Abstract
Human skinned muscle fibers were used to investigate the effects of bovine serum albumin (BSA) on the tension/pCa relationship and on the functional properties of the Ca2+-release channel of the sarcoplasmic reticulum (SR). In both fast- and slow-type fibers, identified by their tension response to pSr 5.0, BSA (0.7-15 µM) had no effect on the Ca2+ affinity of the contractile proteins and elicited no tension per se in Ca2+-loaded fibers. In contrast, BSA (>1.0 µM) potentiated the caffeine-induced tension in Ca2+-loaded fibers, this effect being more intense in slow-type fibers. Thus, BSA reduced the threshold caffeine concentration required for eliciting detectable tension, and increased the amplitude, the rate of rise and the area under the curve of caffeine-induced tension. BSA also potentiated the tension elicited in Ca2+-loaded fibers by low-Mgv solutions containing 1.0 mM free ATP. These results suggest that BSA modulates the response of the human skeletal muscle SR Ca2+-release channel to activators such as caffeine and ATP.
calcium-release channel; sarcoplasmic reticulum; human muscle fiber; bovine serum albumin; caffeine
Braz J Med Biol Res, May 1997, Volume 30(5) 675-678 (Short Communication)
Bovine serum albumin potentiates caffeine- or ATP-induced tension in human skinned skeletal muscle fibers
C.G. Ponte, C.F. Oliveira and G. Suarez-Kurtz
Departamento de Bioquímica Médica, Universidade Federal do Rio de Janeiro, 21941-590 Rio de Janeiro, RJ, Brasil
 Text
Text
 References
References
 Correspondence and Footnotes
Correspondence and Footnotes
Correspondence and Footnotes
Correspondence and Footnotes
Correspondence and Footnotes
Correspondence and Footnotes
Abstract
Human skinned muscle fibers were used to investigate the effects of bovine serum albumin (BSA) on the tension/pCa relationship and on the functional properties of the Ca2+-release channel of the sarcoplasmic reticulum (SR). In both fast- and slow-type fibers, identified by their tension response to pSr 5.0, BSA (0.7-15 µM) had no effect on the Ca2+ affinity of the contractile proteins and elicited no tension per se in Ca2+-loaded fibers. In contrast, BSA (>1.0 µM) potentiated the caffeine-induced tension in Ca2+-loaded fibers, this effect being more intense in slow-type fibers. Thus, BSA reduced the threshold caffeine concentration required for eliciting detectable tension, and increased the amplitude, the rate of rise and the area under the curve of caffeine-induced tension. BSA also potentiated the tension elicited in Ca2+-loaded fibers by low-Mgv solutions containing 1.0 mM free ATP. These results suggest that BSA modulates the response of the human skeletal muscle SR Ca2+-release channel to activators such as caffeine and ATP.
Key words: calcium-release channel, sarcoplasmic reticulum, human muscle fiber, bovine serum albumin, caffeine
In the course of a study of the effects of peptide scorpion toxins on the functional properties of the sarcoplasmic reticulum (SR) (1) it was observed that bovine serum albumin (BSA), which was used to saturate nonspecific binding sites for the toxins, potentiated the caffeine-induced tension in rat skinned muscle fibers. Subsequently, it was shown that BSA increased the binding of ryanodine to, and enhanced the electrophysiological activity of the Ca2+-release channel of skeletal muscle SR (2,3). In the present study we used human skinned muscle fibers to explore the functional interaction of BSA with the Ca2+-release channel.
