Abstracts
The use of monoclonal antibodies for specific pectic epitopes is an important tool in the study of the cell wall. Throughout the development of mucilage cells of Araucaria angustifolia (Bertol.) Kuntze, a gradient of distribution was observed in relation to the pectic de-esterification, as well as to the increase of galactan and arabinan epitope distribution, and to the reduction of arabinogalactans proteins (AGPs) epitope at maturity. AGP and methyl-esterified homogalacturonan (HGA) were present in the mucilage. Galactans and arabinans were also observed in the mucilage, though with weak labelling. Degradation of AGP in the maturity of mucilage cells, in cell wall, as well as in the secretion, could be involved in the programmed cell death (PCD). Different labellings found among parenchyma and mucilage cells suggested differences in the cell wall properties of the mucilage cells.
Araucaria angustifolia; cell development; cell wall; monoclonal antibodies; mucilage cells; pectin
A utilização de anticorpos monoclonais para epitopos pécticos específicos é uma importante ferramenta no estudo da parede celular. Ao longo do desenvolvimento das células de mucilagem de Araucaria angustifolia (Bertol.) Kuntze foi observado um gradiente de distribuição com relação à de-esterificação de pectinas, bem como um aumento na distribuição dos epitopos de galactanos e arabinanos e uma redução na distribuição de arabinogalactanos proteínas (AGPs) na maturidade. AGP e homogalacturonanos (HGA) metil-esterificados foram detectados na mucilagem. Galactanos e arabinanos foram também observados na mucilagem, embora com fraca marcação. A degradação de AGPs na maturidade das células de mucilagem, tanto na parede celular, como na secreção, poderia estar envolvida no processo de morte celular programada (MCP). Diferentes marcações encontradas entre as células de parênquima e células de mucilagem sugerem diferenças nas propriedades da parede celular das células mucilaginosas.
anticorpos monoclonais; Araucaria angustifolia; células de mucilagem; desenvolvimento celular; parede celular; pectinas
ARTICLES
Immunocytochemistry of the mucilage cells of Araucaria angustifolia (Bertol.) Kuntze (Araucariaceae)
Imunocitoquímica das células de mucilagem de Araucaria angustifolia (Bertol.) Kuntze (Araucariaceae)
Alexandra Antunes MastrobertiI,1 1 Corresponding author: amastroberti@yahoo.com ; Jorge Ernesto de Araujo MariathI
IUniversidade Federal do Rio Grande do Sul, Instituto de Biociências, Departamento de Botânica, Av. Bento Gonçalves, 9500 Prédio 43423 sala 210, 91501-970 Porto Alegre, RS. Brazil
ABSTRACT
The use of monoclonal antibodies for specific pectic epitopes is an important tool in the study of the cell wall. Throughout the development of mucilage cells of Araucaria angustifolia (Bertol.) Kuntze, a gradient of distribution was observed in relation to the pectic de-esterification, as well as to the increase of galactan and arabinan epitope distribution, and to the reduction of arabinogalactans proteins (AGPs) epitope at maturity. AGP and methyl-esterified homogalacturonan (HGA) were present in the mucilage. Galactans and arabinans were also observed in the mucilage, though with weak labelling. Degradation of AGP in the maturity of mucilage cells, in cell wall, as well as in the secretion, could be involved in the programmed cell death (PCD). Different labellings found among parenchyma and mucilage cells suggested differences in the cell wall properties of the mucilage cells.
Key words: Araucaria angustifolia, cell development, cell wall, monoclonal antibodies, mucilage cells, pectin
RESUMO
A utilização de anticorpos monoclonais para epitopos pécticos específicos é uma importante ferramenta no estudo da parede celular. Ao longo do desenvolvimento das células de mucilagem de Araucaria angustifolia (Bertol.) Kuntze foi observado um gradiente de distribuição com relação à de-esterificação de pectinas, bem como um aumento na distribuição dos epitopos de galactanos e arabinanos e uma redução na distribuição de arabinogalactanos proteínas (AGPs) na maturidade. AGP e homogalacturonanos (HGA) metil-esterificados foram detectados na mucilagem. Galactanos e arabinanos foram também observados na mucilagem, embora com fraca marcação. A degradação de AGPs na maturidade das células de mucilagem, tanto na parede celular, como na secreção, poderia estar envolvida no processo de morte celular programada (MCP). Diferentes marcações encontradas entre as células de parênquima e células de mucilagem sugerem diferenças nas propriedades da parede celular das células mucilaginosas.
Palavras-chave: anticorpos monoclonais, Araucaria angustifolia, células de mucilagem, desenvolvimento celular, parede celular, pectinas
Introduction
Several authors had described certain unusual cells in the mesophyll of the genus Araucaria Juss. as cells of large volume with a rare presence of chloroplasts, suggesting a function of water storage (Baker & Smith 1910, Griffith 1950, Vasiliyeva 1969, Monteiro et al. 1977). Bamber et al. (1978) observed that these cells had pectic partitions, which subdivided the cell lumen, forming numerous compartments. Owing to these reticulated partitions, the authors described them as " compartmented cells."
Nevertheless, the cytological characteristics observed during the developmental stages described in ultrastructural studies by Mastroberti & Mariath (2003) suggested that compartmented cells of Araucaria angustifolia (Bertol.) Kuntze could be a type of mucilage cell, confirming its function of water storage through apoplastic tracers and histochemical tests. Mastroberti & Mariath (2008) described four developmental stages for these cells. In stage 1, the shoot apex volume increased in some mucilage cells showing that the cells were very young. In stage 2, these cells reached greater volume and became abundant. Mucilage was continuously deposited between the cytoplasm and the vacuole. A cavity was formed by the shrinkage of the tonoplast. In stage 3, the mucilage cells were still abundant and the cytoplasmic amount decreased. In stage 4, the cells were completely filled with pectic mucilage that led to the formation of denser regions. The nucleus and the cytoplasm degenerated, but the cell wall and mucilage integrity were maintained.
