Abstract
Cytotoxin production was studied in 60 Serratia marcescens strains isolated from hospitalized patients. Association of cytotoxic activity with serotype, source of isolation and presence of plasmids was also evaluated. Thirteen of the 60 S. marcescens strains produced a cytotoxic effect on Vero cells. These strains were isolated from distinct clinical sources and classified into seven different serotypes (O1:H7; O4:NM; O10:NT; O19:NM; O6,14:H4; O6,14:NM and O6,14:H1). No relationship was observed between cytotoxic activity and clinical source or serotypes of the strains. Plasmids from five cytotoxin-producing S. marcescens strains were transferred to E. coli K12/711. The transconjugants did not exhibit cytotoxicity, indicating that the cytotoxic effect is not plasmid-mediated among these strains. Although a cytotoxic activity was demonstrated in filtrates of some S. marcescens strains, further studies should be performed to assess the role of this toxin in pathogenesis
cytotoxin production; nosocomial infections; plasmids; Serratia marcescens; virulence factors
Braz J Med Biol Res, November 1997, Volume 30(11) 1291-1298
Detection of cytotoxic activity on Vero cells in clinical isolates of Serratia marcescens
G.V. Carbonell1, A.F. Alfieri2, A.A. Alfieri2, M.C. Vidotto3, C.E. Levy4, A.L.C. Darini5 and R.M. Yanaguita1
1Instituto de Ciências Biomédicas II, Universidade de São Paulo, São Paulo, SP, Brasil
Departamentos de 2Medicina Veterinária Preventiva and 3Patologia Geral, Universidade Estadual de Londrina, Londrina, PR, Brasil
4Laboratório de Microbiologia, Hospital das Clínicas, Faculdade de Medicina de Ribeirão Preto, Universidade de São Paulo, Ribeirão Preto, SP, Brasil
5Departamento de Análises Clínicas, Toxicológicas e Bromatológicas, Faculdade de Ciências Farmacêuticas de Ribeirão Preto, Universidade de São Paulo, Ribeirão Preto, SP, Brasil
 References
References
 Correspondence and Footnotes
Correspondence and Footnotes
Correspondence and Footnotes
Correspondence and Footnotes
Correspondence and Footnotes
Correspondence and Footnotes
Abstract
Cytotoxin production was studied in 60 Serratia marcescens strains isolated from hospitalized patients. Association of cytotoxic activity with serotype, source of isolation and presence of plasmids was also evaluated. Thirteen of the 60 S. marcescens strains produced a cytotoxic effect on Vero cells. These strains were isolated from distinct clinical sources and classified into seven different serotypes (O1:H7; O4:NM; O10:NT; O19:NM; O6,14:H4; O6,14:NM and O6,14:H1). No relationship was observed between cytotoxic activity and clinical source or serotypes of the strains. Plasmids from five cytotoxin-producing S. marcescens strains were transferred to E. coli K12/711. The transconjugants did not exhibit cytotoxicity, indicating that the cytotoxic effect is not plasmid-mediated among these strains. Although a cytotoxic activity was demonstrated in filtrates of some S. marcescens strains, further studies should be performed to assess the role of this toxin in pathogenesis.
Key words: cytotoxin production, nosocomial infections, plasmids, Serratia marcescens, virulence factors
Introduction
Serratia marcescens has been recognized as an important nosocomial pathogen causing respiratory and urinary tract infections (1), bacteremia (2), meningitis (3,4), peritonitis (5), and other disorders (6,7). This bacterium has been considered to be an important agent of nosocomial infections, particularly among newborns and patients submitted to invasive procedures at the University Hospital of the School of Medicine of Ribeirão Preto, SP, Brazil.
Reports in the literature regarding virulence of this bacterium have shown characteristics such as resistance to serum bactericidal activity (8), cell-bound hemolysin (9,10), presence of fimbriae and adherence to uroepithelial cells (11,12), and production of extracellular proteases (13) and nucleases (14). Cytotoxin production has been considered to be an important virulence factor shown by several bacteria (15-18), but as far as we know there are no data on cytotoxin production by S. marcescens strains.
Bacterial toxins are currently detected according to action on mammalian cell lines, evidenced by changes in cell shape. These morphological changes are defined as cytopathic effects and can be useful to classify a cytotoxin. Most bacterial toxins cause initially cytopathic effects but only those denominated cytotoxin lead to death of cultured cells (19). In addition, viability of the cell monolayer treated with a cytotoxin can be determined using the neutral red assay (20,21).
