium spp., B. radicans, e B. calliptera estavam distribuídas em toda a extensão analisada. As variações na amplitude da distribuição vertical das macroalgas do manguezal do Perequê foram relacionadas principalmente aos níveis de marés (média das marés de sizígia e de quadratura), enquanto que as variações observadas no manguezal do Sítio Grande foram relacionadas à salinidade, exceto para B. calliptera e Caloglossa spp., cujas distribuições foram relacionadas aos níveis de marés e à alta irradiância, respectivamente.
Temporal and spatial variations in the structure of macroalgal communities associated with mangrove trees of Ilha do Cardoso, São Paulo state, Brazil
NAIR S. YOKOYA11. Instituto de Botânica, Caixa Postal 4005, 01061-970 São Paulo, SP, Brazil. Instituto de Botânica, Caixa Postal 4005, 01061-970 São Paulo, SP, Brazil. , ESTELA M. PLASTINO21. Instituto de Botânica, Caixa Postal 4005, 01061-970 São Paulo, SP, Brazil. Instituto de Botânica, Caixa Postal 4005, 01061-970 São Paulo, SP, Brazil. , MARIA DO ROSÁRIO A. BRAGA11. Instituto de Botânica, Caixa Postal 4005, 01061-970 São Paulo, SP, Brazil. Instituto de Botânica, Caixa Postal 4005, 01061-970 São Paulo, SP, Brazil. , MUTUE T. FUJII11. Instituto de Botânica, Caixa Postal 4005, 01061-970 São Paulo, SP, Brazil. Instituto de Botânica, Caixa Postal 4005, 01061-970 São Paulo, SP, Brazil. , MARILZA CORDEIRO-MARINO31. Instituto de Botânica, Caixa Postal 4005, 01061-970 São Paulo, SP, Brazil. Instituto de Botânica, Caixa Postal 4005, 01061-970 São Paulo, SP, Brazil. , VERENA R. ESTON11. Instituto de Botânica, Caixa Postal 4005, 01061-970 São Paulo, SP, Brazil. Instituto de Botânica, Caixa Postal 4005, 01061-970 São Paulo, SP, Brazil. and JOSEPH HARARI41. Instituto de Botânica, Caixa Postal 4005, 01061-970 São Paulo, SP, Brazil. Instituto de Botânica, Caixa Postal 4005, 01061-970 São Paulo, SP, Brazil.
(recebido em 11/09/98; aceito em 21/02/99)
ABSTRACT - (Temporal and spatial variations in the structure of macroalgal communities associated with mangrove trees of Ilha do Cardoso, São Paulo state, Brazil). Temporal and spatial variations in species composition and vertical distribution of macroalgal communities growing on mangrove trees were analyzed bimonthly in the Ilha do Cardoso State Park, São Paulo state (25°03'S and 47°55'W), Southeastern Brazil. The macroalgal communities from mangroves of Perequê and Sítio Grande rivers comprised 10 and 18 taxa respectively. Bostrychia radicans (Mont.) Mont. and B. calliptera (Mont.) Mont. were the predominant taxa, present almost throughout the year and in all the sites studied. The species composition of macroalgal communities from both mangroves presented temporal and spatial variations related to environmental factors. The highest number of taxa was observed during colder, drier months, coinciding with the highest means of high water neap and short periods of continuous emersion (April to August). Some mangrove algae such as B. calliptera, Rhizoclonium spp., Caloglossa spp., and Boodleopsis pusilla (Collins) W. Taylor, Joly et Bernatowicz showed a high degree of tolerance to desiccation, being able to tolerate continuous emersion up to six days. The spatial variations in species composition were related to light, as observed in Catenella caespitosa (Withering) L. Irvine, which occurred in well-lit sites. No pattern of vertical zonation was observed, since Rhizoclonium spp., B. radicans, and B. calliptera occur over the entire vertical range. Variations in the range of vertical distribution of macroalgae of Perequê mangrove were mainly related to the variations in the tidal levels (mean high water neap and/or mean high water spring) while those observed in Sítio Grande mangrove were related to salinity variations, except for B. calliptera and Caloglossa spp. related to tidal levels and high irradiance, respectively.
