Abstract
Lactating rat dams were submitted to short episodes (1, 2 or 3 weeks) of nutritional restriction by receiving the "regional basic diet" (RBD, with 8% protein) of low-income human populations of Northeast Brazil. Their pups were then studied regarding the developmental effects on body and brain weights. When the rats reached adulthood, cortical susceptibility to the phenomenon of spreading depression (SD) was evaluated by performing electrophysiological recordings on the surface of the cerebral cortex. SD was elicited at 20-min intervals by applying 2% KCl for 1 min to a site on the frontal cortex and its occurrence was monitored at 2 sites in the parietal region by recording the electrocorticogram and the slow potential change of SD. When compared to control rats fed a commercial diet with 23% protein, early malnourished rats showed deficits in body and brain weights (10% to 60% and 3% to 15%, respectively), as well as increases in velocity of SD propagation (10% to 20%). These effects were directly related to the duration of maternal dietary restriction, with pups malnourished for 2 or 3 weeks presenting more intense weight and SD changes than those malnourished for 1 week. The effects of 1-week restrictions on SD were less evident in the pups malnourished during the second week of lactation and were more evident in pups receiving the RBD during the third week. The results indicate that short episodes of early malnutrition during the suckling period can affect body and brain development, as well as the cortical susceptibility to SD during adulthood. The data also suggest that the third week of lactation is the period during which the brain is most sensitive to malnutrition, concerning the effects on SD
early protein malnutrition; spreading depression; brain growth spurt period
Braz J Med Biol Res, May 1997, Volume 30(5) 663-669
Spreading depression is facilitated in adult rats previously submitted to short episodes of malnutrition during the lactation period
A.P. Rocha-de-Melo and R.C.A. Guedes
Departamento de Nutrição, Universidade Federal de Pernambuco, 50670-901 Recife, PE, Brasil
 Correspondence and Footnotes
Correspondence and Footnotes
Correspondence and Footnotes
Correspondence and Footnotes
Correspondence and Footnotes
Correspondence and Footnotes
Abstract
Lactating rat dams were submitted to short episodes (1, 2 or 3 weeks) of nutritional restriction by receiving the "regional basic diet" (RBD, with 8% protein) of low-income human populations of Northeast Brazil. Their pups were then studied regarding the developmental effects on body and brain weights. When the rats reached adulthood, cortical susceptibility to the phenomenon of spreading depression (SD) was evaluated by performing electrophysiological recordings on the surface of the cerebral cortex. SD was elicited at 20-min intervals by applying 2% KCl for 1 min to a site on the frontal cortex and its occurrence was monitored at 2 sites in the parietal region by recording the electrocorticogram and the slow potential change of SD. When compared to control rats fed a commercial diet with 23% protein, early malnourished rats showed deficits in body and brain weights (10% to 60% and 3% to 15%, respectively), as well as increases in velocity of SD propagation (10% to 20%). These effects were directly related to the duration of maternal dietary restriction, with pups malnourished for 2 or 3 weeks presenting more intense weight and SD changes than those malnourished for 1 week. The effects of 1-week restrictions on SD were less evident in the pups malnourished during the second week of lactation and were more evident in pups receiving the RBD during the third week. The results indicate that short episodes of early malnutrition during the suckling period can affect body and brain development, as well as the cortical susceptibility to SD during adulthood. The data also suggest that the third week of lactation is the period during which the brain is most sensitive to malnutrition, concerning the effects on SD.
Key words: early protein malnutrition, spreading depression, brain growth spurt period
Introduction
Several effects of malnutrition on neural structure and function have been described in human beings, as well as in laboratory animals (1-3). These effects are more severe when nutritional deficiency occurs during the "brain growth spurt period", which corresponds to the time when the brain presents its maximal vulnerability to many types of insults, including malnutrition (3,4). During this period, the brain develops at a maximal rate and its weight increases at the highest velocity. Depending on the animal species, this critical period of intense development of the nervous system occurs at different time points early in life. In the rat, this period corresponds to the first 3 weeks of postnatal life, i.e., the lactation period (4). An inadequate diet imposed on the dams throughout this period can cause nutritional deficiency in the offspring and the effects of this condition on the nervous system will depend on the severity and duration of malnutrition (5).