The purpose of the present study was to investigate the ability of clinical isolates of S. marcescens to produce cytotoxin. The correlation of cytotoxin production with clinical source, serotype of strains and presence of plasmids was studied. Characteristics of the cytotoxic activity such as heat stability and the type of cytopathic effect were also evaluated.
Material and Methods
Bacterial strains
A total of 60 S. marcescens strains supplied by the Microbiology Laboratory of the University Hospital of the School of Medicine of Ribeirão Preto, University of São Paulo, Brazil, were studied. The strains were isolated from surgical wounds (11 isolates), urinary infections (12 isolates), respiratory tract (4 isolates), catheter (3 isolates), abscesses (3 isolates), ocular secretions (4 isolates), bacteremias (7 isolates) and others. E. coli H30 (serogroup O26), which produces verocytotoxin, was used as a positive control in cytotoxicity assays and E. coli K12/711 (nontoxigenic) was used as a negative control and as a plasmid recipient in conjugation experiments.
Characterization of Serratia marcescens
The strains were serotyped at Instituto Adolfo Lutz, SP, Brazil, according to standard methods (22). Resistance to 10 antimicrobial agents (amikacin, ampicillin, chloramphenicol, gentamicin, kanamycin, nalidixic acid, tetracycline, sulfamethoxazole-trimethoprim, carbenicillin and streptomycin) was determined by the method of Bauer et al. (23).
Clinical data
The clinical records of all patients were reviewed. Only those patients who became infected 48 h after admission to the hospital were considered to have nosocomial infections. Colonization is defined as the situation where isolation of S. marcescens occurred without evidence of overt disease.
Bacterial filtrates
The strains were cultured in 10 ml of Trypticase Soy Broth (TSB, Difco Lab., Detroit, MI) at 37oC for 18 h, with shaking. Bacterial culture filtrates were obtained by centrifugation (15,000 rpm for 10 min at 4oC) and the supernatants were sterilized by filtration through 0.2-µm filters (Millipore).
Cytotoxicity assay
The cytotoxicity assay was performed as described by Konowalchuk et al. (24). Vero (African green monkey kidney) cells were cultivated in tissue culture flasks with Eagle's modified essential medium (MEM; Gibco, Grand Island, NY), supplemented with 10% fetal calf serum, 0.75 mM L-glutamine, 40 µg/ml gentamicin and 1 µg/ml amphotericin B. Confluent monolayers were removed with trypsin EDTA, resuspended to approximately 4 x 105 cells/ml in MEM and 0.1-ml samples were pipetted into each well of a 96-well microtiter plate. After incubation at 37oC in 5% CO2 for 72 h, the medium was replaced with 180 µl MEM and 20 µl of the bacterial filtrate was added to each well. As negative controls, some wells received only MEM, TSB or E. coli K12/711 filtrates and E. coli H30 filtrates were used as positive control. Each sample was tested in triplicate. Vero monolayer morphology was observed under the inverted microscope and checked for cytotoxic effect for five days.
Neutral red assay
Cell viability was quantified by the neutral red cytotoxicity assay (20). Briefly, after the cytotoxicity assay (incubation with filtrates for 24-96 h), the medium containing the bacterial culture filtrates was removed and the cultures were washed with phosphate-buffered saline, pH 7.4. Two hundred µl MEM containing 50 µg/ml neutral red was added to each well and the plate was incubated for 3 h at 37oC. The media containing the dye were removed and each well was washed for 2-3 min with formol-calcium (40% formaldehyde, 10% anhydrous calcium chloride) to remove non-incorporated neutral red. Finally, 0.2 ml of an acetic acid-ethanol mixture (1.0 ml glacial acetic acid in 100 ml 50% ethanol) was added to each well and the plate was kept for 15 min at room temperature in order to remove the dye from the viable cells. Plates were transferred to a spectrophotometer (Titertek Multiskan model 340) and read with a 540-nm filter. Two wells on the first row received medium without neutral red and served as blanks. Control cultures, 8 wells located in different areas of the plate, received normal medium without test filtrates. Cell viability was determined by comparison to the absorbance values obtained for control wells (without toxin), which were taken as 100% cell viability. The cytotoxicity assay and the quantitative colorimetric assay were carried out on the same cell culture plate.
Heat stability
Filtrates that induced morphological changes in Vero cells were tested for heat stability. One-ml samples of bacterial culture filtrates were incubated for 30 min in sealed tubes in a thermostatically controlled water bath at 40, 50, 60, 70, 80 or 90oC, prior to the cytotoxicity assay. The cytotoxic activity of the filtrates was determined by cell viability measured by the neutral red assay, as described above.