Experiments were performed at 19 ± 1oC on single skinned fibers prepared from biopsies of human skeletal muscles (quadriceps and vastus lateralis), obtained during programmed surgery at the Hospital Universitário Clementino Fraga Filho, Rio de Janeiro, Brazil. The study protocol was approved by the Hospital Ethics Committee. The procedures employed for a) chemically skinning the muscle fibers during overnight soaking in EGTA-containing skinning solution, b) storing the skinned fibers at -20oC in glycerol-containing relaxing solution, c) recording isometric tension from a single fiber segment at a sarcomere length of 2.6 ± 0.05 µm, d) studying the tension/pCa relationship in fibers whose SR was functionally disrupted by pretreatment with the non-ionic detergent Brij-58, e) loading Ca2+ into the SR, f) releasing the SR-stored Ca2+ with caffeine, and g) calculating the composition of the experimental solutions have been previously described (4-6). The control bathing medium ("washing" solution, designated W) had the following composition: 185 mM K propionate, 2.5 mM K2Na2ATP, 2.5 mM Mg acetate and 10 mM imidazole, pH 7.0; 50 µM K2EGTA was added to this solution to reduce the concentration of contaminating Ca2+. The skinning solution and the standard "relaxing" solution (designated R) had the same composition: 170 mM K propionate, 2.5 mM K2Na2ATP, 2.5 mM Mg acetate, 5 mM K2EGTA and 10 mM imidazole. Solutions used to load Ca2+ into the SR ("loading" solutions of pCa 6.5-7.0) or to activate the myofibrils directly ("activating" solutions of pCa 6.2-4.5) were prepared by replacing K2EGTA in the relaxing solution with CaK2EGTA to obtain different K2EGTA/CaK2EGTA ratios, while keeping the total EGTA concentration constant at 5 mM. An activating solution of pSr 5.0 was prepared by replacing K2EGTA in the relaxing solution with SrK2EGTA. BSA, caffeine base, ATP and imidazole were obtained from Sigma Chemical Co. (St. Louis, MO). All other reagents were analytical grade. Molar concentrations of BSA were calculated assuming Mr to be 69,000; thus, 1 mg/ml or 0.1% = 15 µM.
Fiber type (slow or fast) was identified at the beginning of each experiment by comparing the tension elicited by activating solutions of pSr 5.0 or pCa 4.4 (7). pCa 4.4 induces maximum tension (Po) in both fiber types, whereas pSr 5.0 elicits no tension in human fast-type fibers but induces >80% Po in slow-type fibers. Experiments during which Po declined by 20% or more were excluded from analysis. Each protocol was repeated on a minimum of four fibers. Data from pooled experiments are reported as means ± SEM and the Student t-test was used for statistical analysis.
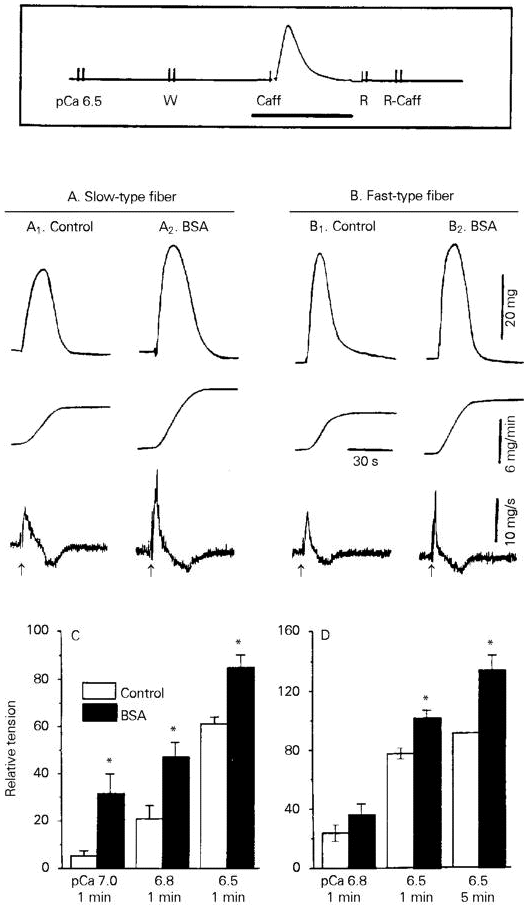
BSA (0.15-15 µM) had no effect on the resting tension of Ca2+-loaded human skinned fibers, but potentiated the caffeine-induced tension in both fast- and slow-type fibers (Figure 1). Potentiation was detected as increases in the peak amplitude, maximum rate of rise (dP/dTmax) and area under the curve (AUC) of the caffeine-induced tension (Figure 1A,B). The AUC, which provides a good functional correlate of the caffeine-induced release of SR-stored Ca2+ (8), was used for quantification of the data. The data in Figure 1C,D show that 15 µM BSA, a maximally active concentration, increased the AUC of the caffeine-induced tension in slow-type fibers at various levels of SR Ca2+ loading; in fast-type fibers, however, significant increases in AUC occurred only when the SR was heavily loaded, i.e., following soaking for 1 or 5 min in a loading solution of pCa 6.5.
