Abstract
Achyrocline satureioides (Lam.) DC. (Compositae) is a medicinal herb used in Argentina, Uruguay, Brazil and Paraguay for its choleretic, antispasmodic and hepatoprotective properties. The presence of the flavonoid quercetin and its derivatives, and of different phenolic acids such as caffeic, chlorogenic and isochlorogenic acids in the aerial parts of this plant has led us to study the antioxidant activity of its extracts using different bioassays. The inhibition of luminol-enhanced chemiluminescence by the aqueous and methanolic extracts was used to show that their total reactive antioxidant potential index (TRAP; in µM Trolox equivalents) was 91.0 ± 15.4 and 128.1 ± 20.1 µM, respectively, while the total antioxidant reactivity index (TAR) was calculated to be 1537 ± 148 and 1910 ± 171 µM. Only the methanolic extract was capable of reducing iron (II)-dependent DNA damage. Lipid peroxidation was assessed by two different methods. The aqueous extract reduced hydroperoxide-initiated chemiluminescence in rat liver homogenates at all concentrations in a dose-dependent manner, with a calculated IC50 = 225 µg/ml, while the methanolic extract was only effective at higher concentrations (100 and 1000 µg/ml). Both aqueous and methanolic extracts were capable of reducing the production of thiobarbituric acid reactive substances (TBARS) in rat liver homogenates, with an IC50 >1000 µg/ml. The results obtained suggest that the extracts of A. satureioides possess significant free radical scavenging and antioxidant activity in vitro, a fact that should encourage future in vivo studies.
Achyrocline satureioides; antioxidant activity; luminol-enhanced chemiluminescence; thiobarbituric acid-reactive substances; hydroperoxide-initiated chemiluminescence; lipid peroxidation; DNA damage; reactive oxygen species
Braz J Med Biol Res, September 1998, Volume 31(9) 1163-1170
Antioxidant and free radical scavenging effects in extracts of the medicinal herb Achyrocline satureioides (Lam.) DC. ("marcela")
C. Desmarchelier1, J. Coussio2 and G. Ciccia1
1Cátedra de Microbiología Industrial y Biotecnología, 2Cátedra de Farmacognosia (IQUIMEFA/CONICET), Facultad de Farmacia y Bioquímica, Universidad de Buenos Aires, Buenos Aires, Argentina
Abstract
Introduction
Material and Methods
Results and Discussion
References
 Correspondence and Footnotes
Correspondence and Footnotes
Correspondence and Footnotes
Correspondence and Footnotes
Correspondence and Footnotes
Correspondence and Footnotes

Achyrocline satureioides (Lam.) DC. (Compositae) is a medicinal herb used in Argentina, Uruguay, Brazil and Paraguay for its choleretic, antispasmodic and hepatoprotective properties. The presence of the flavonoid quercetin and its derivatives, and of different phenolic acids such as caffeic, chlorogenic and isochlorogenic acids in the aerial parts of this plant has led us to study the antioxidant activity of its extracts using different bioassays. The inhibition of luminol-enhanced chemiluminescence by the aqueous and methanolic extracts was used to show that their total reactive antioxidant potential index (TRAP; in µM Trolox equivalents) was 91.0 ± 15.4 and 128.1 ± 20.1 µM, respectively, while the total antioxidant reactivity index (TAR) was calculated to be 1537 ± 148 and 1910 ± 171 µM. Only the methanolic extract was capable of reducing iron (II)-dependent DNA damage. Lipid peroxidation was assessed by two different methods. The aqueous extract reduced hydroperoxide-initiated chemiluminescence in rat liver homogenates at all concentrations in a dose-dependent manner, with a calculated IC50 = 225 µg/ml, while the methanolic extract was only effective at higher concentrations (100 and 1000 µg/ml). Both aqueous and methanolic extracts were capable of reducing the production of thiobarbituric acid reactive substances (TBARS) in rat liver homogenates, with an IC50 >1000 µg/ml. The results obtained suggest that the extracts of A. satureioides possess significant free radical scavenging and antioxidant activity in vitro, a fact that should encourage future in vivo studies.