Plasmid isolation and conjugation
The cytotoxin-producing S. marcescens strains selected for plasmid isolation and conjugation assays were susceptible to nalidixic acid and resistant to ampicillin, chloramphenicol and tetracycline, among other drugs. Plasmid DNA was isolated according to the extraction method described by Kado and Liu (25) and electrophoresed on 0.7% agarose gel. The molecular mass of each plasmid was determined by comparison with plasmids of known molecular masses: pR27 (110 MDa), pJPN11 (66 MDa) and pRK (13.2 MDa). The conjugation experiments were performed as described elsewhere (26) using E. coli K12/711 as recipient. Briefly, mating mixtures consisting of 107 donor cells/ml sensitive to nalidixic acid and 108 recipient cells/ml were incubated at 37oC. After incubation for 24 or 72 h, the bacterial mixtures were plated onto MacConkey's agar (Difco, Detroit, MI) containing nalidixic acid (50 µg/ml). Depending upon the antibiotic sensitivity of the donor strain, the bacterial mixture and appropriate controls were plated onto MacConkey's-nalidixic acid agar containing one of the following antibiotics: chloramphenicol (20 µg/ml), carbenicillin (20 µg/ml), tetracycline (20 µg/ml), streptomycin (25 µg/ml) or ampicillin (50 µg/ml). The isolated transconjugants were submitted to plasmid extraction and electrophoresis on agarose gels in order to confirm the plasmid transfer. All transconjugants were analyzed for antibiotic resistance and cytotoxicity.
Results
Cytotoxicity of the Serratia marcescens strains
Cytopathic effects were observed in 13 culture filtrates of S. marcescens strains. After incubation with culture filtrates there was a change from spindle-shaped cells characteristic of normal Vero cells to round and shriveled cells, and these changes were followed by gradual destruction of the monolayer (Figure 1).
- Cytopathic effect of S. marcescens filtrates on Vero cells. A, Negative control (E. coli K12); B, cytopathic effect after 24 h. Magnification: 100X.
Determination of cell viability
Cell viability was determined for all S. marcescens strains and the results obtained were closely similar. Figure 2 illustrates the results obtained for three S. marcescens strains, Serratia 1 (serotype O19:NM, isolated from urinary infection), Serratia 4 (serotype O6,14:H4, isolated from the respiratory tract) and Serratia 5 (serotype O1:H7, isolated from a surgical wound), and the negative control E. coli K12.
- Determination of the viability of Vero cells treated with filtrates of the S. marcescens strains numbers 1, 4 and 5, and negative control (E. coli K12) after different incubation times.
Heat stability
Tests for heat stability showed that the cell monolayer lost approximately 30% cell viability when the bacterial filtrates were heated to 60oC, and no cytopathic effect was observed at 70oC, as shown by maintenance of the viability of Vero cells (Figure 3).
- Effect of heat treatment of Serratia marcescens cytotoxin. The filtrates were submitted to different temperatures and tested on Vero cells. The viability of the cell culture was determined by the neutral red assay.
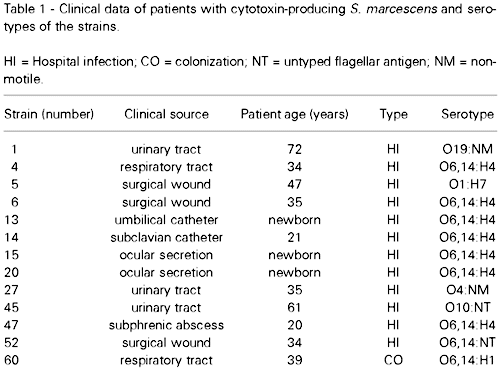
Clinical data
The clinical data correlating the patients and the cytotoxin-producing S. marcescens strains are shown in Table 1. The strains were obtained from ocular infection (2 strains), pneumonia (2 strains), urinary infection (3 strains), abscess (1 strain), surgical wound (3 strains) and catheter (2 strains). The majority of these infections (92%) were considered to be nosocomially acquired, and one was only colonization. The age of patients ranged from newborn to 72 years.