- Effect of BSA on caffeine-induced tension in human skinned muscle fibers. The experimental protocol (box) consisted of initially loading Ca2+ into the SR during 1-5-min exposure to a solution of pCa 6.5-7.0. This was followed by a 1-min soak in the "washing" solution W, and by a challenge with 20 mM caffeine (Caff) in solution W. The fiber was subsequently transferred to the "relaxing" solution R, and to caffeine-containing solution R in order to deplete SR Ca2+. After two rinses with W, a new loading cycle was initiated. The thick horizontal line indicates the segment of the experimental protocol that is shown in A1,2 and B1,2. A and B, The tracings show (from top to bottom) isometric tension, integrated AUC and dP/dT recorded from single slow- or fast-type fibers in the absence (control) or in the presence of 15 µM BSA. BSA was added to solution W before and during exposure to caffeine. Caffeine was applied at the arrows. Loading conditions: pCa 6.5, 1 min. C and D, The AUC of the caffeine-induced tension was plotted against the loading conditions (pCa, loading time). Data are reported relative to the AUC of the tension induced by 20 mM caffeine after a 5-min load at pCa 6.5.
In addition to its potentiating effect on the tension elicited by 20 mM caffeine, BSA reduced the threshold caffeine concentration required for eliciting detectable tension in maximally Ca2+-loaded fibers. This is shown in Figure 2 for both fiber types. In the absence of albumin (control), no tension was elicited in the fast fiber during exposure to 2 or 5 mM caffeine, whereas the slow fiber developed a small (<5% Po) tension when challenged with 5 mM caffeine. Increasing the caffeine concentration to 20 mM evoked responses from both fibers (Figure 2A1,B1). In the presence of albumin (15 µM; Figure 2A2,B2) the slow-type fiber developed a barely detectable tension when exposed to 2 mM caffeine, while 5 mM caffeine induced large (>50% Po) tensions in both fiber types. Significantly, the tension recorded during the subsequent challenge with 20 mM caffeine was markedly reduced in relation to the control responses of both fibers. We propose that this reduction is caused by partial depletion of SR-stored Ca2+ due to the increased release of Ca2+ during the previous exposure to 5 mM caffeine in the presence of albumin.
- Effects of BSA on the threshold caffeine concentration required to elicit tension (A, B) and on the tension/pCa curves (C). A and B show recordings of isometric tension from a slow- and a fast-type fiber, respectively. The experimental protocol was similar to that depicted in the box in Figure 1, except that the Ca2+-loaded fibers were successively challenged with 2, 5 and 20 mM caffeine (Caff). In A2 and B2, BSA (15 µM) was added to the "washing" solution W before and during the caffeine tests. C shows the tension/pCa relationship of 5 slow- and 5 fast-type fibers, whose SR was previously disrupted by pretreatment with Brij-58 (2% w/v in the "relaxing" solution R for 10 min). Data are reported as means ± SEM relative to control Po elicited by pCa 4.4. The curves were obtained by fitting the experimental control data points to the Hill equation: P/Po = [Ca]h/(Kh + [Ca]h). For better visualization, some data points were displaced by 0.03 pCa units along the abscissa.
Ca2+-loaded fast-type fibers develop tension when exposed to low-Mg2+ (<200 µM) washing solutions, containing 1 mM free ATP. This ATP-induced tension was also enhanced by BSA (15 µM), as indicated by the reduced latency for tension development, increased dP/dTmax and larger AUC of the tension curve (data not shown).
To determine whether increased Ca2+ affinity of the contractile proteins contributes to the potentiation of the caffeine- or the ATP-induced tension by BSA, tension/pCa curves were constructed. The plots in Figure 2C show that the curves obtained in the absence or in the presence of BSA (15 µM) were superimposable. Thus, BSA does not affect the Ca2+ affinity for troponin-C under our experimental conditions.