Key words: Achyrocline satureioides, antioxidant activity, luminol-enhanced chemiluminescence, thiobarbituric acid-reactive substances, hydroperoxide-initiated chemiluminescence, lipid peroxidation, DNA damage, reactive oxygen species
Recent years have witnessed a renewed interest in plants as pharmaceuticals. This interest has been focused not only on the discovery of new biologically active molecules by the pharmaceutical industry, but also on the adoption of crude extracts of plants, such as infusions, for self-medication by the general public (1). Within this context, considerable interest has arisen in the possibility that the impact of several diseases may be either ameliorated or prevented by improving the dietary intake of natural nutrients with antioxidant properties, such as vitamin E, vitamin C, ß-carotene and plant phenolics such as tannins and flavonoids (2).
Achyrocline satureioides (Lam.) DC. (Compositae), known as "marcela" or "yateí caá", is a medicinal plant widely used in Argentina, Uruguay, Brazil and Paraguay for its choleretic, antispasmodic and hepatoprotective properties (3). Pharmacological evaluations of different extracts of this plant concerning their possible antispasmodic, anti-inflammatory, analgesic and sedative activities, together with their effects on intestinal transit and acute toxicity, have been reported (4,5), and phytochemical studies have confirmed the presence of the flavonoid quercetin and its derivatives. Similarly, the presence of caffeic, chlorogenic and isochlorogenic acids has also been reported in the aerial parts (6).
Different studies have demonstrated that flavonoids present interesting levels of anti-inflammatory activity (7,8). Likewise, caffeic acid and its derivatives have shown a choleretic and hepatoprotective action in different pharmacological studies (9). The high content of polyphenolic compounds in the aerial parts of A. satureioides has led us to study the antioxidant properties of the extracts of this medicinal plant. For this purpose the aqueous and methanolic extracts were submitted to different bioassays in order to determine their free radical scavenging activity and their capacity to reduce lipid peroxidation and iron (II)-dependent DNA damage.
Chemicals
tert-Butyl hydroperoxide (t-BOOH), catechin, thiobarbituric acid (TBA), butylated hydroxytoluene (BHT), phosphotungstic acid, bovine serum albumin acid, trichloroacetic acid (TCA), calf thymus DNA (Sigma Chemical Co., St. Louis, MO), sodium dodecyl sulfate (SDS) (Mann Research Laboratories Inc., New York, USA), dichloromethane (Dorwill, Buenos Aires, Argentina), methanol, ethanol and ferrous ammonium sulfate (Mallinckrodt, New York, USA), dimethylsulfoxide (DMSO), n-butanol and trichloroacetic acid (Merck, Buenos Aires, Argentina) and 2,2'-azo bis(2-amidinopropane) (ABAP) and luminol (Acros, Geel, Belgium) were of analytical grade.
Plant material
Plant material was collected in the province of Entre Ríos, Argentina. Botanical identification was made by Dr. A.A. Gurni in the Cátedra de Farmacobotánica, Facultad de Farmacia y Bioquímica, Universidad de Buenos Aires, where a voucher specimen is deposited.
Plant extracts
Extracts were prepared following the recommendations of CYTED (10). Infusions were made by pouring 100 ml of boiling water on 5 g of powdered plant material placed in a stoppered flask. The mixture was left to stand for 20 min and then filtered. The resulting infusion was freeze-dried in a Gamma A lyophilizer (Chriss, Germany). The resulting powder was considered to be the aqueous extract.