RESUMO - (Variações temporal e espacial na estrutura de comunidades de macroalgas associadas a árvores de manguezais na Ilha do Cardoso, estado de São Paulo, Brasil). A composição de espécies e a distribuição vertical das comunidades de macroalgas associadas às espécies arbóreas de manguezais do Parque Estadual da Ilha do Cardoso, estado de São Paulo (25°03'S e 47°55'W), Brasil, foram analisadas bimestralmente. As comunidades de macroalgas dos manguezais dos rios Perequê e Sítio Grande apresentaram 10 e 18 táxons, respectivamente. Bostrychia radicans (Mont.) Mont. e B. calliptera (Mont.) Mont. foram os táxons predominantes, presentes durante quase todo o ano e em todos os locais estudados. A composição em espécies das comunidades de macroalgas dos dois manguezais apresentaram variações temporal e espacial relacionadas aos fatores ambientais. O maior número de táxons foi observado durante os meses mais frios e secos, coincidentes com as maiores médias das marés de quadratura e curtos períodos de emersão (abril a agosto). Algumas macroalgas, tais como B. calliptera, Rhizoclonium spp., Caloglossa spp., e Boodleopsis pusilla (Collins) W. Taylor, Joly et Bernatowicz apresentaram um alto grau de tolerância à dessecação, com períodos de emersão contínua de até seis dias. As variações espaciais na composição em espécies foram relacionadas à luz, como observadas em Catenella caespitosa (Withering) L. Irvine, encontrada em locais muito iluminados. Não foi observado um padrão de zonação vertical, uma vez que Rhizoclonium spp., B. radicans, e B. calliptera estavam distribuídas em toda a extensão analisada. As variações na amplitude da distribuição vertical das macroalgas do manguezal do Perequê foram relacionadas principalmente aos níveis de marés (média das marés de sizígia e de quadratura), enquanto que as variações observadas no manguezal do Sítio Grande foram relacionadas à salinidade, exceto para B. calliptera e Caloglossa spp., cujas distribuições foram relacionadas aos níveis de marés e à alta irradiância, respectivamente.
Key words - Ilha do Cardoso, mangroves, macroalgae, vertical distribution
Introduction
Mangrove forests cover about 25000 km2 in Brazil (Saenger et al. 1983), extending South to 28°30'S (Oliveira 1984). These mangroves occur bordering estuaries, coves and lagoons, with high water turbidity and large salinity variations. In these environments, algal communities have low diversity (Oliveira 1984).
There have been extensive studies of mangrove algal communities in tropical and subtropical coasts from Latin-America (West 1991, Cordeiro-Marino et al. 1992, West et al. 1992, Pedroche et al. 1995), Africa (Lambert et al. 1987, Steinke & Naidoo 1990, Phillips et al. 1994, 1996), Australia (Davey & Woelkerling 1980, Beanland & Woelkerling 1982, King & Wheeler 1985, King 1990, King & Puttock 1994), Japan (Tanaka & Chihara 1984, 1987), and the Philippines (Cordero 1978). However, little information about the temporal variation in species composition of algal communities associated with mangroves is available (Narasimha Rao 1995). On the other hand, the spatial variation in species composition can be correlated with some environmental factors such as shade produced by the tree canopy (Almodovar & Biebl 1962, Almodovar & Pagan 1971, Eston et al. 1991).
Vertical zonation of mangrove algae has been described in Puerto Rican (Almodovar & Biebl 1962, Almodovar & Pagan 1971) and Japanese mangroves (Tanaka & Chihara 1987). Oliveira (1984) described a common pattern of vertical distribution of algae found in Brazilian mangroves, and suggested that the vertical distribution can be the result of different adaptation or tolerance to the variation in several environmental factors, as well as the result of the biological interactions among the populations. However, ecological studies conducted to determine the effects of environmental factors on the temporal and spatial variations in the vertical distribution of mangrove macroalgae are uncommon.
The purpose of the present study is to provide information about the temporal and spatial variation of the species composition and the vertical distribution of mangrove macroalgal communities, assessing if there is a correlation with the changes in some environmental factors.
Materials and methods
Study areas - Macroalgal communities growing on mangrove trees located in the Perequê and Sítio Grande estuaries of the Ilha do Cardoso State Park, São Paulo state, Brazil (25°03'S and 47°55'W) were studied (figure 1). In the Perequê estuary, an area near the river mouth was selected and divided into three sub-areas: (PE 1) next to the river, (PE 2) intermediate, and (PE 3) next to the permanently emersed area. Two trees were randomly marked in each sub-area. Three areas along the Sítio Grande estuary (SG) were delimited: (SG 1) upstream, (SG 2) intermediate, and (SG 3) near the river mouth. Four trees were randomly marked in each area. These mangroves are composed by the following angiosperm species: Rhizophora mangle L., Laguncularia racemosa (L.) Gaertn., and Avicennia schaueriana Stap. et Leech. Site SG 1 is an exception, being formed predominantly by dwarf L. racemosa.
Study areas: Perequê (sites PE 1-3) and Sítio Grande rivers (sites SG 1, SG 2, and SG 3), Ilha do Cardoso State Park, Southeastern Brazil.
Environmental factors - The photosynthetically active radiation (PAR) was measured simultaneously with two spherical quantum sensors (LICOR, model LI-193) connected to a datalogger (LICOR, model LI-1000). One sensor was positioned above the canopy and the other at ground level. Percentage of incident irradiance was calculated as the ratio between PAR at ground level and above the canopy, using the average of 25 data points. Measurements were made on sunny days and between 10:00 and 14:00 h.