Mucilage cells are known to occur in several families, e.g., Lauraceae (Bakker & Gerritsen 1989, Bakker et al. 1991), Cactaceae (Trachtenberg & Fahn 1981, Trachtenberg & Mayer 1981a, b, 1982a, b), Malvaceae (Bakker & Gerritsen 1992), and others. The secreted mucilage accumulates between the cell wall and the protoplast in Cinnamomum burmanni Blume, Opuntia ficus-indica (L.) Mill. and Hibiscus schizopetalus (Mast.) Hook., which is not identical to the secretory process observed in A. angustifolia, in which the mucilage accumulates between the cytoplasm and the vacuole (Mastroberti & Mariath 2008).
Mucilages are complex acidic or neutral polysaccharide polymers of high molecular weight. They may be associated with various functions: serving as a food reserve, helping in water retention, acting as a lubricant of the growing root tip, as adhesive in seed dispersal, and aiding in capturing of insects in carnivorous plants. They also regulate the germination of seeds, and may probably be useful for other unknown purposes (Fahn 1979).
According to Esau (1965) and Fahn (1979), polysaccharides contribute to the plants' resistance to dry climate. It is well known that the pectins are polysaccharides of hydrophilic nature, and these substances are the base of mucilage composition.
Pectic polymers are classified into three major domains: homogalacturonans (HGA), which have a methyl-esterification variation, responsible for the ionic regulation, hydration, and gel states of the cell wall matrix; rhamnogalacturonans II (RG-II), which occur in all primary cell walls in the form of complex side chains of the HGA backbone; and the rhamnogalacturonans I (RG-I) formed by alternation of HGA by the rhamnoses (Ridley et al. 2001). Approximately 80% of the residues of rhamnoses, in RG-I undergo substitutions by side chains, rich in arabinans and galactans (Krishnamurthy 1999, Scheller et al. 1999, Pérez et al. 2000). It is generally accepted that pectins are synthesized in a highly methyl-esterified form (Micheli 2001). The degree of pectin esterification results in varying states of pectin. In the same wall of a cell, there are many domains where this degree is modified. Thus, the methyl-esterification degree and the change in the pectic molecule are important in the functional properties of the plant cell wall (Knox 1997).
In general, pectins have an important physiological role, contributing to the cell wall hydration, strength and flexibility of non-lignified bodies, fruit maturing, signalizing to wound and to pathogen-host interactions, morphogenetic effects (regulatory effect in hormonal action and synthesis) and intercellular adhesion. Consequently, pectin could be considered not only as a structural polymer, but also as the main informative polymer of the plant cell wall, because of its influence in the calcium entrance regulation, which in turn regulates the plasmalemma permeability (Van Cutsem & Messiaen 1994).
In addition to the major polysaccharides, the growing plant cell wall also contains structural proteins (Showalter 1993). Several classes of cell wall structural proteins have been described in the plants, classified into hydroxyproline-rich glycoprotein (HRGP), glycine-rich protein (GRP), proline-rich protein (PRP), etc. Unlike the structural proteins listed above, arabinogalactan proteins (AGPs) are soluble and heavily glycosylated (Fincher et al. 1983, Showalter 1993). Multiple AGP forms are found in plant tissues, either in the cell wall or associated with the plasmalemma, and they display tissue- and cell-specific expression patterns (Pennel et al. 1989). The AGPs have a sticky quality and show specific binding to pectins, being involved in the cell wall adhesion (Cosgrove 1997). The AGPs are also implicated in the growth, nutrition, and other developmental processes (Pennel & Roberts 1990).
Immunocytochemical studies have contributed to the study of identifying, defining, and understanding the special distribution of pectins in the cell and in the apoplast. Several immunocytochemical studies have been conducted in many plants, showing that during cell development, modifications were observed in pectin esterification (Fujino & Itoh 1998, Sutherland et al. 1999, Bush et al. 2001), in the presence of galactans and arabinans (Jones et al. 1997, Bush et al. 2001, Willats et al. 2001) and in the calcium binding (Liners et al. 1994, His et al. 1997).
Monoclonal antibodies (MAbs) are important tools to show the spatial location and distribution of different pectic and AGPs epitopes of the cell wall (Knox 1997, Bush et al. 2001). However, very little is known about the mucilage immunolocalization besides those secreted in seeds, as in Arabidopsis thaliana (Willats et al. 2001).
A. angustifolia is a native species from Brazil and anatomical studies pertaining to this species should be encouraged. Besides, immunocytochemical studies in gymnosperms and in mucilage cells are uncommon (Mogami et al. 1999). Hence, it is important to point out that this is the first report on the immunocytochemistry of an idioblast cell wall, as well as investigation of the process of mucilage formation in the stages of leaf development.
In this work, five MAbs have been used to investigate the distribution of different pectic epitopes and AGPs during leaf development in A. angustifolia, using epifluorescence microscopy.
Material and methods
Seeds of Brazilian Pine (A. angustifolia (Bertol.) Kuntze) were collected from São Francisco de Paula National Forest (FLONA de São Francisco de Paula, 29º23' and 29º27' S and 50º23' and 50º25' W) in the state of Rio Grande do Sul, Brazil. The seeds were cultivated in a seedbed in full sunlight. The leaves were collected when the young plants reached about 4 months of age (100-210 mm height). Planting was undertaken in the green house at the Genetic Department of the Federal University of Rio Grande do Sul (UFRGS). Three stages of leaf development were observed and studied. Immature leaves (10-15 mm length) were collected from the shoot apex to observe stages 1 and 2. Mature leaves (30-45 mm length) were collected from the middle portion of the stem.