Methanolic extracts were prepared by previously defatting 5 g of dry powdered plant material for 24 h at room temperature in a stoppered flask containing 50 ml of dichloromethane. The resulting marc was extracted with methanol under the same conditions as described for dichloromethane, and the extract was filtered and concentrated under reduced pressure at 43oC in a Savant Speed Vac Plus SC210A concentrator. DMSO was used to pre-solubilize the methanolic extract (1% v/v).
Total reactive antioxidant potential (TRAP) and total antioxidant reactivity (TAR)
TRAP and TAR indices were measured by luminol-enhanced chemiluminescence (11). For TRAP determinations, the reaction medium consisted of 100 mM sodium phosphate buffer, pH 7.4, 20 mM ABAP, 10 µM luminol, and increasing volumes (2-10 µl) of the extracts (0.1 mg/ml). Light intensity was measured at room temperature in a Wallac WinSpectral 1414 liquid scintillation counter with the circuit coincidence out of mode. ABAP is a source of free radicals which reacts with luminol yielding chemiluminescence. The system was calibrated using the a-tocopherol analog Trolox (150 µM). A comparison of the induction times after the addition of known concentrations of Trolox and antioxidants allows to obtain TRAP values as equivalents of Trolox concentration necessary to suppress the emitted luminescence by employing the following equation:
TRAP (µM Trolox) = µl total/µl sample x di sample/diTrolox x diTrolox (1 µM)
where µl total is the final volume (3000 µl), µl sample is the volume of the sample added to the reaction, di sample is the induction time observed for the different volumes of the sample, diTrolox is the induction time observed for the reference compound (Trolox), and diTrolox (1 µM) is the induction time observed for the reference compound at a 1 µM final concentration.
TAR values were determined by measuring the initial decrease of the luminol luminescence after addition of small aliquots of the sample at room temperature using a Wallac WinSpectral 1414 liquid scintillation counter with the circuit coincidence out of mode. The conditions of the experiment were the following: 100 mM phosphate buffer, pH 7.4, 30 mM ABAP and 50 µM luminol. Trolox (150 µM) was used as a standard.
The TAR index is defined by:
TAR (µM Trolox) = µl total/µl sample x Io/I sample/Io/I Trolox x Io/I Trolox (1 µM)
where µl total is the final volume (3000 µl), µl sample is the volume of the sample added to the reaction, Io is the initial emission of chemiluminescence (before the addition of the antioxidant), and I is the instantaneous chemiluminescence after the addition of the different aliquots of the sample or the reference compound (Trolox). TAR indices reflect the capacity of the additive to engage in the electron transfer processes to luminol-derived radicals.
Assay of deoxyribose damage
Deoxyribose damage was assayed by the method of Halliwell and Gutteridge (12), with modifications. The reaction mixture in a total volume of 1.2 ml contained 0.5 ml calf thymus DNA (1 mg/ml of 0.15 M NaCl), 0.5 ml 0.1 M sodium phosphate buffer, pH 7.4, 0.2 ml 4.8 mM ferrous ammonium sulfate and 1000, 100 and 10 µg/ml of the methanolic plant fraction. The reaction mixture was incubated for 1 h at 37oC in a water bath shaker. After incubation, 1 ml 1% TBA (w/v) plus 1 ml 2.8% TCA (w/v) were added to the reaction mixture which was kept in a boiling water bath for 15 min. The thiobarbituric acid-reactive substances (TBARS) thus generated were extracted into 2 ml n-butanol. After centrifugation, the fluorescence of the butanol layer was measured as described above. The iron (II)-dependent deoxyribose damage inhibition values are expressed as the ratio of TBARS in the presence of plant extracts to that in their absence (control). Catechin was used as a positive control, and Finney's (13) statistical method of Probit analysis was used to calculate the IC50.
Preparation of rat liver homogenates
Adult Wistar rats weighing 180-200 g fed on a standard laboratory diet and receiving water ad libitum were used. The livers were excised, perfused and homogenized with 120 mM KCl, 50 mM phosphate buffer, pH 7.4 (1:10 w/v). The samples were centrifuged at 700 g for 10 min at 0-4oC. The supernatant fraction was kept at -20oC until the time for use. Protein concentration was measured by the method of Lowry et al. (14) using bovine serum albumin as a standard.