Variation in salinity at each site was measured by using a refractometer (American Optical, model 10419). The water was collected in vials of 15 ml, which were spaced vertically every 5 cm on 100 cm high wooden poles. In order to avoid the addition of rainfall in the vials, a plastic cover was fixed above each vial. The poles were attached to the trunks with the lowest vial at ground level. Each collection was made over one tidal cycle.
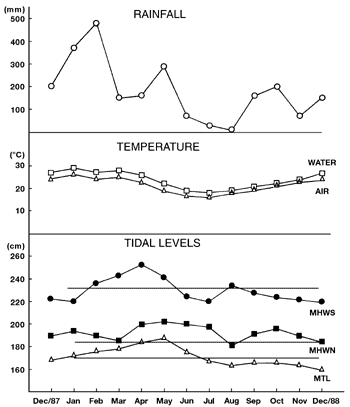
Daily variations in air and surface water temperatures, rainfall and hourly data for daily mean tidal level computations (Mesquita & Harari 1988) were recorded by the Environmental Station of the Oceanographic Institute of University of São Paulo, in Cananéia, São Paulo state situated close to the study areas (figure 2).
Monthly mean of temperature (air and water), rainfall and tidal levels during the period of study. Horizontal lines represent the annual mean for each tidal level. MHWS = mean high water spring; MHWN = mean high water neap; MTL = mean tidal level.
The tidal regime at the collected sites was semidiurnal (Por et al. 1984). The terminology used for tides in this paper was: mean tidal level (MTL), mean high water neap (MHWN), and mean high water spring (MHWS).
Algal observations - Mangrove macroalgae growing on trunks previously marked were observed bimonthly from December 1987 to December 1988. Vertical distribution of each taxon was observed in loco on the side of the trunks facing the river. Whenever it was necessary to check taxon identification under the microscope, small samples were taken. The highest and lowest position of each taxon were measured in the field and standardized taking into consideration the slope of the trunk, by means of the equation: H = hcosa; where: H = standardized height; h = height measured; a = angle formed by the trunk and the perpendicular line in relation to the ground.
The levels of the marked trees in relation to tidal height were determined by comparing the height of the predicted high tide (from the tide-gauge data in Cananéia) with the highest water level measured in the vials used for salinity determination.
In order to obtain the time of emersion of algal communities, the level of two trees (numbered 3 and 5) from Perequê mangrove were plotted against the harmonic curves of the daily data on tidal levels. This procedure allowed determining when the water did not reach the basis of the trees, and periods of emersion equal to or higher than one day were recorded. These trees were located at the highest level of the mangrove, supplying data for the greatest period of emersion.
Bostrychia radicans was considered as a complex of taxa, which included B. radicans f. radicans, B. radicans f. moniliforme, Bostrychia sp., and Stictosiphonia kelanensis. The Rhizoclonium spp. complex included R. africanum, R. kerneri, R. riparium and R. tortuosum, and monostromatic chlorophytes included Ulvaria oxyspermum and Monostroma sp. The rationale to consider different species of Bostrychia and Rhizoclonium as a complex was based on the observations that they always occurred in association, and specific identification in the field was not possible. The differences between U. oxyspermum and Monostroma sp. are concerned with the ontogeny of the thallus and limiting temperatures of survival (Braga et al. 1997).
Statistical analysis - The environmental factors used in multivariate analysis were water temperature, rainfall, percentage of incident irradiance, minimum and maximum values of salinity, sampling sites and period, and tidal levels (MHWS and MHWN) recorded in the same month as well as one month before recording algal data. The rationale for comparing those two periods is based on the assumption that environmental change may be manifested in the organism only after a change has occurred (Ang 1986).
For multivariate analysis, the data of the range of vertical distribution of each taxon and environmental factors were standardized to have a mean of zero and a variance of one, in order to give each variable an equivalent weight. The principal component analysis (PCA) was used to detect combinations of those variables that explain most of the variability. The multivariate methods used in this study are part of the program package STATISTICA (version 5.0).
Results
Environmental factors - The shadiest sites were PE 1 and SG 3, while PE 2, PE 3, SG 1, and SG 2 were well-lit sites (table 1). The salinity varied during the year, and the largest range in salinity variation in Perequê mangroves was 17 (PE 2 and PE 3), while in Sítio Grande mangroves the range was 30 (SG 2, table 1). Rainfall and air and surface-water temperatures varied seasonally, showing a colder, drier period (June to August) and a warmer, wetter, period (February and March, figure 2).
Species composition The macroalgal communities growing on mangrove trees in the Perequê and Sítio Grande mangroves are composed of 10 and 18 taxa, respectively (table 2). However, the number of taxa varied during the year, between sites and between trees at the same site, mainly in the Sítio Grande mangrove.