Serotyping
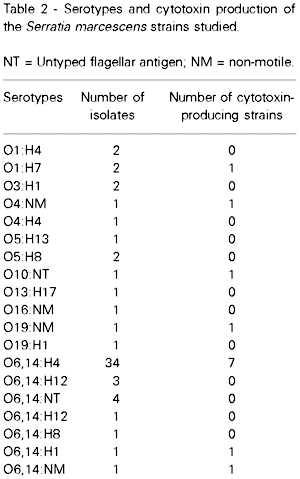
The serotypes of the S. marcescens isolates are shown in Table 2. Bacterial strains that showed untyped flagellar antigen or that were non-motile are referred to as NT and NM, respectively. The cytotoxin-producing strains were classified into 7 different serotypes: O6,14:H4 (7 strains), O1:H7 (1 strain), O4:NM (1 strain), O10:NT (1 strain), O19:NM (1 strain), O6,14:NM (1 strain) and O6,14:H1 (1 strain).
Plasmids and transconjugants
Plasmids were found in 8 cytotoxin-producing S. marcescens strains, but only 5 of these strains transferred plasmids to E. coli K12/711 (Figure 4). Serratia isolates numbers 15, 20 and 27 showed the same antibiotic resistance pattern. These isolates were resistant to ampicillin, carbenicillin, gentamicin, streptomycin, sulfamethoxazole-trimethoprim, kanamycin and tobramycin and all transferred one plasmid (~60 MDa) that carries resistance to carbenicillin, ampicillin, streptomycin and tobramycin. Strain number 45 was resistant to ampicillin, carbenicillin, gentamicin, streptomycin, sulfamethoxazole-trimethoprim, kanamycin, tobramycin and chloramphenicol and transferred another plasmid of ~66 MDa that showed resistance to ampicillin, carbenicillin, kanamycin, streptomycin, and sulfamethoxazole-trimethoprim. Strain number 1 showed a resistance pattern similar to that of number 45, with the addition of tetracycline. Only this isolate transferred two plasmids to E. coli K12/711: one plasmid (~54 MDa) that carries resistance to tetracycline and chloramphenicol and another (~66 MDa) carrying resistance to ampicillin, carbenicillin, streptomycin, kanamycin and sulfamethoxazole-trimethoprim. Transconjugants are reported as donor number/recipient number. Culture filtrates obtained from transconjugants did not produce morphological changes of Vero cells and cell viability always reached 100%.
Figure 4 - Agarose gel electrophoresis of plasmids from cytotoxin-producing Serratia marcescens transferred to E. coli K12/711. A, E. coli K12/711 (recipient); B, Serratia 1 (donor); C, 711/1a (transconjugant); D, 711/1b; E, Serratia 15; F, 711/15; G, Serratia 20; H, 711/20; I, Serratia 27; J, 711/27; K, Serratia 45; L, 711/45; M, pR27; N, pJPN11; O, pRK. pR27, pJPN11 and pRK are molecular mass standards.
Discussion
In the present study, 13 culture filtrates of 60 (21.7%) Serratia marcescens strains showed cytotoxic activity on Vero cells. These strains were isolated from various clinical sources, and from patients of different ages. Although no association between cytotoxic activity and isolation source was observed, most cytotoxin-producing strains (92%) were isolated from nosocomial infections.
The cytopathic effects of the S. marcescens isolates on Vero cells were characterized by cell rounding and detachment. Cell rounding without swelling can occur when the cytoskeleton is altered in the absence of plasma membrane damage. Toxins that produce this effect are classified as cytoskeleton-altering toxins (18).
The criterion used here to define cell injury was the loss of cell culture viability measured by the neutral red assay. The results showed that the monolayer of Vero cells treated with S. marcescens culture filtrates was completely damaged after 72-96 h, causing the death of the cell culture, when compared to the negative control.
The cytotoxic activity of the S. marcescens culture filtrates was inactivated at 70oC, a result similar to that reported for some E. coli cytotoxins such as verocytotoxins (27) and Shiga toxin (28).
In E. coli, some serogroups are generally isolated from verocytotoxin-producing strains (29). In the present study, no relationship between serotype and cytotoxin production was demonstrable. S. marcescens strains were classified into seven different serotypes and the O6,14:H4 serotype was the most common among the isolates studied, independent of the production of cytotoxin.
Furthermore, other virulence characteristics such as hemolysin production (10) and resistance to serum bactericidal activity (8) were detected in 97% and 92% of the strains, respectively. However, no association with cytotoxin production was observed, since these characteristics were also found in most of the non-producing strains.
Five cytotoxin-producing strains transferred plasmids to E. coli K12/711. The transconjugants received plasmids carrying antibiotic resistance but they did not produce cytotoxins, suggesting that the genetic information for this activity is not carried on these plasmids.