Hydroperoxide-initiated chemiluminescence
Hydroperoxide-initiated chemiluminescence (QL) of liver homogenates (15) was measured in a Wallac WinSpectral 1414 liquid scintillation counter with the circuit coincidence out of mode, at room temperature. Rat liver homogenates adjusted to a final protein concentration of 0.5 mg/ml in 120 mM KCl, 50 mM sodium phosphate buffer, pH 7.4, and to a final volume of 2 ml were placed in 10 x 35-mm glass tubes, which were inserted into low potassium 25 x 50-mm glass vials. Plant extracts were solubilized in distilled water and adjusted to the reaction medium at 10, 100 and 1000 µg/ml. Chemiluminescence was initiated by the addition of 3 mM tert-butyl hydroperoxide, and was expressed as total counts. Chemiluminescence inhibition is reported as the ratio of the maximum emission in the presence of plant extracts to that in their absence (control). Catechin was used as a positive control or standard due to its high antioxidant activity (16). Finney's (13) statistical method of Probit analysis was used to calculate the concentration of the extract that inhibited chemiluminescence by 50%, i.e., the IC50.
Thiobarbituric acid-reactive substance assay
TBARS were determined as described by Fraga et al. (17). Rat liver homogenates, adjusted to 10 mg protein/ml in 120 mM KCl, 50 mM phosphate buffer, pH 7.4, were incubated with 1000, 100 and 10 µg dry weight/ml of plant extract at 37oC for 15 min. Sodium dodecyl sulfate (0.2 ml of 3% (w/v)) and 0.05 ml of 4% BHT in ethanol were added. After mixing, 2 ml of 0.1 N HCl, 0.3 ml of 10% (w/v) phosphotungstic acid and 1 ml of 0.7% (w/v) 2-thiobarbituric acid were added. The mixture was heated for 60 min in boiling water, and TBARS were extracted into 5 ml n-butanol. After centrifugation, the fluorescence of the butanol layer was measured at 515-nm excitation and 555-nm emission using a Hitachi F-3010 fluorescence spectrophotometer. The values are reported as the ratio of the amount of TBARS formed in the presence of plant extracts compared to control. Catechin was used as standard. The extract concentration that would inhibit by 50% the production of thiobarbituric acid-reactive substances, i.e., the IC50 with a 95% confidence interval, was calculated using Finney's (13) statistical method of Probit analysis.
Total reactive antioxidant potential and total antioxidant reactivity
In order to determine the TRAP and TAR indices of the extracts of A. satureioides, a simple method based on luminol-enhanced chemiluminescence was used, which is based on the measurement of induction times in the oxidation of ABAP, a free radical source (11). This method is based on the trapping of peroxyl radicals (ROO·), and is capable of detecting most of the significant compounds with antioxidant activity present in complex mixtures of antioxidants such as plant extracts. Since TRAP measurements indicate the quantity of antioxidants present in the plant extracts, the TAR was also determined in order to measure the quality (given by the reactivity) in those extracts which showed antioxidant activity (11).
The addition of increasing concentrations of the aqueous and methanolic extracts to the reaction medium resulted in a reduction of luminol-enhanced chemiluminescence, indicating their capacity of scavenging peroxyl radicals. The induction times observed were almost proportional to the concentration of the additive (Figure 1), allowing to determine the TRAP and TAR indices for the extracts expressed as µM Trolox equivalents (Table 1). The results obtained indicate that TAR values are considerably higher than TRAP values. This may be due to the presence of antioxidants of relatively high reactivity, thus suggesting that some of the compounds present in A. satureioides could be considerably more reactive than Trolox.