The algal community in the Perequê mangrove is composed of Bostrychia calliptera, B. radicans, and Rhizoclonium spp., which are generally present throughout the year (figure 3). The number of taxa increases in June (austral winter), in the colder, drier period, coincinding with higher values of mean high water neap (figure 2), and short periods of continuous emersion (table 3). Some of these mangrove taxa (Bostrychia calliptera, Rhizoclonium spp., Caloglossa spp., and Boodleopsis pusilla) can tolerate continuous emersion up to six days, as observed in the Perequê mangrove (table 3).
Figure 3. Vertical distribution of macroalgal species in the Perequê mangrove in relation to mean sea level. The discontinuous line corresponds to the ground level of trees 2 to 6. Av = Avicennia schaueriana; Lg = Laguncularia racemosa; Rh = Rhizophora mangle; T = tree.  Bostrychia radicans;
Bostrychia radicans;  Bostrychia calliptera;
Bostrychia calliptera;  Rhizoclonium spp.;
Rhizoclonium spp.;  Caloglossa spp.;
Caloglossa spp.;  Boodleopsis pusilla.
Boodleopsis pusilla.
Bostrychia calliptera and B. radicans were the most abundant taxa in the Sítio Grande mangrove (figures 4-6 ). The spatial variations in species composition observed in the Sítio Grande mangrove may be related to variations in light (table 1). Bostrychia montagnei occurred only in shaded sites, while Catenella caespitosa was found in well-lit ones. Moreover, the community at SG 1 was composed of only two taxa (B. calliptera and B. radicans), except for tree 4, where the number of taxa varied from two to four, with the predominance of Caloglossa spp. and B. radicans (figure 4). The highest number of taxa during one year was observed at SG 2 (10 taxa, figure 5). However, the highest number of taxa within a month was observed at SG 3 (six and seven taxa growing on tree 4 in April and tree 2 in June, respectively, figure 6 ) corresponding to austral autumn and winter, with high MHWN, low temperatures, and low rainfall (figure 2).
Figure 4. Vertical distribution of macroalgal species in the Sítio Grande mangrove, SG 1 site, in relation to mean sea level. The discontinuous line corresponds to the ground level of trees 1 to 3. Lg = Laguncularia racemosa; Rh = Rhizophora mangle; T = tree.  Bostrychia radicans;
Bostrychia radicans;  Bostrychia calliptera;
Bostrychia calliptera;  Rhizoclonium spp.;
Rhizoclonium spp.;  Caloglossa spp.;
Caloglossa spp.;  Catenella caespitosa;
Catenella caespitosa;  Monostromatic chlorophytes.
Monostromatic chlorophytes.
Figure 5. Vertical distribution of macroalgal species in the Sítio Grande mangrove, SG 2 site, in relation to mean sea level. Lg = Laguncularia racemosa; Rh = Rhizophora mangle; T = tree.  Bostrychia radicans;
Bostrychia radicans;  Bostrychia calliptera;
Bostrychia calliptera;  Rhizoclonium spp.;
Rhizoclonium spp.;  Caloglossa spp.;
Caloglossa spp.;  Catenella caespitosa; Monostromatic chlorophytes;
Catenella caespitosa; Monostromatic chlorophytes;  Enteromorpha sp.;
Enteromorpha sp.;  Cladophoropsis membrancea;
Cladophoropsis membrancea;  Boodleopsis pusilla;
Boodleopsis pusilla;  Bostrychia montagnei.
Bostrychia montagnei.
Figure 6. Vertical distribution of macroalgal species in the Sítio Grande mangrove, SG 3 site, in relation to mean sea level. The discontinuous line corresponds to the ground level of trees 2 to 4. Av = Avicennia schaueriana; Rh = Rhizophora mangle; T = tree.  Bostrychia radicans;
Bostrychia radicans;  Bostrychia calliptera;
Bostrychia calliptera;  Rhizoclonium spp.;
Rhizoclonium spp.;  Caloglossa spp.;
Caloglossa spp.;  Cladophoropsis membrancea;
Cladophoropsis membrancea;  Boodleopsis pusilla;
Boodleopsis pusilla;  Bostrychia montagnei.
Bostrychia montagnei.
Maximum height and range of the vertical distribution - Variations in maximum height and range of the vertical distribution of the macroalgae showed no distinct seasonal pattern at any of the mangrove sites (figures 3-6 ). A pattern of vertical zonation was not observed either, since Bostrychia radicans, B. calliptera, and Rhizoclonium spp. occurred over the entire vertical range.
Bostrychia radicans, B. calliptera, and Rhizoclonium spp. showed the broadest range of vertical distributions, whereas Caloglossa spp. and Cladophoropsis membranacea were always found on the lower part of the trunks.