Material processing for immunofluorescence The middle portions of the immature and mature leaves from young plants were fixed in 2.5% glutaraldehyde, 2% formaldehyde, in 0.1 M sodium phosphate buffer, pH 7.2 (Roland & Vian 1991), dehydrated in an ethanol-graded series (20, 30, 50, 60, 70, 80, 90, and 100%), and embedded in LR White " Hard Grade" (London Resin Company). The semithin sections (350-500 nm) were cut on a Leica Ultracut UCT microtome with a glass knife, and adhered to glass slides recovered with poly-L-lysine (Sigma). The sections observed in the bright field microscope were stained with 1% Toluidine Blue O (C.I. 52040), pH 8-9 (Souza 1998). Sections were incubated with the MAbs JIM5, JIM7, JIM13, LM5, and LM6 (provided by Dr. Keith Roberts, John Innes Centre, and Dr. Paul Knox, Centre for Plant Sciences, University of Leeds, UK), which recognize the epitopes described in table 1.
Incubation of the sections for immunofluorescence Sections were hydrated with saline phosphate buffer (PBS) of pH 7.1 (Harris 1994), blocked with a centrifuged solution with 3% of no-fat milk powder in PBS, and incubated with the primary antibody in PBS for 1-2 h. Primary antibody incubation was left out for controls. The material was washed in PBS and then incubated with the secondary antibody Goat anti-rat FITC (Sigma) in PBS for 1-2 h in a dark room. After washing with PBS, sections were mounted in 0.1% para-phenylenediamine (PPD) (Sigma), 10 mM of PBS with 10% of 0.15 M NaCl and 90% glycerol anti-fade solution, and observed on a Leica DMR microscope equipped with epifluorescence (excitation filter 450-490 nm).
Results
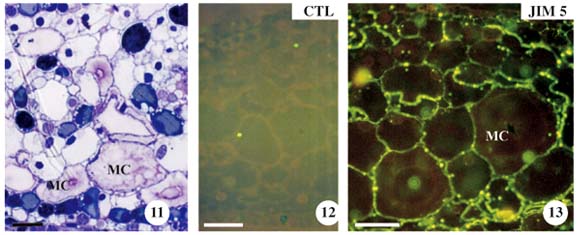
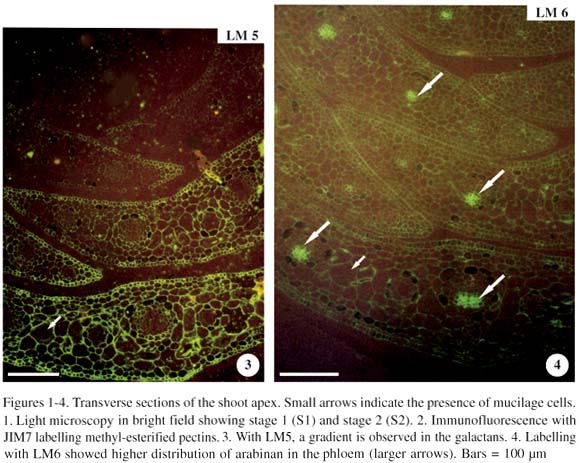
Stages 1 and 2 (immature leaves) were observed in transverse section of the shoot apex (figure 1). In stage 1, immature leaves had an undistinguished mesophyll, and mucilage cells were not observed (data not shown). In stage 2, the immature leaf possessed mucilage cells. Material incubated with JIM7 (methyl-esterified pectins) had strong and homogeneous labelling (figure 2). With LM5 (galactans), there was a gradient observed throughout the leaf development in which the galactan epitope increased with the leaf maturity (figure 3). On the other hand, labelling with LM6 (arabinans) had a homogeneous, low distribution of these chains throughout the leaf development. However, a strong labelling of the arabinan epitope was observed in the region of the phloem (figure 4, arrows).
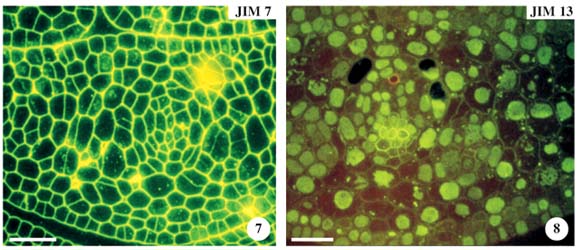
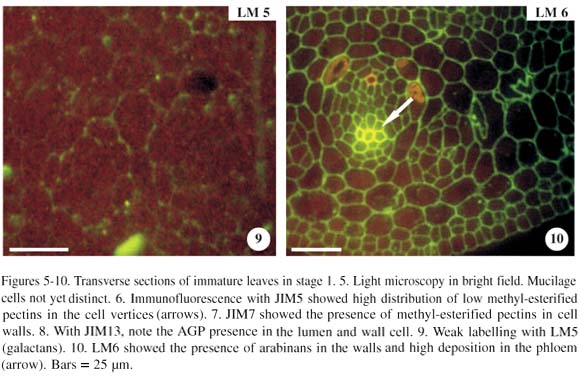
In the transverse section, a leaf in stage 1 (figure 5), when incubated with JIM5 (pectins with low methyl-esterification) resulted either in a weak or no labelling in the mesophyll, making it impossible to distinguish the differences in labelling of other mucilage cells. Epidermal and hypodermal cells were not labelled with JIM5. These chains of low methyl-esterification, however, were abundant in the cell vertices (figure 6, arrows). Observations with JIM7, differently from JIM5, showed that the cell walls were strong and uniformly labelled in all tissues (figure 7). With JIM13 (AGPs), cells were weakly labelled, except the phloem cells. Nevertheless, we observed that the cell lumen had high labelling with AGPs (figure 8). The anti-AGP MAb, JIM13, binds to plasmalemma, but in the figures, this epitope in the plasmalemma and cell wall cannot be easily distinguished.