- Induction times as a function of additive volumes (µl) of two extracts of A. satureioides and the reference compound Trolox. Initial concentrations of the extracts were 150 µM for Trolox (closed squares), 0.1 mg/ml for the methanolic extract (open squares), and 0.1 mg/ml for the aqueous extract (closed circles). The initial reaction mixture before addition of the samples was 3 cc of 0.1 M sodium phosphate buffer, pH 7.4, containing 10 µM luminol and 20 mM ABAP. N = 2.
Inhibition of deoxyribose damage
The effect of A. satureioides on the inhibition of free radical-mediated deoxyribose damage was assessed by means of the iron (II)-dependent DNA damage assay. The Fenton reaction generates hydroxyl radicals (OH·) which degrade DNA deoxyribose, using Fe2+ salts as an important catalytic component (12). Oxygen radicals may attack DNA either at the sugar or the base, giving rise to a large number of products. Attack at a sugar ultimately leads to sugar fragmentation, base loss, and strand break with a terminal fragmented sugar residue (18). Addition of low concentrations of transition metal ions such as iron to DNA causes degradation of the sugar into malondialdehyde and other related compounds which form a chromogen with TBARS. Table 2 shows the effect of the extracts on the iron (II)-dependent deoxyribose damage. The methanolic extract was the only one capable of reducing DNA damage at all concentrations. Catechin, used as a standard, was highly effective in inhibiting the oxidative DNA damage, showing an IC50 = 5 µg/ml.
Inhibition of lipid peroxidation
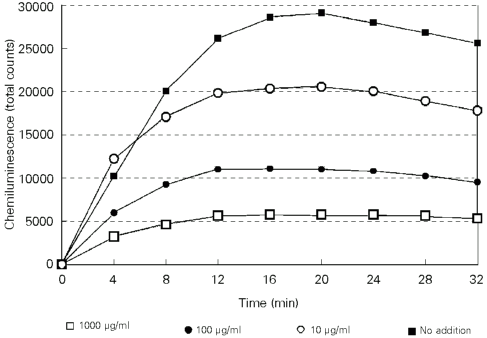
Peroxyl and hydroxyl radicals are important agents that mediate lipid peroxidation, thereby damaging cell membranes (19). In order to determine if the extracts of A. satureioides are capable of reducing in vitro oxidative stress, these were further tested using the hydroperoxide-initiated chemiluminescence assay in rat liver homogenates (15). The results obtained for the aqueous and methanolic extract are shown in Figures 2 and 3. Table 3 shows the antioxidant activity expressed as percentage of inhibition evaluated at maximum emission (20), while negative values are the effect of increasing chemiluminescence. The aqueous extract was able to reduce the emission of chemiluminescence at all concentrations in a dose-dependent manner, thus allowing to calculate an IC50 = 225 µg/ml. On the other hand, the methanolic extract was only effective at higher concentrations (100 and 1000 µg/ml), showing an increase in chemiluminescence at lower concentrations (10 µg/ml). Hydroperoxide-initiated chemiluminescence in rat liver homogenates is a qualitatively different method, since it is used to determine oxidative stress associated with the existence of an imbalance between antioxidant and prooxidant compounds present in the reaction mixture (15). The prooxidant activity observed at a lower concentration of the methanolic extract could therefore be due to the gradual disappearance of antioxidant compounds in the reaction mixture, in contrast to the persistence of substances with prooxidant activity. Furthermore, it has been determined that the lipid peroxidation that occurs in the hydrophobic domains of the membranes accounts for a large part of the emission of chemiluminescence (15). In contrast, the major antioxidant components of A. satureioides are mostly hydrophilic, and this could reduce their ability to reach these domains.