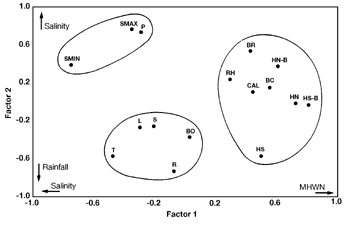
In the Perequê mangrove, the principal component analysis showed that groups fell into discrete areas (figure 7). The first component correlated the effect of tidal levels on the ranges of vertical distribution of Bostrychia radicans, B. calliptera, Caloglossa spp., and Rhizoclonium spp. (figure 7). In the second component, the range of vertical distribution of Boodleopsis pusilla was correlated with rainfall, sampling sites, percentage of incident irradiance, and temperature (figure 7).
Figure 7. Scatter diagram of plots on the first two principal component analysis axes of the algal and environmental factor data in Perequê mangrove. The first two components accounted for 43.4% of total variance. BC = Bostrychia calliptera; BR = B. radicans; BO = Boodleopsis pusilla; CAL = Caloglossa spp.; RH = Rhizoclonium spp.; HN and HN-B = mean high water neap recorded in the same month and one month before recording algal data, respectively; HS and HS-B = mean high water spring recorded in the same month and one month before recording algal data, respectively; L = percentage of incident irradiance; R = rainfall; SMAX and SMIN = maximum and minimum values of salinity, respectively; S and P = sampling sites and periods, respectively; T = mean of water temperature.
In the Sítio Grande mangrove, the principal component analysis showed three groups (figure 8). The first component correlated the range of vertical distribution of Catenella caespitosa with percentage of incident irradiance, MHWS measured in the same month and rainfall. It correlated salinity variation, sampling sites and sampling periods with Rhizoclonium spp., Cladophoropsis membranacea, Caloglossa spp., Bostrychia montagnei, Enteromorpha spp., Boodleopsis pusilla, and monostromatic chlorophytes (figure 8). In the second principal component, the range of vertical distribution of Bostrychia calliptera was correlated with MHWS measured one month before and MHWN measured in the same month as well one month before (figure 8).
Figure 8. Scatter diagram of plots on the first two principal component analysis axes of the algal and environmental factor data in Sítio Grande mangrove. The first two components accounted for 31.7% of total variance. BC = Bostrychia calliptera; BM = B. montagnei; BR = B. radicans; BO = Boodleopsis pusilla; CAL = Caloglossa spp.; CC = Catenella caespitosa; CM = Cladophoropsis membranacea; E = Enteromorpha sp.; M = monostromatic chlorophytes; RH = Rhizoclonium spp.; HN and HN-B = mean high water neap recorded in the same month and one month before recording algal data, respectively; HS and HS-B = mean high water spring recorded in the same month and one month before recording algal data, respectively; L = percentage of incident irradiance; R = rainfall; SMAX and SMIN = maximum and minimum values of salinity, respectively; S and = sampling sites and periods, respectively; T = mean of water temperature.
Discussion
The species composition of macroalgal communities growing on trees in the Perequê and Sítio Grande mangroves corresponds to the Bostrychietum association (sensu Post 1968), with a predominance of Bostrychia radicans and B. calliptera.
The numbers of taxa recorded, 10 taxa in the Perequê mangrove and 18 taxa in the Sítio Grande mangrove, are higher than, or similar to the numbers recorded by other authors who investigated Brazilian mangroves (Mitchell et al. 1974 - eight taxa, Hadlich 1984 - six taxa, Hadlich & Bouzon 1985 - nine taxa, Paula et al. 1989 - eight taxa). These mangroves occur in an estuarine environment with a muddy substratum, high water turbidity and large salinity variations, and they exhibit a relative low macroalgal species diversity (Oliveira 1984). Observations on Northeastern Brazilian mangroves reported higher species diversity, due to the presence of species that are normally present on rocky shores (Miranda 1986, Pinheiro-Joventino & Lima-Verde 1988). The high diversity of algal species in these mangroves is related to hard substratum, high water transparency and stable salinity, as observed in Caribbean mangroves (Cordeiro-Marino et al. 1992). The higher species diversity observed in one of the mangroves may be explained by anthropogenic impact due to housing development in an area near the Perequê river, which altered the hydrographic regime, and increased the sediment loading and nutrients from sewage.
The species composition from both mangroves presented temporal variations. The highest number of species was recorded during colder, drier months, coincinding with higher MHWN and short periods of continuous emersion. During this period, the algal communities were exposed to a short periods of adverse environmental conditions, which allowed the growth of less tolerant species.
Bostrychia radicans is the predominant taxon, present almost throughout the year and at all the sites studied. Moreover, B. radicans presented a broad range of vertical distribution, while Caloglossa spp. (including C. leprieurii) grew only on the lower part of the trunks. These observations may be explained by physiological tolerance of each species to different environmental parameters. Mann & Steinke (1988) suggested that B. radicans is better adapted to higher temperature, desiccation and to both lower and higher irradiance, than Caloglossa leprieurii, based on observations of photosynthetic and respiratory rates. B. radicans has also presented the ability to maintain low respiratory rates under higher temperatures, and to return to normal levels of photosynthetic and respiratory rates after high levels of desiccation, as well as to tolerate higher levels of irradiance (Mann & Steinke 1988); these characteristics may allow this species' broad vertical distribution. Additionally, B. radicans has an euryhaline nature (Yarish et al. 1980, Karsten & Kirst 1989a) and is able to tolerate extreme salinity values, growing in a range of 5.3 to 70.0 (Karsten et al. 1994).