Like other conditions that change brain excitability, early malnutrition also influences the phenomenon of spreading depression (SD) of cerebral electrical activity in adult rats (6,7). SD has been initially characterized as a decrease of spontaneous electrical activity of the cerebral cortex in response to electrical, chemical or mechanical stimulation of one point on the surface of the tissue (8). The "wave" of depression of electrical activity spreads concentrically from the stimulated point, while the originally depressed region starts to recover. Usually, a depressed area takes about 5-10 min to recover completely (8-10). Previous data from our laboratory have shown a facilitatory effect on brain susceptibility to SD in adult rats submitted to malnutrition during the gestation + suckling period (7,11-13).
During the brain growth spurt period, however, distinct developmental processes (for example, neurogenesis, gliogenesis and myelination) present peaks of maximal intensity at different time points, depending on the brain region to be considered. Thus, it is possible that short episodes of malnutrition acting at a certain time point during the lactation period may affect some neural structures and processes more effectively than others (3,5,14). Since, to our knowledge, there are no systematic studies investigating this possibility concerning the SD phenomenon, the present study was undertaken to address this issue. Our data show that short episodes of maternal malnutrition (1 or 2 weeks) during the lactation period followed by nutritional rehabilitation can induce changes in body and brain development in the pups, as well as long-lasting alterations in the cortical susceptibility to SD, evaluated when the pups become adults.
Material and Methods
The experiments were performed on 166 Wistar rat pups of both sexes, obtained from 33 litters (6-8 pups per litter). Short episodes of malnutrition during the lactation period were provoked by feeding the dams a diet prepared with the foodstuffs that constitute the daily fare of low-income human populations in the Northeast of Brazil, the so-called "regional basic diet" (RBD), containing 8% protein and 315.6 kcal% (i.e. 100 g of the diet provides 315.6 kcal) (11,12,15). According to the weeks of lactation during which malnutrition was imposed on the dams, 5 groups of pups were obtained, as follows: 3 groups of pups whose dams received the RBD for one week during the lactation period: during the first (group 1; N = 13; 6 males), second (group 2; N = 17; 8 males) or third (group 3; N = 15; 7 males) week of lactation; 2 groups of pups whose dams received the RBD for two weeks during the lactation period: either during the first plus second (group 1+2; N = 12; 7 males) or second plus third (group 2+3; N = 8; 4 males) week of lactation. After weaning, pups were fed a commercial diet (Anderson Clayton do Brasil) with 23% protein and 299.5 kcal% until adulthood.
During treatment with the RBD, half of the pups in each group were separated (S) daily from the mother for 2 to 8 h according to age, as described by Abel (2), to provoke a more severe restriction of food intake in these newborns at that respective time point. These pups were used to form five additional groups called 1S (N = 18; 8 males), 2S (N = 16; 7 males), 3S (N = 12; 8 males), 1+2S (N = 9; 3 males) and 2+3S (N = 10; 8 males), respectively.
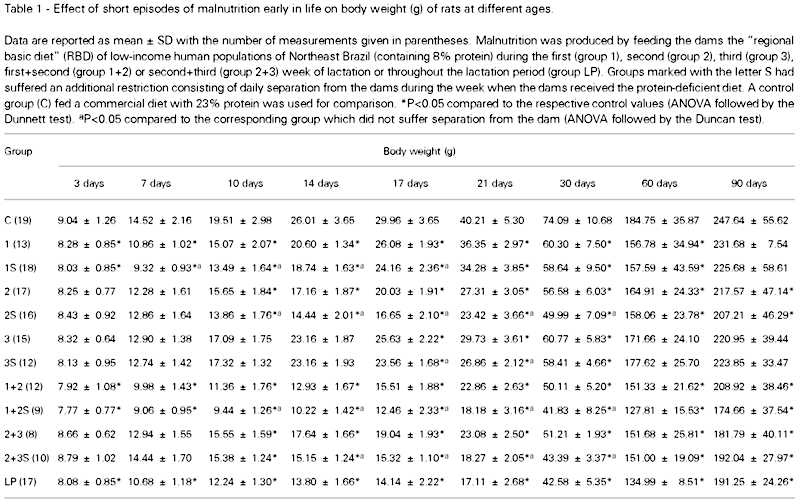
These 10 groups were compared with the following 2 reference groups which were not separated from the mothers: control group (N = 19; 9 males) consisting of pups whose dams received the commercial diet, and LP group (N = 17; 9 males) consisting of pups whose dams were fed the RBD throughout the lactation period. The animals were weighed on postnatal days 3, 7, 10, 14, 17, 21, 30, 60 and 90.