- Effect of A. satureioides (aqueous extract) on the time course of hydroperoxide-initiated chemiluminescence of rat liver homogenates. Zero time was the moment of tert-butyl hydroperoxide addition. The reaction mixture was a 2-ml final volume of rat liver homogenate (final protein concentration, 0.5 mg/ml) in 120 mM KCl, 50 mM phosphate buffer, pH 7.4. Plant extracts were adjusted to the reaction medium at 1000 (open squares), 100 (closed circles), 10 (open circles) µg/ml, and control (no addition) (closed squares). Chemiluminescence was initiated by the addition of 3 mM tert-butyl hydroperoxide and is reported as total counts. The values are means of 2 determinations.
- Effect of A. satureioides (methanolic extract) on the time course of hydroperoxide-initiated chemiluminescence of rat liver homogenates. No addition (closed squares), 1000 (open squares), 100 (closed circles) and 10 (open circles) µg/ml. Experimental conditions are as described in the legend to Figure 2. Zero time was the moment of tert-butyl hydroperoxide addition. The values are means of 2 determinations.
Because hydroperoxide-initiated chemiluminescence in rat liver homogenates determines the free radical trapping activity in a chain reaction that occurs both in the hydrophilic and the hydrophobic domains of the biological membranes (15), a second assay that determines the production of malondialdehyde and related compounds was performed. TBARS are produced as by-products of lipid peroxidation that occur only in the hydrophobic core of biomembranes (17). The results obtained suggest that both the aqueous and methanolic extracts of A. satureioides are capable of reducing the lipid peroxidation that occurs in these domains (Table 4).
Catechin, used as a reference compound in the determination of the inhibition of lipid peroxidation, was highly effective in reducing both chemiluminescence and TBARS, showing an IC50 = 1 µg/ml in the first assay, and an IC50 = 46 µg/ml in the second.
Based on the results described, we may conclude that the aqueous and methanolic extracts of A. satureioides possess significant free radical scavenging and antioxidant activity in vitro. It has been stated that chemiluminescence methods are more sensitive than TBARS production in the determination of antioxidant activity (21-23). This could explain the absence of activity observed in the aqueous extract in inhibiting DNA damage. In a similar way, the methanolic extract was less active when tested by fluorescence methods. These results should encourage future in vivo studies, which could ultimately lead to the inclusion of this medicinal herb in different antioxidant pharmaceutical formulations.
1. Houghton P (1995). The role of plants in traditional medicine and current therapy. Journal of Alternative and Complementary Medicine, 1: 131-143.
2. Haslam E (1996). Natural polyphenols (vegetable tannins) as drugs: possible modes of action. Journal of Natural Products, 59: 205-215.
3. Toursarkissian M (1980). Plantas Medicinales de la Argentina. Hemisferio Sur, Argentina.
4. Filot Da Silva L & Langeloh A (1994). Comparative study of antispasmodic activity of hydroalcoholic 80% (v/v) extracts of Achyrocline satureioides (Lam.) DC. (Asteraceae) with papaverive and atropine on rat isolated jejunum. Acta Farmaceutica Bonaerense, 13: 35-40.
5. Simões C, Schenkel E, Bauer L & Langeloh A (1988). Pharmacological investigations on Achyrocline satureioides (Lam.) DC. (Compositae). Journal of Ethnopharmacology, 22: 281-293.
6. Broussalis A, Ferraro G, Gurni A & Coussio J (1989). Aspectos fitoquímicos de especies argentinas del género Achyrocline. Acta Farmaceutica Bonaerense, 8: 11-16.
7. Ferrándiz M & Alcaraz M (1991). Anti-inflammatory activity and inhibition of arachidonic acid metabolism by flavonoids. Agents and Actions, 32: 283-288.
8. Middleton E (1996). Biological properties of plant flavonoids: an overview. International Journal of Pharmacognosy, 34: 344-348.
9. López P, Broussalis A, Rodríguez M, Coussio J & Ferraro G (1996). Análisis de muestras comerciales de "marcela" (Achyrocline satureioides). Acta Farmaceutica Bonaerense, 15: 243-249.