Although most bacterial toxins seem to be located on the chromosome, toxins such as CNF2 and heat-labile (LT) and heat-stable (ST) enterotoxins of E. coli (17,30,31) have been associated with plasmid presence. However, the controlling genes for verocytotoxin production are phage-encoded in several E. coli strains (32).
Some cytotoxins are homologous between different bacterial species, as observed for Shiga-like toxins produced by E. coli and Shiga toxin (33), or for Shiga-like toxin II-related toxins produced by Citrobacter freundii (34). Thus, characteristics such as source of isolation, heat stability and type of cytopathic effect are preliminary tests that can be useful for differentiation of a cytotoxin.
In conclusion, the present results describe a cytotoxic activity on Vero cells induced by clinical isolates of S. marcescens, and may provide impetus for subsequent work on the characterization of this cytotoxin.
Acknowledgments
We are grateful to the personnel of the Laboratory of Microbiology, School of Medicine of Ribeirão Preto (USP), who provided the bacterial strains, to Rosa H.A.R. Gironi for technical assistance, and also to the Instituto Adolfo Lutz, SP, Brazil, for serotyping the isolates.
Address for correspondence: A.L.C. Darini, Departamento de Análises Clínicas, Toxicológicas e Bromatológicas, FCFRP, USP, Av. do Café, s/n, 14040-903 Ribeirão Preto, SP, Brasil. Fax: 55 (016) 633-1092. E-mail: aldarini@gly.fcfrp.usp.br
Research supported by CNPq. Publication supported by FAPESP. Received October 22, 1996. Accepted September 10, 1997.
- 1. Okuda T, Endo N & Osada Y (1981). Outbreak of nosocomial urinary tract infections caused by Serratia marcescens Journal of Clinical Microbiology, 20: 691-695.
- 2. Volkow-Fernandez P, Ponce de Léon-Rosales S, Sifuentes-Orsonio J, Calva-Mercado JJ, Ruiz-Palacios GM & Cerbon MA (1993). Epidemia de bacteremias primarias por una cepa endémica de Serratia marcescens en una unidad de terapia intensiva. Salud Pública de México, 35: 440-447.
- 3. Zaidi M, Sifuentes-Orsonio J, Bobadilha M, Moncada D & Ponce de Léon S (1989). Epidemic of Serratia marcescens bacteremia and meningitis in a neonatal unit in Mexico City. Infection Control and Hospital Epidemiology, 10: 14-20.
- 4. Campbell JR, Diacovo T & Baker CJ (1992). Serratia marcescens meningitis in neonates. Pediatric Infectious Disease Journal, 11: 381-386.
- 5. Bizette GA, Lindberg JS & Figueroa JE (1995). Serratia marcescens peritonitis in a patient receiving chronic ambulatory peritoneal dialysis complicated by osteomyelitis. Journal of the Louisiana State Medical Society, 147: 64-67.
- 6. Pagani L, Luzzaro F, Ronza P, Rossi A, Micheletti P, Porta F & Romero E (1994). Outbreak of extended-spectrum beta-lactamase producing Serratia marcescens in an intensive care unit. FEMS Immunology and Medical Microbiology, 10: 39-46.
- 7. Stephen M & Lalitha MK (1993). An outbreak of Serratia marcescens infection among obstetric patients. Indian Journal of Medical Research, 97: 202-205.
- 8. Carbonell GV, Levy CE & Vidotto MC (1992). Virulence factors in Serratia marcescens: human serum resistance, serogroup and pathogenicity for mice. Revista de Microbiologia, 23: 72-75.
- 9. Ruan Y & Braun V (1990). Hemolysin as a marker for Serratia. Archives of Microbiology, 154: 221-225.
- 10. Carbonell GV & Vidotto MC (1992). Virulence factors in Serratia marcescens: cell-bound hemolysin and aerobactin. Brazilian Journal of Medical and Biological Research, 25: 1-8.
- 11. Yamamoto T, Ariyoshi A & Amako K (1985). Fimbria-mediated adherence of Serratia marcescens US5 strain to human urinary bladder surface. Microbiology and Immunology, 29: 677-681.
- 12. Parment PA, Svanborg-Edén C, Chaknis MJ, Sawant AD, Hadberg L, Wilson LA & Ahearn DG (1992). Hemagglutination (fimbriae) and hydrophobicity in adherence of Serratia marcescens to urinary tract epithelium and contact lenses. Current Microbiology, 25: 113-118.