Bostrychia calliptera, Caloglossa spp., Rhizoclonium spp., and Boodleopsis pusilla can tolerate continuous emersion for up to six days in the Perequê mangrove. Our results show a high degree of tolerance to desiccation for some mangrove algal species. Several studies showed that the red algae Bostrychia radicans, Stictosiphonia sp. and Caloglossa sp., have the ability to produce polyols, D-sorbitol, D-dulcitol and D-mannitol, which have a role in osmoacclimation during stress caused by desiccation and salinity variations (Karsten & Kirst 1989b, Karsten et al. 1990, Karsten et al. 1992a,b, West et al. 1992, Pedroche et al. 1995). The regulation mechanism for salinity variation and tolerance to desiccation in some Brazilian mangrove algae would be a relevant subject for further studies, since these algae are able to tolerate salinity variation from 0 to 30 (as observed at SG 2) and continuous emersion for up to six days.
Regarding the vertical distribution of macroalgae in the Perequê and Sítio Grande mangroves, no pattern was observed for the dominant algae, since Rhizoclonium spp., Bostrychia radicans and/or B. calliptera occur over the entire vertical range. This result could be related to the absence of competitive interactions for bare space among mangrove macroalgae during colonization processes as observed by Eston et al. (1992) in a study in the same mangrove macroalgal communities. Interspecific competition has been considered the primary determinant in open coast vertical algal zonation (Chapman 1973). Wilkinson (1980) has suggested that the interspecific competition could be reduced by number of species, resulting in less well-defined zonation in estuarine algal community. On the other hand, Almodovar & Pagan (1971) observed a clear zonation pattern in Puerto Rican mangroves, and Tanaka & Chihara (1987) in Japanese mangroves. However, the temporal variation of vertical distribution of the algal community was not considered in those studies.
The ability to withstand emersion is considered the major determinant of the presence of the species and controls the upper distributional limits in some intertidal species (Davison & Pearson 1996). This could explain the results of PCA and cluster analysis which showed that the range of vertical distribution of algal community from Perequê mangrove and of Bostrychia calliptera from Sítio Grande mangrove were related to tidal levels (MHWN and MHWS). The vertical distributions of the majority of species from Sítio Grande mangrove were correlated with salinity variations, since the sampling sites presented a great range of salinity variations. The distribution of Catenella was correlated with high percentage of incident irradiance, in opposition with the observation made by Oliveira (1984) who reported that C. caespitosa has been found in shaded spots.
In conclusion, this work showed that species composition of macroalgal communities from both mangroves presented temporal and spatial variations related to environmental factors. Temporal variations are related to temperature, tidal levels and tolerance of each species to withstand emersion, whereas spatial variations are related to salinity and light. Tidal levels controlled the range of vertical distribution of macroalgae from Perequê mangrove while salinity variations were a determinant factor for the majority of the species from Sítio Grande mangrove.
Acknowledgements - The authors are grateful to John A. West, Édison J. Paula, Eurico C. Oliveira, and Tim Moulton for critically reading the manuscript; José Domingos for helping with field measurements, and Norga M. M. dos Santos for drawing the illustrations. This work was funded by a CNPq grant as part of the Ecosystem Mangrove Project (Proc. n 407.872/86-ZO-XC) and by a fellowship to V.R.E. (Proc. n 150.007/87-6).
2. Instituto de Biociências, Universidade de São Paulo, Caixa Postal 11461, 05422-970 São Paulo, SP, Brazil.
3. In memoriam
4. Instituto Oceanográfico, Universidade de São Paulo, Caixa Postal 9075, 05508-900 São Paulo, SP, Brazil.
- ALMODOVAR, L.R. & BIEBL, R. 1962. Osmotic resistance of mangrove algae around La Parguera, Puerto Rico. Rev. algol. 6:203-208.
- ALMODOVAR, L.R. & PAGAN, F.A. 1971. Notes on a mangrove lagoon and mangrove channels at la Parguera, Puerto Rico. Nova Hedwigia 21:241-253.
- ANG, P.O. 1986. Analysis of the vegetation structure of a Sargassum community in the Philippines. Mar. Ecol. Prog. Ser. 28:9-19.
- BEANLAND, W.R. & WOELKERLING, W.J. 1982. Studies on Australian mangrove algae: II. Composition and geographic distribution of communities in Spencer Gulf, South Australia. Proc. R. Soc. Vict. 94:86-106.