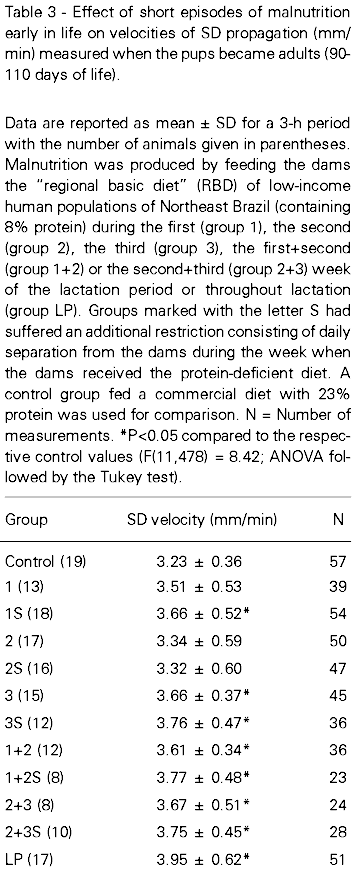
When the pups became adults (90-110 days) the phenomenon of SD was recorded continuously for 3 h according to the following protocol: under anesthesia with 1g/kg urethane plus 40 mg/kg chloralose, ip, two trephine holes (3 mm in diameter) were drilled into the right parietal bone. The spontaneous cortical electrical activity (ECoG) and the slow potential change accompanying SD were recorded simultaneously at these 2 points on the cortical surface using Ag-AgCl agar-Ringer electrodes (1 mm tip diameter; one electrode in each hole). A common reference electrode of the same type was placed on the nasal bones. SD was elicited at 20-min intervals through a third hole (2 mm in diameter) drilled into the right frontal bone by applying a small cotton pledget (1-2 mm in diameter) soaked in 2% KCl solution for 1 min. The three holes were aligned in the anteroposterior direction and parallel to the midline. The velocity of SD propagation was calculated based on the time required for an SD wave to cross the distance (range: 3.8 to 4.2 mm) between the 2 recording electrodes. During the recording session, which lasted 3 h, rectal temperature was maintained at 37 ± 1oC by means of a heating blanket. At the end of the session, the animal was killed with an overdose of anesthetic and the brain (including the cerebellum and excluding the olfactory bulb) was immediately removed and weighed (wet weight). The brain was then kept at 100oC and weighed daily until it reached a constant weight (dry weight). Statistical analysis was performed using ANOVA followed by post hoc tests when indicated.
Results
Body and brain weights
The pups submitted to short episodes of early nutritional restriction gained less weight than the controls. From the respective restriction period up to 30 days of life, the body weights of all malnourished groups were significantly lower than those of the control group. At 90 days, the differences in body weights were still significant in groups 2, 2S, 1+2, 1+2S, 2+3, 2+3S and LP as compared to the control. The following S-groups (which suffered separation from the dams during the lactation period) displayed significant reductions in body weight, as compared to the corresponding group which was not separated from the respective dams: group 1S differed from group 1 from day 7 to day 17 of life; group 2S differed from group 2 from day 10 to 30; group 3S differed from group 3 from day 17 to 21; group 1+2S differed from group 1+2 from day 10 to 30, and group 2+3S differed from group 2+3 from day 14 to 30. ANOVA provided the following F(11,154) values: 2.25 at day 3; 21.52 at day 7; 30.71 at day 10; 76.05 at day 14; 70.99 at day 21; 26.47 at day 30; 4.70 at day 60 and 2.97 at day 90. Data on body weights are shown in Table 1.
At 90-110 days, the wet brain weights of the following early malnourished groups were significantly lower than the control: 2S, 1+2, 1+2S, 2+3, 2+3S and LP (F(11,124) = 6.98, P<0.05). The dry brain weights were significantly lower in group 1+2S (F(11,73) = 4.58, P<0.05). Mean values for brain weights are shown in Table 2.