10. Anonymous (1995). Manual de Técnicas de Investigación. Programa Iberoamericano de Ciencias y Tecnología para el Desarrollo, Madrid.
11. Lissi E, Pascual C & Castillo M (1992). Luminol luminescence induced by 2,2'-azo-bis(2-amidinopropane) thermolysis. Free Radical Research Communications, 17: 299-311.
12. Halliwell B & Gutteridge J (1981). Formation of a thiobarbituric-acid-reactive substance from deoxyribose in the presence of iron salts. The role of superoxide and hydroxyl radicals. FEBS Letters, 128: 347-352.
13. Finney D (1971). Probit Analysis. Cambridge University Press, Cambridge.
14. Lowry O, Rosebrough A, Farr A & Randall R (1951). Protein measurement with the Folin phenol reagent. Journal of Biological Chemistry, 193: 265-275.
15. Gonzalez Flecha B, Llesuy S & Boveris A (1991). Hydroperoxide-initiated chemiluminescence: an assay for oxidative stress in biopsies of heart, liver and muscle. Free Radicals in Biology and Medicine, 10: 93-100.
16. Fraga C, Martino V, Ferraro G, Coussio J & Boveris A (1987). Flavonoids as antioxidants evaluated by in vitro and in situ liver chemiluminescence. Biochemical Pharmacology, 36: 717-720.
17. Fraga C, Leibovitz B & Tappel A (1987). Halogenated compounds as inducers of lipid peroxidation in tissue slices. Free Radicals in Biology and Medicine, 3: 119-123.
18. Imlay J & Linn S (1988). DNA damage and oxygen radical toxicity. Science, 240: 1302-1309.
19. Boik J (1996). Cancer and Natural Medicine. Oregon Medical Press, Princeton, MN.
20. Cadenas E & Sies H (1982). Low level chemiluminescence of liver microsome fractions initiated by tert-butyl hydroperoxide. Relation to microsomal haemoproteins, oxygen dependence and lipid peroxidation. European Journal of Biochemistry, 124: 349-356.
21. Fernandez V, Llesuy S, Solari L, Kipreos K, Videla L & Boveris A (1988). Chemiluminescent and respiratory response related to thyroid hormone-induced liver oxidative stress. Free Radical Research Communications, 5: 77-84.
22. Lavagno C, Giulivi C & Boveris A (1991). Betamethasone effects on Paraquat lung toxicity. Xenobiotica, 21: 1003-1011.
23. Videla L, Fraga C, Koch O & Boveris A (1983). Chemiluminescence of the in situ rat liver after acute ethanol intoxication. Effect of (+)-cyanidanol-3. Biochemical Pharmacology, 32: 2822-2825.
Address for correspondence: C. Desmarchelier, Cátedra de Microbiología Industrial y Biotecnología, Facultad de Farmacia y Bioquímica, Universidad de Buenos Aires, Junín 956, (1113) Capital Federal, Argentina. Fax: (541) 961-7637. E-mail: cdesmar@huemul.ffyb.uba.ar
Research supported by the International Foundation for Science (IFS), in Stockholm, Sweden (No. F/2628-1), BID-CONICET (No. PMT-SID0370) and University of Buenos Aires (No. UBACYT FAO12/J). Received December 10, 1997. Accepted May 25, 1998.
- 1. Houghton P (1995). The role of plants in traditional medicine and current therapy. Journal of Alternative and Complementary Medicine, 1: 131-143.
- 2. Haslam E (1996). Natural polyphenols (vegetable tannins) as drugs: possible modes of action. Journal of Natural Products, 59: 205-215.
- 3. Toursarkissian M (1980). Plantas Medicinales de la Argentina Hemisferio Sur, Argentina.
- 4. Filot Da Silva L & Langeloh A (1994). Comparative study of antispasmodic activity of hydroalcoholic 80% (v/v) extracts of Achyrocline satureioides (Lam.) DC. (Asteraceae) with papaverive and atropine on rat isolated jejunum. Acta Farmaceutica Bonaerense, 13: 35-40.