- BRAGA, M.R.A., FUJII, M.T. & CORDEIRO-MARINO, M. 1997. Monostromatic green algae (Ulvales, Chlorophyta) of Săo Paulo and Paraná states (Brazil): distribution, growth, and reproduction. Revta brasil. Bot. 20:197-203.
- CHAPMAN, A.R.O. 1973. A critique of prevailing attitudes towards the control of seaweed zonation on the seashore. Bot. Mar. 16:80-82.
- CORDERO, P.A. 1978. Phycological observations. VI. Mangrove-associated algae from Aklan, Philippines. Philipp. J. Biol. 7:275-296.
- CORDEIRO-MARINO, M., BRAGA, M.R.A., ESTON, V.R., FUJII, M.T. & YOKOYA, N.S. 1992. Mangrove macroalgal communities of Latin America: the state of art and perspectives. In Coastal Plant Communities of Latin America (U. Seeliger, ed.). Academic Press, New York, p.51-64.
- DAVEY, A. & WOELKERLING, W.J. 1980. Studies on Australian mangrove algae. I. Victorian communities: composition and geographic distribution. Proc. R. Soc. Vict. 91:53-66.
- DAVISON, I.R. & PEARSON, G.A 1996. Stress tolerance in intertidal seaweeds. J. Phycol. 32:197-211.
- ESTON, V.R., BRAGA, M.R.A., CORDEIRO-MARINO, M., FUJII, M.T. & YOKOYA, N.S. 1992. Macroalgal colonization patterns on artificial substrates inside Southeastern Brazilian mangroves. Aquat. Bot. 42:315-325.
- ESTON, V.R., YOKOYA, N.S., FUJII, M.T., BRAGA, M.R.A., PLASTINO, E.M. & CORDEIRO-MARINO, M. 1991. Mangrove macroalgae in Southeastern Brazil: spatial and temporal patterns. Rev. Brasil. Biol. 51:829-837.
- HADLICH, R.M. 1984. Contribuiçăo ao levantamento taxonômico das algas marinhas bentônicas do mangue do Itacorubi Florianópolis Ilha de Santa Catarina Brasil. Ínsula 14:121-138.
- HADLICH, R.M. & BOUZON, Z.L. 1985. Contribuiçőes ao levantamento taxonômico das algas marinhas bentônicas do mangue do Itacorubi - Florianópolis - Ilha de Santa Catarina - Brasil. II - Rhodophyta. Ínsula 15:89-116.
- KARSTEN, U. & KIRST, G.O. 1989a. The effect of salinity on growth, photosynthesis and respiration in the estuarine red alga Bostrychia radicans Mont. Helgol. Meeresunters. 43:61-66.
- KARSTEN, U. & KIRST, G.O. 1989b. The role of inorganic ions and D-sorbitol in the osmotic adjustment of the estuarine red alga Bostrychia radicans Mont. (Ceramiales, Rhodomelaceae). Crypt. Bot. 1:226-229.
- KARSTEN, U., KING, R.J. & KIRST, G.O. 1990. The distribution of D-sorbitol and D-dulcitol in the red algal genera Bostrychia and Stictosiphonia (Rhodomelaceae, Rhodophyta) - a re-evaluation. Br. Phycol. J. 25:363-366.
- KARSTEN, U., WEST, J.A., MOSTAERT, A., KING, R.J., BARROW, K. & KIRST, G.O. 1992a. Mannitol in the red alga Caloglossa J. Plant Physiol. 140:292-297.
- KARSTEN, U., WEST, J.A. & ZUCCARELLO, G. 1992b. Polyol content of Bostrychia and Stictosiphonia (Rhodomelaceae, Rhodophyta) from field and culture. Bot. Mar. 35:11-19.
- KARSTEN, U., WEST, J.A., ZUCARELLO, G. & KIRST, G.O. 1994. Physiological ecotypes in the marine alga Bostrychia radicans (Ceramiales, Rhodophyta) from the east coast of the U.S.A. J. Phycol. 30:174-182.
- KING, R.J. 1990. Macroalgae associated with the mangrove vegetation of Papua New Guinea. Bot. Mar. 33:55-62.
- KING, R.J. & PUTTOCK, C.F. 1994. Macroalgae associated with mangroves in Australia: Rhodophyta. Bot. Mar. 37:181-191.
- KING, R.J. & WHEELER, M.D. 1985. Composition and geographic distribution of mangrove macroalgal communities in New South Wales. Proc. Linn. Soc. N.S.W. 108:97-117.
- LAMBERT, G., STEINKE, T.D. & NAIDOO, Y. 1987. Algae associated with mangroves in southern African estuaries. I. Rhodophyceae. S. Afr. J. Bot. 53:349-361.
- MANN, F.D. & STEINKE, T.D. 1988. Photosynthetic and respiratory responses of the mangrove-associated red algae, Bostrychia radicans and Caloglossa leprieurii S. Afr. J. Bot. 54:203-207.