Velocity of SD propagation
Figure 1 shows electrophysiological recordings representative of the control and of the different malnourished groups. The ECoG and slow potential recordings confirmed the presence of SD after each stimulation with 2% KCl. Animals malnourished early in life presented higher velocities of SD propagation when compared to control rats. The mean SD velocities per hour ranged as follows: control group, from 3.15 to 3.30 mm/min; group 1, from 3.47 to 3.60 mm/min; group 1S, from 3.63 to 3.69 mm/min; group 2, from 3.26 to 3.42 mm/min; group 2S, from 3.27 to 3.36 mm/min; group 3, from 3.58 to 3.78 mm/min; group 3S, from 3.70 to 3.81 mm/min; group 1+2, from 3.54 to 3.69 mm/min; group 1+2S, from 3.64 to 3.85 mm/min; group 2+3, from 3.59 to 3.77 mm/min; group 2+3S, from 3.69 to 3.80 mm/min and group LP, from 3.84 to 4.06 mm/min. As shown in Table 3, when comparing SD propagation in the groups malnourished for 1 week, the least affected groups were those malnourished during the second week of lactation and the most affected ones were those malnourished during the third week of lactation. Comparison of groups previously malnourished for 2 weeks with those restricted for 1 week revealed that the former were more affected than the latter. However, the S-groups did not differ significantly from the corresponding groups submitted to malnutrition during the same weeks and suffering no separation from the mother during the restriction period.
- Examples of electrocorticogram (ECoG) and slow potential change (P) of SD in adult rats. C = Control rats fed a commercial diet with 23% protein. Short episodes of malnutrition were provoked by feeding the dams a protein-deficient diet (RBD, with 8% protein; see Material and Methods) during the first, second, or third week of the lactation period, or during the first+second and second+third weeks. LP = Rats submitted to malnutrition throughout the lactation period. The right inset shows the recording positions 1 and 2 and the position of the reference electrode (R). SD was elicited with 2% KCl applied to the frontal cortex at the times indicated by the arrows. The vertical calibration bar, which indicates 10 mV for the P recordings, also equals 1 mV for the ECoG recordings. A 1-min application of KCl usually elicited a single wave of SD as seen in the control rat. In some previously malnourished rats (e.g., groups 1, 1+2 and 2+3), the same stimulus elicited more than one SD wave. This fact is considered to be an indication of increased susceptibility of the cortical tissue to SD, in addition to the significantly higher velocities of SD propagation shown in Table 3.
Discussion
The major conclusion of the present study is that short episodes (1 or 2 weeks) of maternal nutritional restriction by consuming the RBD during the "brain growth spurt period" were sufficient to produce lasting alterations in the body and brain weights of the pups, as well as in cortical susceptibility to SD. The use of both male and female pups is unlikely to have been responsible for the differences in body weights between previously malnourished and control rats for two main reasons: 1) most weight differences occurred between 7 and 30 days of life, a period during which body weight is not much influenced by gender, and 2) except for groups 1+2S (which contained only 33.33% males) and 1+2, 3S and 2+3S (containing 58.33%, 66.67% and 80% males, respectively), the remaining 8 groups contained an approximately equal number of males and females (see ). Therefore, it is pertinent to note that even in the malnourished groups with a predominance of males the effect of early malnutrition on body weight was easily recognizable. Let us consider, for example, the mean body weights at 90 days of age of the groups 1+2 (208.92 ± 38.46 g), 2+3 (181.79 ± 40.11 g) and 2+3S (192.04 ± 27.97 g). These groups contained 58.33%, 50% and 80% males, respectively, in contrast to the control group (only 47.4% males). Furthermore, these groups displayed significant decreases in mean body weights compared to control (247.64 ± 55.62 g).
The short episodes of early malnutrition facilitated SD propagation when the rats became adults, as evaluated by the higher SD velocities compared to the control group. These effects were qualitatively similar to those previously observed in rats submitted to early malnutrition for longer periods of time (gestation + lactation or until adulthood; 7,12) and can be attributed to the nutritional deficiency imposed early in life, since protein-deficient diets administered during adulthood do not influence SD (12).