Results with LM5 (galactans) showed weak or no labelling in the leaf tissues in stage 1 (figure 9). The LM6 (arabinans) showed that labelling in the walls was moderate and uniform, except for the phloem cells, which had strong labelling (figure 10, arrow).
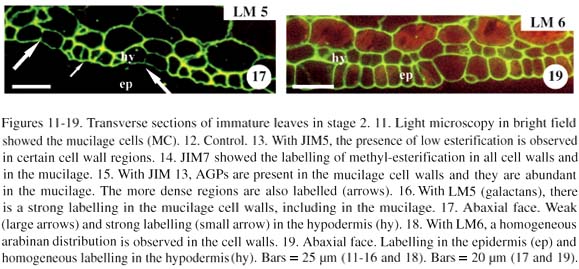
In stage 2, the leaf was still immature, although young mucilage cells were already observed (figure 11). The control was demonstrated without the primary antibody (figure 12). With JIM5, mesophyll cell wall, epidermal cells were labelled only in some regions (figure 13). With JIM7, the labelling was similar as in stage 1 (figure 14). Results with JIM13 showed an increase in AGP labelling in the cell wall of different cell tissues (figure 15). With LM5, the tissues were strongly labelled, increasing the galactan epitope from stage 1 (figure 9) to stage 2 (figure 16). The absence of galactans was observed in the epidermal cell wall. On the other hand, galactans occurred in varied distributions in the hypodermis, in the zones of decreased cell elongation (figure 17, arrows). The LM6 showed an arabinan distribution similar to the galactans (figure 18), except for the presence of arabinans that is observed in the epidermal cell wall (figure 19).
Mucilage cells and the neighbouring parenchyma cells had the same strong labelling in the cell wall when the antibodies JIM5 (figure 13), JIM7 (figure 14), and LM6 (figure 17) were used, but with JIM13 and LM5, the mucilage cell wall showed stronger labelling than the parenchyma cell wall (figures 15 and 16).
With JIM7, mucilage of the mucilage cells had moderate labelling only in some regions of the cell lumen (figure 14). With JIM13, there was a moderate detection of AGP in the mesophyll cell wall, which was stronger in the mucilage cell wall (figure 15). Moreover, with this antibody, a strong mucilage labelling was also observed in the denser regions of the mucilage (figure 15, arrows). With JIM5, LM5, and LM6, the pectin with low esterification, galactans, and arabinans, respectively, presented weak labelling in the mucilage (figures 13, 16, and 18).
The observed mature leaves (stage 3) were those in which the mucilage cell lost its polygonal outline and in which the protoplast degenerated (figure 20). A control test was conducted without the primary antibody and this can be observed in figure 21. The JIM5 showed weak labelling of low methyl-esterified pectins in the leaf tissue walls, except in specific regions, where the presence of these chains was strong (figure 22). Nevertheless, cell walls of all tissues were labelled strongly with JIM7, where the mucilage was also labelled with this antibody, thus revealing a clear presence of methyl-esterified chains (figure 23, arrow). When incubated with JIM13, a decrease in AGP in the mucilage cell wall and an increase in this compound in the neighbouring parenchyma cell wall (figure 24, arrow) was observed, showing an inversion in the AGP labelling when compared with stage 2. Results with LM5 showed that mucilage cells also had a strong labelling, compared with the neighbouring cells with moderate labelling of galactan chains in the spongy parenchyma cell wall and an absence in the palisade parenchyma (figure 25). With LM6, there was a strong and uniform distribution of arabinans in the cell wall of different leaf tissues (figure 26).
In relation to the mucilage, the occurrence of methyl-esterified pectins (figure 23, arrow) and a severe reduction in the AGP distribution were also observed (figure 24, arrow). Moderate labelling with LM5 and LM6 showed that galactans and arabinans, respectively, were present in the mucilage of these cells and that they increased following the leaf maturity (figures 25 and 26, arrow).
The unstructured secretion stage lasts until a denser organized net stage is resulted from the cell development owing to biochemical alterations that the mucilage undergoes.
Table 2 shows the synthesis of different labellings obtained in the analyzed stages of the leaves.
Discussion
The results indicated the differences in the distribution of pectic epitopes between immature and mature leaves. In the mucilage cell wall, a gradient was observed throughout the development, in relation to methyl-esterification of pectins and distribution of galactans, arabinans, and AGP. Spongy parenchyma cells (neighbouring cells) also showed a gradient in relation to the reduction of these pectic epitopes.
There was an increase in the presence of low and high methyl-esterification pectins (JIM5 and JIM7), not only with regard to the mucilage cells, but also the other cells of the spongy parenchyma, observed in stages 1 and 2 (shoot apex). The high methyl-esterification (JIM7) did not present any variation during leaf or cellular maturation. These data are in accordance with previous findings that showed that pectins are synthesized in a highly methyl-esterified type in the Golgi (Knox 1997, Bush et al. 2001).
The JIM5 and JIM7 antibodies had been characterized by Willats et al. (2000) and Clausen et al. (2003), who found that JIM5 showed a binding with antigens of more than 40% methyl-esterified chains. Thus, the results with JIM5 should be considered with caution in terms of an indicator for low esterification.