- 5. Simőes C, Schenkel E, Bauer L & Langeloh A (1988). Pharmacological investigations on Achyrocline satureioides (Lam.) DC. (Compositae). Journal of Ethnopharmacology, 22: 281-293.
- 6. Broussalis A, Ferraro G, Gurni A & Coussio J (1989). Aspectos fitoquímicos de especies argentinas del género Achyrocline Acta Farmaceutica Bonaerense, 8: 11-16.
- 7. Ferrándiz M & Alcaraz M (1991). Anti-inflammatory activity and inhibition of arachidonic acid metabolism by flavonoids. Agents and Actions, 32: 283-288.
- 8. Middleton E (1996). Biological properties of plant flavonoids: an overview. International Journal of Pharmacognosy, 34: 344-348.
- 9. López P, Broussalis A, Rodríguez M, Coussio J & Ferraro G (1996). Análisis de muestras comerciales de "marcela" (Achyrocline satureioides). Acta Farmaceutica Bonaerense, 15: 243-249.
- 10. Anonymous (1995). Manual de Técnicas de Investigación Programa Iberoamericano de Ciencias y Tecnología para el Desarrollo, Madrid.
- 11. Lissi E, Pascual C & Castillo M (1992). Luminol luminescence induced by 2,2'-azo-bis(2-amidinopropane) thermolysis. Free Radical Research Communications, 17: 299-311.
- 12. Halliwell B & Gutteridge J (1981). Formation of a thiobarbituric-acid-reactive substance from deoxyribose in the presence of iron salts. The role of superoxide and hydroxyl radicals. FEBS Letters, 128: 347-352.
- 13. Finney D (1971). Probit Analysis Cambridge University Press, Cambridge.
- 14. Lowry O, Rosebrough A, Farr A & Randall R (1951). Protein measurement with the Folin phenol reagent. Journal of Biological Chemistry, 193: 265-275.
- 15. Gonzalez Flecha B, Llesuy S & Boveris A (1991). Hydroperoxide-initiated chemiluminescence: an assay for oxidative stress in biopsies of heart, liver and muscle. Free Radicals in Biology and Medicine, 10: 93-100.
- 16. Fraga C, Martino V, Ferraro G, Coussio J & Boveris A (1987). Flavonoids as antioxidants evaluated by in vitro and in situ liver chemiluminescence. Biochemical Pharmacology, 36: 717-720.
- 17. Fraga C, Leibovitz B & Tappel A (1987). Halogenated compounds as inducers of lipid peroxidation in tissue slices. Free Radicals in Biology and Medicine, 3: 119-123.
- 18. Imlay J & Linn S (1988). DNA damage and oxygen radical toxicity. Science, 240: 1302-1309.
- 19. Boik J (1996). Cancer and Natural Medicine Oregon Medical Press, Princeton, MN.
- 20. Cadenas E & Sies H (1982). Low level chemiluminescence of liver microsome fractions initiated by tert-butyl hydroperoxide. Relation to microsomal haemoproteins, oxygen dependence and lipid peroxidation. European Journal of Biochemistry, 124: 349-356.
- 21. Fernandez V, Llesuy S, Solari L, Kipreos K, Videla L & Boveris A (1988). Chemiluminescent and respiratory response related to thyroid hormone-induced liver oxidative stress. Free Radical Research Communications, 5: 77-84.
- 22. Lavagno C, Giulivi C & Boveris A (1991). Betamethasone effects on Paraquat lung toxicity. Xenobiotica, 21: 1003-1011.
Correspondence and Footnotes
Publication Dates
-
Publication in this collection
21 Sept 1998 -
Date of issue
Sept 1998
History
-
Accepted
25 May 1998 -
Received
10 Dec 1997