The mechanisms responsible for these effects still need to be identified. However, early malnutrition is known to reduce brain myelination, to increase brain cell packing density and to alter synaptic neurotransmitters (5). All of these changes have been postulated to participate in the effects of malnutrition on SD (6,7,12,13). Stern et al. (16) reported significantly higher brain norepinephrine and serotonin concentrations in early malnourished rats compared to controls, which could be considered as an indicator of increased brain packing density, occurring with different intensities at distinct time points during the rat "brain growth spurt period" (3,5,14). Concerning the effects on SD observed in the present study, it seems reasonable to consider the involvement of the above postulated alterations. Furthermore, it is known that the processes responsible for gliogenesis and myelination largely occur during early postnatal life and can be affected by malnutrition (14). This fact is of relevance for the present results, since the participation of glial cells and myelin in SD has been considered by some authors on the basis of experimental data (13,17-19). Interestingly, in rats, both processes present their peak of development at the beginning of the third week of lactation and, according to the present results, rats malnourished during this week were found to be more affected than those malnourished during the first or second week of the lactation period.
Based on the present data, we emphasize the view that pediatricians and nutrition researchers should become more aware of situations in which short episodes of early malnutrition could occur in children, since the possibility exists that these episodes, sometimes almost unnoticed, produce lasting impairment of brain structure and function.
References
1. Ballabriga A (1989). Some aspects of clinical and biochemical changes related to nutrition during brain development in humans. In: Evrard P & Minkowski A (Editors), Developmental Neurobiology. Raven Press, New York, 271-286.
2. Abel EL (1990). Effects of paternal and maternal undernutrition on growth of offspring in rats. Growth, Development, and Aging, 54: 125-129.
3. Morgane PJ, Austin-Lafrance RJ, Bronzino JD, Tonkiss J & Galler JR (1992). Malnutrition and developing central nervous system. In: Isaacson RL & Jensen KF (Editors), The Vulnerable Brain and Environmental Risks. Plenum Press, New York, 2-42.
4. Dobbing J (1968). Vulnerable periods in developing brain. In: Davison AN & Dobbing J (Editors), Applied Neurochemistry. Blackwell, Oxford, 287-316.
5. Morgane PJ, Miller M, Kemper T, Stern W, Forbes W, Hall R, Bronzino J, Kissane J, Hawrylewicz E & Resnick O (1978). The effects of protein malnutrition on the developing central nervous system in the rat. Neuroscience and Biobehavioral Reviews, 2: 137-230.
6. De Luca B, Cioffi LA & Bures J (1977). Cortical and caudate spreading depression as an indicator of neural changes induced by early malnutrition in rats. Activitas Nervosa Superior, 19: 130-131.
7. Guedes RCA (1984). On some conditions that influence cortical spreading depression. Anais da Academia Brasileira de Ciências, 56: 446-453.
8. Leão AAP (1944). Spreading depression of activity in the cerebral cortex. Journal of Neurophysiology, 7: 359-390.
9. Leão AAP (1972). Spreading depression. In: Purpura DP, Penry K, Tower DB, Woodbury DM & Walter RD (Editors), Experimental Models of Epilepsy. Raven Press, New York, 173-195.
10. Martins-Ferreira H (1983). Spreading depression in chicken retina. In: Ookawa T (Editor), The Brain and Behavior of the Fowl. Japan Scientific Society Press, Tokyo, 317-333.
11. Andrade AFD, Guedes RCA & Teodósio NR (1990). Enhanced rate of cortical spreading depression due to malnutrition: prevention by dietary protein supplementation. Brazilian Journal of Medical and Biological Research, 23: 889-893.
12. Guedes RCA, Andrade AFD & Cabral-Filho JE (1987). Propagation of cortical spreading depression in malnourished rats: facilitatory effect of dietary protein deficiency. Brazilian Journal of Medical and Biological Research, 20: 639-642.
13. Guedes RCA, Cabral-Filho JE & Teodósio NR (1992). GABAergic mechanisms involved in cortical spreading depression in normal and early malnourished rats. In: Do Carmo RJ (Editor), Spreading Depression. Experimental Brain Research Series, 23: 17-26.