The detection of high methyl-esterified pectins in primary walls by using the JIM7 MAb was already described by Knox et al. (1990). Also, Vandenbosh et al. (1989) and Knox et al. (1990) demonstrated that with JIM5, there was strong labelling in the cell vertices and intercellular spaces. This was observed in the immature leaves from stage 1 in A. angustifolia, where the labelling in the vertice regions was very clear. Furthermore, this process occurred quickly as de-esterification occurs in the primordium leaf.
Van Cutsem & Messiaen (1994) and Morris et al. (2000) observed that when the cell wall rigidity was lower, there was a higher degree of methyl-esterification, thus making the cell wall expansion easier during the growth process. The leaves of stage 1 presented a weak labelling with JIM5 in the elongation regions, with more intensity in the cell vertices. Furthermore, the leaf continued its growth, in the absence or rare occurrence of low methyl-esterified pectins in the epidermal and hypodermal cell walls of immature leaves. These results were in accordance with previous observations of the epidermal cells on the epicotyl of Pisum sativum L., where the JIM5 epitope was distributed in regions of the cell wall that did not elongate (Fujino & Itoh 1998).
In mature leaves (stage 3), labelling with JIM5 seemed to be similar to stage 2 (immature leaves) because the de-esterification process was observed in the shoot apex (between stages 1 and 2). In addition, the distribution of pectins with low methyl-esterification (JIM5) was not uniform. Some regions seen through the wall with strong labelling could be pit regions. Casero & Knox (1995) and Sutherland et al. (1999) showed that JIM5 had relevant labelling in the pit regions, which were observed in the form of rays in the inner wall layer. Methyl-esterified pectins (JIM7) remain abundant and uniformly distributed in the mature leaves. Similarly, Bush et al. (2001) observed the development of the potato tubercle stolons (Solanum tuberosum) in which the cell walls were rich in highly methyl-esterified pectins.
Cell wall rigidity is also related to the presence of side chains of galactans and arabinans in the RG-I (Jones et al. 1997). In immature leaves of A. angustifolia, no labelling with LM5 in the epidermal cells was observed. In contrast, galactans occurred in different distributions in the hypodermis in the regions of decreased cell elongation. Jones et al. (1997) also observed that these pectic epitopes were absent in the epidermal and sub-epidermal cells in tomato fruits (Lycopersicon esculentum Mill.). The side chains decreased the ability of the pectins to form more resistant gels. Thus, the absence of galactans in the epidermis and the different distribution in the hypodermis of this epitope in stages 1 and 2 are believed to correlate with the maintenance of the wall integrity during leaf elongation. According to Willats et al. (1999) and Bush et al. (2001), galactans were not detected in the cell walls of meristematic regions of roots or shoot apex, but appeared in certain stages of tissue development. In A. angustifolia, this aspect was clearly observed in the transverse section of the shoot apex, where there was an increase in galactan epitope between stages 1 and 2. In stages 2 and 3, the galactan epitope distribution was higher in the mucilage cell walls than in the parenchyma cells. McCartney et al. (2000) and McCartney & Knox (2002) suggested that the presence of these chains could be related to mechanical properties necessary for cell elongation. McCartney et al. (2003) also described that besides the elongation, this epitope marks the acceleration of this process, contributing to the elasticity of the cell wall. In the mucilage cells of A. angustifolia, there was a faster cell elongation process than in others, suggesting that the galactans responsible for the decrease in cell wall rigidity contribute to the increase in volume of these cells at maturity and also to their elasticity while storing water.
The LM6 is also linked to RG-I epitope of the side chains, or to the arabinans. In stages 1 and 2, the arabinan (LM6) epitope was distributed uniformly with moderate labelling in the cell walls of different tissues, when compared with the distribution of galactans. In stage 3, arabinan distribution was slightly higher in the parenchyma cells, in contrast to the LM5 labelling. Both galactan and arabinan epitopes may occur in different regions of the cell wall of one single cell (Orfila & Knox 2000). Moreover, they have been already observed separately in different developing cells (Willats et al. 1999, Willats et al. 2000). Bush et al. (2001) observed that the arabinan epitope was restricted to cell walls from the youngest regions of the stolon of S. tuberosum; and in mature cell walls the arabinan epitopes were dramatically reduced. The authors suggested that different side chains might be attached to RG-I backbones at different times, probably in response to cell development. In this way, it was possible to explain the differential distributions of these side chains between mucilage and other neighbouring cells in A. angustifolia, which showed differences in the development process, similar to the increase in volume of mucilage cells, cell wall elasticity at maturity, and senescence.
Most often, type II arabinogalactans (AG II) are associated with proteins forming AGPs. It has not yet been clarified if this polysaccharide is a part of the pectin complex. Knox et al. (1991) described an antibody with anti-AGP binding in the plasmalemma that had different distributions during cell development. In the figures presented in our work, the labelling of the plasmalemma and cell wall cannot be easily distinguished.
In immature leaves, a higher AGP presence was observed in the cell walls of mucilage cells in comparison with the mature mucilage cells, and an increase in distribution of the neighbouring parenchyma cells was also observed.
One of the most widespread functions of the AGP, mainly in the plasmalemma, would be the formation of the cell wall (Mogami et al. 1999). The AGPs are involved in different aspects of plant growth and development, including embryogenesis and cell proliferation (Majewska-Sawka & Nothnagel 2000), but the molecular basis of their actions is still unclear (Letarte et al. 2006).