BSA has been shown to stimulate the electrophysiological activity of the SR Ca2+-release channel in the presence of agonists such as caffeine and ATP (2,3). These data are consistent with, and provide a tentative mechanistic explanation for the potentiation of the caffeine- and the ATP-induced tension by BSA in human muscle fibers, which we now report. Lu et al. (3) have shown that BSA potentiates ryanodine binding to the Ca2+-release channel of both skeletal and cardiac muscle by increasing the binding capacity (Bmax) without significantly affecting the binding constant (KD). Increased binding of caffeine or ATP to their receptors in the Ca2+-release channel could account for the potentiation of the tension induced by these ligands in human skinned fibers. Changes in surface charge density due to BSA adsorption to the SR membrane might contribute to the effects of BSA on the release channel. BSA carries a net negative charge at physiological pH, and its adsorption to the SR membrane could increase the local concentration of Ca2+, which is a potent physiological activator of the release channel and which interacts synergistically with both caffeine and ATP to stimulate Ca2+ release. Changes in surface charge density have been previously proposed to account for the BSA-induced potentiation of Ca2+ channel currents in neuroblastoma x glioma cells (9).
Address for correspondence: G. Suarez-Kurtz, Departamento de Bioquímica Médica, Instituto de Ciências Biomédicas, CCS, Universidade Federal do Rio de Janeiro, 21941-590 Rio de Janeiro, RJ, Brasil. Fax: 55 (021) 270-8647. E-mail: kurtz@server.bioqmed.ufrj.br
Research supported by CNPq (No. 522.714/95), FAPERJ (No. 170.209.95) and FINEP (No. 43.89.0630-00). G. Suarez-Kurtz is a Senior Investigator of CNPq. Received November 27, 1996. Accepted February 21, 1997.
- 1. Suarez-Kurtz G, Ponte CG & Vianna-Jorge R (1995). Charybdotoxin activates the SR Ca2+-release channel and potentiates the effect of caffeine in skinned fibers. Abstracts of the X Annual FESBE Meeting, 315.
- 2. Catinot MP, Suarez-Kurtz G & Mounier Y (1996). Activity of the calcium release channel of sarcoplasmic reticulum from skeletal muscle in the presence of albumin. Journal of Muscle Research and Cell Motility, 16: 17 (Abstract).
- 3. Lu X, Xu L & Meissner G (1994). Activation of the skeletal muscle calcium release channel by a cytoplasmic loop of the dihydropyridine receptor. Journal of Biological Chemistry, 269: 6511-6516.
- 4. Wood DS, Zollman J, Reuben JP & Brandt PW (1975). Human skeletal muscle: Properties of the "chemically skinned" fiber. Science, 187: 1075-1076.
- 5. Trachez MM, Sudo RT & Suarez-Kurtz G (1990). Alterations in the functional properties of skinned fibers from denervated rabbit skeletal muscle. American Journal of Physiology, 259 (Cell Physiology, 28): C503-C506.
- 6. Suarez-Kurtz G, Catinot MP, Ponte CG, Vianna-Jorge R & Mounier Y (1995). Effects of uridine triphosphate on skinned skeletal muscle fibers of the rat. Canadian Journal of Physiology and Pharmacology, 73: 1451-1457.
- 7. Takagi A, Yonemoto K & Sugita H (1978). Single-skinned human muscle fibers: Activation by calcium and strontium. Neurology, 28: 497-499.
- 8. Endo M & Kitazawa T (1981). The effect of ATP on calcium release mechanisms in the sarcoplasmic reticulum of skinned muscle fibers. Proceedings of the Japanese Academy of Sciences, 52: 595-598.
- 9. Schmitt H & Meves H (1994). Bovine serum albumin selectively increases the low-voltage-activated calcium current of NG108-15 neuroblastoma x glioma cells. Brain Research, 656: 375-380.
Correspondence and Footnotes
Publication Dates
-
Publication in this collection
13 Oct 1998 -
Date of issue
May 1997
History
-
Accepted
21 Feb 1997 -
Received
27 Nov 1996