14. Morgane PJ, Austin-Lafrance R, Bronzino J, Tonkiss J, Díaz-Cintra S, Cintra L, Kemper T & Galler JR (1993). Prenatal malnutrition and development of the brain. Neuroscience and Biobehavioral Reviews, 17: 91-128.
15. Teodósio NR, Lago ES, Romani SAM & Guedes RCA (1990). A regional basic diet from northeast Brazil as a dietary model of experimental malnutrition. Archivos Latinoamericanos de Nutricion, 40: 533-547.
16. Stern WC, Forbes WB, Resinick O & Morgane PJ (1974). Seizure susceptibility and brain amine levels following protein malnutrition during development in the rat. Brain Research, 79: 375-384.
17. Sugaya E, Takato M & Noda Y (1975). Neuronal and glial activity during spreading depression in cerebral cortex of cat. Journal of Neurophysiology, 38: 822-841.
18. Szerb JC (1991). Glutamate release and spreading depression in the fascia dentata in response to microdialysis with high K+: role of glia. Brain Research, 542: 259-265.
19. Guedes RCA, Andrade AFD & Cavalheiro EA (1988). Excitatory amino acids and cortical spreading depression. In: Cavalheiro EA, Lehman J & Turski L (Editors), Frontiers in Excitatory Amino Acid Research. Liss, New York, 667-670.
Address for correspondence: R.C.A. Guedes, Departamento de Nutrição, Universidade Federal de Pernambuco, 50670-901 Recife, PE, Brasil.
Research supported by CAPES, and FACEPE (No. 0782-4.05/93). R.C.A. Guedes is the recipient of a CNPq fellowship (No. 52.1706/94). This work is part of a Master's thesis presented by A.P. Rocha-de-Melo to the Departamento de Nutrição, Universidade Federal de Pernambuco. Received February 22, 1996. Accepted February 14, 1997.
- 2. Abel EL (1990). Effects of paternal and maternal undernutrition on growth of offspring in rats. Growth, Development, and Aging, 54: 125-129.
- 6. De Luca B, Cioffi LA & Bures J (1977). Cortical and caudate spreading depression as an indicator of neural changes induced by early malnutrition in rats. Activitas Nervosa Superior, 19: 130-131.
- 7. Guedes RCA (1984). On some conditions that influence cortical spreading depression. Anais da Academia Brasileira de Cięncias, 56: 446-453.
- 8. Leăo AAP (1944). Spreading depression of activity in the cerebral cortex. Journal of Neurophysiology, 7: 359-390.
- 11. Andrade AFD, Guedes RCA & Teodósio NR (1990). Enhanced rate of cortical spreading depression due to malnutrition: prevention by dietary protein supplementation. Brazilian Journal of Medical and Biological Research, 23: 889-893.
- 12. Guedes RCA, Andrade AFD & Cabral-Filho JE (1987). Propagation of cortical spreading depression in malnourished rats: facilitatory effect of dietary protein deficiency. Brazilian Journal of Medical and Biological Research, 20: 639-642.
- 14. Morgane PJ, Austin-Lafrance R, Bronzino J, Tonkiss J, Díaz-Cintra S, Cintra L, Kemper T & Galler JR (1993). Prenatal malnutrition and development of the brain. Neuroscience and Biobehavioral Reviews, 17: 91-128.
- 15. Teodósio NR, Lago ES, Romani SAM & Guedes RCA (1990). A regional basic diet from northeast Brazil as a dietary model of experimental malnutrition. Archivos Latinoamericanos de Nutricion, 40: 533-547.
- 16. Stern WC, Forbes WB, Resinick O & Morgane PJ (1974). Seizure susceptibility and brain amine levels following protein malnutrition during development in the rat. Brain Research, 79: 375-384.
- 17. Sugaya E, Takato M & Noda Y (1975). Neuronal and glial activity during spreading depression in cerebral cortex of cat. Journal of Neurophysiology, 38: 822-841.
- 18. Szerb JC (1991). Glutamate release and spreading depression in the fascia dentata in response to microdialysis with high K+: role of glia. Brain Research, 542: 259-265.
Correspondence and Footnotes
Publication Dates
-
Publication in this collection
13 Oct 1998 -
Date of issue
May 1997