Knox et al. (1989) used AGP as molecular label to observe future cells, which die in the meristem cell population. By using an anti-AGP for the tobacco leaf (Nicotiana tabacum L.) and corn coleoptiles (Zea mays), respectively, Herman & Lamb (1992) and Schindler et al. (1995) showed the autophagy of cells and proved AGP degeneration at maturity. In corn coleoptiles, high levels of AGP associated with plasmalemma have been found in young cells, while more mature cells showed low levels of AGP. This concept could also be applied to the mucilage cells of A. angustifolia. The increase in AGP in the plasmalemma in the mucilage cells during its secretion stage and the drastic reduction in the mature cells lead us to suggest that these epitopes would indicate a participation in the PCD process in accordance with the observations in previous ontogenetic studies of these cells (Mastroberti & Mariath 2003). Gao & Showalter (1999) stated that AGPs were possibly involved in the control of PCD in Arabidopsis cell culture. Letarte et al. (2006) suggested that AGPs could play an important role in the prevention of PCD of microspores. However, interaction of AGPs in the plasmalemma and extracellular matrix, to induce or to prevent the PCD, deserves more detailed attention as there are only a few studies pertaining to it.
The LM5 and LM6 epitope distribution showed a gradual increase of galactans and arabinans epitopes, respectively, throughout the mucilage cell development in A. angustifolia. This was in contrast to Arabidopsis thaliana seeds, which lacked these chains (Willats et al. 2001). Therefore, the presence of these side chains in the rhamnose would be feasible, forming a part of the mucilage constitution in A. angustifolia.
Willats et al. (2001) observed pectic polysaccharides in the mucilage secreted by A. thaliana seeds, proving the presence of HGA. These results were also observed for the mucilage cell secretions of A. angustifolia.
In contrast, AGP detection with JIM13 was strongest in the immature cell mucilage, with drastic reduction in the mature cells. The AGPs occurring in plant secretions and mucilage have been extensively studied in terms of their highly varied carbohydrate chemistry, and possess great potential for carrying information (Fincher et al. 1983). Clarke et al. (1979) suggested that AGPs could contribute and protect against damage caused by low temperatures or freezing. According to these authors, AGPs could also be associated with water storage function and drought resistance, when found in gums and mucilage. For this reason, the presence of high methyl-esterification and AGP in the mucilage could act as a shield in young plants from desiccation in adverse periods.
In conclusion, the morphological aspects of the mucilage cell have been established by Bamber et al. (1978) and Mastroberti & Mariath (2003), showing an initial granular secretion evolving to a more organized structure, which reflects developmental stages of this particular cell, owing to biochemical alterations of the mucilage. Immunocytochemical studies showed such changes throughout the pectic epitopes and AGP analysis during the leaf development.
Moreover, the methyl-esterification of pectins was always present in the walls, and the de-esterification occurred throughout the leaf development, corroborating with other dicotyledon studies. However, arabinans and galactans showed different distributions during the leaf development. Their functions are still argued, but it is possible to outstand that pectic gradients were generated by the temporal difference in which galactans and arabinans side chains bind with RG-I. Besides, the increased galactans in these cells, in comparison with the neighbouring cells, indicated expansion acceleration on the mucilage cells. The AGPs decreased in these cells, which is associated to the expansion and cell death, as described for other cellular types of dicotyledons. Thus, different labelling found among parenchyma and mucilage cells suggested the differences in the cell wall properties of mucilage cells.
Acknowledgments We are grateful to the Plant Anatomy Laboratory of the Universidade Federal do Rio Grande do Sul (UFRGS) and CNPq for financial resources and grants obtained. Thank also go to the Prof. J. Paul Knox and Prof. Keith Roberts (University of Leeds, UK) for the monoclonal antibodies kindly provided.
(received: November 09, 2006; accepted: October 11, 2007)
- BAKER, R.T. & SMITH, H.G. 1910. A research on the pines of Australia. Government Printer, Sydney.
- BAKKER, M.E. & GERRITSEN, A.F. 1989. A suberized layer in the cell wall of the mucilage cells of Cinnamomum Annals of Botany 63:441-448.
- BAKKER, M.E. & GERRITSEN, A.F. 1992. The development of mucilage cells in Hibiscus schizopetalus Acta Botanica Neerlandica 41:31-42.
- BAKKER, M.E., GERRITSEN, A.F. & VAN DER SCHAAF, P.J. 1991. Development of oil and mucilage cells in Cinnamomum burmanni An ultrastructural study. Acta Botanica Neerlandica 40:339-356.
- BAMBER, R.K., SUMMERVILLE, R. & GREGORY, J. 1978. Unusual cells in the mesophyll zone of leaves of Araucaria Australian Journal of Botany 26:177-187.
- BUSH, M.S., MARRY, M., HUXHAM, I.M., JARVIS, M.C. & McCANN, M.C. 2001. Developmental regulation of pectic epitopes during potato tuberisation. Planta 213:869-880.
- CASERO, P.J. & KNOX, J.P. 1995. The monoclonal antibody JIM5 indicates patterns of pectin deposition in relation to pit fields at the plasma-membrane-face of tomato pericarp cell walls. Protoplasma 188:133-137.
- CLARKE, A.E., ANDERSON, R.L. & STONE, B.A. 1979. Form and function of arabinogalactans and arabinogalactan-proteins. Phytochemistry 18:521-540.
- CLAUSEN, M.H., WILLATS, W.G.T. & KNOX, J.P. 2003. Synthetic methyl hexagalacturonate hapten inhibitors of antihomogalacturonan monoclonal antibodies LM5, JIM5 and JIM7. Carbohydrate Research 338:1797-1800.