- 13. Matsumoto K, Maeda H, Takata K, Kamata R & Okamura R (1984). Purification and characterization of four proteases from a clinical isolate of Serratia marcescens. Journal of Bacteriology, 157: 225-232.
- 14. Hines DA, Saurugger PN, Ihler GM & Benedik MJ (1988). Genetic analysis of extracellular proteins of Serratia marcescens. Journal of Bacteriology, 170: 4141-4146.
- 15. O'Brien AD & Holmes RK (1987). Shiga and Shiga-like toxins. Microbiological Reviews, 51: 206-220.
- 16. Finlay BB & Falkow S (1989). Common themes in microbial pathogenicity. Microbiological Reviews, 53: 210-230.
- 17. De Rycke J (1991). Les colibacilles producteurs de cytotoxines: importance en médecine vétérinaire et en santé publique. Annales de Recherches Veterinaires, 22: 105-126.
- 18. Sears CL & Kaper JB (1996). Enteric bacterial toxins: mechanisms of action and linkage to intestinal secretion. Microbiological Reviews, 60: 167-215.
- 19. Thelestam M & Florin I (1994). Assay of cytopathogenic toxins in cultured cells. Methods in Enzymology, 235: 679-690.
- 20. Borenfreund E & Puerner JA (1984). A simple quantitative procedure using monolayer cultures for cytotoxicity assays. Journal of Tissue Culture Methods, 9: 7-9.
- 21. Repetto G & Sanz P (1993). Neutral red uptake, cellular growth and lysosomal function; in vitro effects of 24 metals. Alternative to Laboratory Animals, 21: 501-507.
- 22. Traub WH & Kleber I (1977). Serotyping of Serratia marcescens: evaluation of Le Minor's H-immobilization test and description of three new flagellar H antigens. Journal of Clinical Microbiology, 5: 115-121.
- 23. Bauer AW, Kirby WMM, Sherris JC & Turck M (1966). Antibiotic susceptibility testing by standardized single disk method. American Journal of Clinical Pathology, 45: 493-496.
- 24. Konowalchuk J, Speirs JI & Stravic S (1977). Vero response to a cytotoxin of Escherichia coli Infection and Immunity, 18: 775-779.
- 25. Kado CI & Liu ST (1981). Rapid procedure for the detection and isolation of large and small plasmids. Journal of Bacteriology, 145: 1365-1373.
- 26. Hedges RW, Rodriguez-Lemoine V & Datta N (1975). R factors from Serratia marcescens. Journal of General Microbiology, 86: 88-92.
- 27. Konowalchuk J, Dickie N & Stravic S (1978). Comparative studies of five heat-labile toxic products of Escherichia coli Infection and Immunity, 22: 644-648.
- 28. Eiklid K & Olsnes S (1983). Animal toxicity of Shigella dysenteriae cytotoxin: evidence that the neurotoxic and cytotoxic activities are due to one toxin. Journal of Immunology, 130: 380-384.
- 29. Thomas A, Chart H, Cheasty T, Smith HR, Frost JA & Rowe B (1993). Vero cytotoxin-producing Escherichia coli, particularly serogroup O157, associated with human infections in United Kingdom: 1989-91. Epidemiology and Infection, 110: 591-600.
- 30. Silva RM, Giraldi R, Keller R, Campos LC & Guth BEC (1996). Diffuse adherence, ST-I enterotoxin and CFA/IV colonization factor are encoded by the same plasmid in Escherichia coli O29:H21 strain. Brazilian Journal of Medical and Biological Research, 29: 969-976.
- 31. Echeverria P & Murphy JR (1980). Enterotoxigenic Escherichia coli carrying plasmids coding for antibiotic resistance and enterotoxin production. Journal of Infectious Diseases, 142: 273-278.
- 32. Smith H, Green P & Parseli Z (1983). Vero cell toxins in Escherichia coli and related bacteria: transfer by phage and conjugation and toxic action in laboratory animals, chickens and pigs. Journal of General Microbiology, 129: 3121-3127.
- 33. Gyles CL (1992). Escherichia coli cytotoxins and enterotoxins. Canadian Journal of Microbiology, 38: 734-746.
- 34. Schmidt H, Montag M, Bockemuhl J, Heesemann J & Karch H (1993). Shiga-like toxin II-related cytotoxins in Citrobacter freundii strains from humans and beef samples. Infection and Immunity, 61: 534-543.
Correspondence and Footnotes
Publication Dates
-
Publication in this collection
07 Oct 1998 -
Date of issue
Nov 1997
History
-
Accepted
10 Sept 1997 -
Received
22 Oct 1996