- MESQUITA, A.R. & HARARI, J. H. 1988. Tides and tide gauges of Cananéia and Ubatuba, Brazil (lat 24°). Relatório Interno do Instituto Oceanográfico da Universidade de Săo Paulo, n.11.
- MIRANDA, P.T.C. 1986. Composiçăo e distribuiçăo das macroalgas bentônicas no manguezal do Rio Ceará (Estado do Ceará - Brasil). Dissertaçăo de mestrado, Universidade Federal de Pernambuco, Recife.
- MITCHELL, G.J., MONTEIRO, D.F. & MEDINA, R.S. 1974. Observaçőes ficológicas no manguezal de Piedade. Leandra 4-5:137-142.
- NARASIMHA RAO, G.M. 1995. Seasonal growth, biomass, and reproductive behavior of three species of red algae in Godavari estuary, India. J. Phycol. 31:209-214.
- OLIVEIRA, E.C. 1984. Brazilian mangrove vegetation with special emphasis on the seaweeds. In Hydrobiology of the mangrove (F.D. Por & I. Dor, eds.). W. Junk Publishers, The Hague, p.55-65.
- PAULA, E.J., UGADIM, Y. & KANAGAWA, A.I. 1989. Macroalgas de Manguezais da Ilha de Maracá - Estado do Amapá, Brasil. Ínsula 19:95-114.
- PEDROCHE, F.F., WEST, J.A., ZUCCARELLO, G.C., SENTIES, G.A. & KARSTEN, U. 1995. Marine red algae of the mangroves in Southern Pacific México and Pacific Guatemala. Bot. Mar. 38:111-119.
- PHILLIPS, A., LAMBERT, G., GRANGER, J.E. & STEINKE, T.D. 1994. Horizontal zonation of epiphytic algae associated with Avicennia marina (Forssk.) Vierh. pneumatophores at beachwood mangroves nature reserve, Durban, South Africa. Bot. Mar. 37:567-576.
- PHILLIPS, A., LAMBERT, G., GRANGER, J.E. & STEINKE, T.D. 1996. Vertical zonation of epiphytic algae associated with Avicennia marina (Forssk.) Vierh. pneumatophores at beachwood mangroves nature reserve, Durban, South Africa. Bot. Mar. 39:167-175.
- PINHEIRO-JOVENTINO, F. & LIMA-VERDE, N.G. 1988. Ocorręncia e distribuiçăo de macroalgas no Estuário do Rio Cocó, Fortaleza, Brasil. Arq. Cięn. Mar. 27:83-89.
- POR, F. D., ALMEIDA PRADO, M.S. & OLIVEIRA, E.C. 1984. The mangal of the estuary and lagoon system of Cananéia (Brazil). In Hydrobiology of the mangrove (F.D. Por & I. Dor, eds.). W. Junk Publishers, The Hague, p.211-228.
- POST, E. 1968. Zur verbreitungs-okologie des Bostrychietum. Hydrobiologia 31:241-316.
- SAENGER, P., HEGERL, P.J. & DAVIE, J.D.S. 1983. Global status of mangrove ecosystems. The Environmentalist 3, Suppl. n. 3.
- STEINKE, T.D. & NAIDOO, Y. 1990. Biomass of algae epiphytic on pneumatophores of the mangrove, Avicennia marina, in the St. Lucia estuary. S. Afr. J. Bot. 56:226-232.
- TANAKA, J. & CHIHARA, M. 1984. Taxonomic studies of Japanese mangrove macroalgae. I. Genus Bostrychia (Ceramiales, Rhodophyceae) (2). Bull. Natn. Sci. Mus. 10:115-126.
- TANAKA, J. & CHIHARA, M. 1987. Species composition and vertical distribution of macroalgae in brackish waters of Japanese mangrove forests. Bull. Natn. Sci. Mus. 13:141-150.
- WEST, J.A. 1991. New records of marine algae from Perú. Bot. Mar. 34:459-464.
- WEST, J.A., ZUCCARELLO, G.C., PEDROCHE, F.F. & KARSTEN, U. 1992. Marine red algae of the mangroves in Pacific México and their polyol content. Bot. Mar. 35:567-572.
- WILKINSON, M. 1980. Estuarine benthic algae and their environment: a review. In The shore environment (J.H. Price, E.G. Irvine & W.F. Farnham, eds.). Academic Press, Oxford, v.2, p.425-486.
- YARISH, C., EDWARDS, P. & CASEY, S. 1980. The effects of salinity, and calcium and potassium variations on the growth of two estuarine red algae. J. Exp. Mar. Biol. Ecol. 47:235-249.
Publication Dates
-
Publication in this collection
28 Oct 1999 -
Date of issue
Aug 1999
History
-
Received
11 Sept 1998 -
Accepted
21 Feb 1999