- COSGROVE, D.J. 1997. Assembly and enlargement of the primary cell wall in plants. Annual Review of Cell and Developmental Biology 13:71-201.
- ESAU, K. 1965. Plant anatomy. John Wiley, New York.
- FAHN, A. 1979. Secretory tissues in plants. Academic Press, London.
- FINCHER, G.B., STONE, B.A. & CLARKE, A.E. 1983. Arabinogalactan-proteins: structure, biosynthesis and function. Annual Review of Plant Physiology 34:47-70.
- FUJINO, T. & ITOH, T. 1998. Changes in pectin structure during epidermal cell elongation in pea (Pisum sativum) and its implications for cell wall architecture. Plant and Cell Physiology 39:1315-1323.
- GAO, M. & SHOWALTER, A.M. 1999. Yariv reagent treatment induces programmed cell death in Arabidopsis cell cultures and implicates arabinogalactan protein involvement. Plant Journal 19:21-331.
- GRIFFITH, M.M. 1950. A study of the shoot apex and leaf histogenesis in certain species of Araucaria Tese de doutorado, University of California, Berkeley.
- HARRIS, N. 1994. Immunocytochemistry for light and electron microscopy. In Plant Cell Biology. A practical approach (N. Harris & K.J Oparka, eds.). Oxford University Press, London, p.157-176.
- HERMAN, E.M. & LAMB, C.J. 1992. Arabinogalactan-rich glycoproteins are localized on the cell surface and in intravacuolar multivesicular bodies. Plant Physiology 98:264-272.
- HIS, I., DRIOUICH, A. & JAUNEAU, A. 1997. Distribution of cell wall matrix polysaccharides in the epidermis of flax hypocotyl seedlings: calcium induced-acidification of pectins. Plant Physiology and Biochemistry 35: 631-644.
- JONES, L., SEYMOUR, G.B. & KNOX, J.P. 1997. Localization of pectic galactan in tomato cell walls using a monoclonal antibody specific to (1 ® 4) b-D-galactan. Plant Physiology 113:1405-1412.
- KNOX, J.P. 1997. The use of antibodies to study the architecture and the developmental regulation of plant cell walls. International Review of Cytology 171:79-120.
- KNOX, J.P., DAY, S. & ROBERTS, K. 1989. A set of surface glycoproteins forms an early marker of cell position, but not cell type, in the root apical meristem of Daucus carota L. Development 106:47-56.
- KNOX, J.P., LINSTEAD, P.J., KING, J., COOPER, C. & ROBERTS, K. 1990. Pectin esterification is spatially regulated both within cell walls and between developing tissues of root apices. Planta 181:512-521.
- KNOX, J.P., LINSTEAD, P.J., PEART, J., COOPER, C. & ROBERTS, K. 1991. Developmentally regulated epitopes of cell surface arabinogalactan proteins and their relation to root tissue pattern formation. Plant Journal 1:317-326.
- KRISHNAMURTHY, K.V. 1999. Methods in cell wall cytochemistry. CRC Press Boca Raton.
- LETARTE, J., SIMION, E., MINER, M. & KASHA, K.J. 2006. Arabinogalactans and arabinogalactan-proteins induce embryogenesis in wheat (Triticum aestivum L.) microspore culture. Plant Cell Reports 24:691-698.
- LINERS, F., GASPAR, T. & VAN CUTSEM, P. 1994. Acetyl- and methyl-esterification of pectins of friable and compact sugar-beet calli: consequences for intercellular adhesion. Planta 192:545-556.
- MAJEWSKA-SAWKA, A. & NOTHNAGEL, E.A. 2000. The multiple roles of arabinogalactan proteins in plant development. Plant Physiology 122:3-9.
- MASTROBERTI, A.A. & MARIATH, J.E.A. 2003. Compartmented cells in the mesophyll of Araucaria angustifolia (Araucariaceae). Australian Journal of Botany 51:267-274.
- MASTROBERTI, A.A. & MARIATH, J.E.A. 2008. Development of the mucilage cells of Araucaria angustifolia (Araucariaceae). Protoplasma. http://www.springerlink.com/ content/107724/?Content+ Status-Accepted (acessed 2008 February).
- McCARTNEY, L. & KNOX, J.P. 2002. Regulation of pectic polysaccharide domains in relation to cell development and cell properties in the pea testa. Journal of Experimental Botany 53:707-713.
- McCARTNEY, L., ORMEROD, A.P., GIDLEY, M.J. & KNOX, J.P. 2000. Temporal and spatial regulation of pectic (1-4) b-D-galactan in cell walls of developing pea cotyledons implications for mechanical properties. Plant Journal 22:105-113.
- McCARTNEY, L., STEELE-KING, C.G., JORDAN, E. & KNOX, J.P. 2003. Cell wall pectin (1-4) b-D-galactan marks the acceleration of cell elongation in the Arabidopsis seedling root meristem. Plant Journal 33:447-454.
- MICHELI, F. 2001. Pectin methylesterases: cell wall enzymes with important roles in plant physiology. Trends in Plant Science 6:414-419.
- MOGAMI, N., NAKAMURA, S. & NAKAMURA, N. 1999. Immunolocalization of the cell wall components in Pinus densiflora pollen. Protoplasma 206:1-10.
- MONTEIRO, S.M., FERREIRA, A.G. & FLORES, F.E.V. 1977. Anatomia da plântula de Araucaria angustifolia (Bert.) O. Ktze. In XXI Congresso Nacional de Botânica, Curitiba, 393-399.
- MORRIS, G.A., FOSTER, T.J. & HARDING, S.E. 2000. The effect of the degree of esterification on the hydrodynamic properties of citrus pectin. Food Hydrocolloid 14:227-235.
- ORFILA, C. & KNOX, J.P. 2000. Spatial regulation of pectic polysaccharides in relation to pit fields in cell walls of tomato fruit pericarp. Plant Physiology 122:775-781.
- PENNELL, R.I. & ROBERTS, K. 1990. Sexual development in pea is presaged by altered expression of arabinogalactan protein. Nature 344:547-549.
- PENNELL, R. I., KNOX, J.P., SCOFIELD, G.N., SELVENDRAN, R.R. & ROBERTS, K. 1989. A family of abundant plasma mebrane-associated glycoproteins related to the arabinogalactan proteins is unique to flowering plants. Journal of Cell Biology 108:1967-1977.
- PÉREZ, S., MAZEAU, K., HERVÉ, C. & DU PENHOAT, C.H. 2000. The three-dimensional structures of the pectic polysaccharides. Plant Physiology and Biochemistry 38:37-55.
- RIDLEY, B.L., O'NEILL, M.A. & MOHNEN, D. 2001. Pectins: structure, biosynthesis, and oligogalacturonide-related signaling. Phytochemistry 57:929-967.
- ROLAND, J.C. & VIAN, B. 1991. General preparation and staining of thin sections. In Electron Microscroscopy of Plant Cells (J.L Hall & E. Hawes, eds.). Academic Press, London, p. 2-66.
- SCHELLER, H.V., DOONG, R.L., RIDLEY, B.L. & MOHNEN, D. 1999. Pectin biosynthesis: a solubilized a-1,4-galacturonosyltransferase from tobacco catalyzes the transfer of galacturonic acid from UDP-galacturonic acid onto the non-reducing end of homogalacturonan. Planta 207:512-517.
- SCHINDLER, T., BERGFELD, R. & SCHOPFER, P. 1995. Arabinogalactan proteins in maize coleoptiles: developmental relationship to cell death during xylem differentiation but not to extension growth. Plant Journal 7:25-36.
- SHOWALTER, A.M. 1993. Structure and function of plant cell wall proteins. Plant Cell 5:9-23.
- SOUZA, W. 1998. Introdução à imunocitoquímica. In Técnicas básicas de microscopia eletrônica aplicadas às Ciências Biológicas (W. Souza, ed.). UENF, Rio de Janeiro, p. 104-105.
- SUTHERLAND, P., HALLET, I., REDGWELL, R., BENHAMOU, N. & MACRAE, E. 1999. Localization of cell wall polysaccharides during kiwifruit (Actinidia deliciosa) ripening. International Journal of Plant Science 160:1099-1109.
- TRACHTENBERG, S. & FAHN, A. 1981. The mucilage cells of Opuntia ficus-indica (L.) Mill.-development ultrastructure, and mucilage secretion. Botanical Gazette 142:206-213.
- TRACHTENBERG, S. & MAYER, A.M. 1981a. Calcium oxalate crystals in Opuntia ficus-indica (L.) Mill. Development and relation to mucilage cells a stereological analysis. Protoplasma 109:271-283.
- TRACHTENBERG, S. & MAYER, A.M. 1981b. Composition and properties of Opuntia ficus-indica mucilage. Phytochemistry 20:2665-2668.
- TRACHTENBERG, S. & MAYER, A.M. 1982a. Biophysical properties of Opuntia ficus-indica mucilage. Phytochemistry 21:2835-2843.
- TRACHTENBERG, S. & MAYER, A.M. 1982b. Mucilage cells, calcium oxalate crystals and soluble calcium in Opuntia ficus-indica Annals of Botany 50:549-557.
- VAN CUTSEM, P. & MESSIAEN, J. 1994. Biological effects of pectic fragments in plant cells. Acta Botanica Neerlandica 43:231-245.
- VANDENBOSH, K.A., BRADLEY, D.J., KNOX, J.P., PEROTTO, S., BUTCHER, G.W. & BREWIN, N. 1989. Common components of the infection thread matrix and the intercellular space identified by immunocytochemical analysis of pea nodules and uninfected roots. EMBO Journal 8:335-342.
- VASILIYEVA, G.V. 1969. A contribution to the comparative anatomy of leaves of the species of Araucaria Juss. Botanicheskij Zhurnal 54:448-459.
- WILLATS, W.G.T., MARCUS, S.E. & KNOX, J.P. 1998. Generation of a monoclonal antibody specific to (1-5)-a-L-arabinan. Carbohydrate Research 308:149-152.
- WILLATS, W.G.T., STEELE-KING, C.G., MARCUS, S.E. & KNOX, J.P. 1999. Side chains of pectic polysaccharides are regulated in relation to cell proliferation and cell differentiation. Plant Journal 20:619-628.
- WILLATS, W.G.T, LIMBERG, G., BUCHHOLT, H.C., VAN ALEBEECK, G.-J., BENEN, J., CHRISTENSEN, T.M. I.E., VISSER, J., VORAGEN, A., MIKKELSEN, J.D. & KNOX, J.P. 2000. Analysis of pectic epitopes recognised by hybridoma and phage display monoclonal antibodies using defined oligosaccharides, polysaccharides, and enzymatic degradation. Carbohydrate Research 327:309-320.
- WILLATS, W.G.T., McCARTNEY, L. & KNOX, J.P. 2001. In-situ analysis of pectic polysaccharides in seed mucilage and at the root surface of Arabidopsis thaliana Planta 213:37-44.
Publication Dates
-
Publication in this collection
23 June 2008 -
Date of issue
Mar 2008
History
-
Accepted
11 Oct 2007 -
Received
09 Nov 2006