Abstract
The characterization of proteins from Brucella spp, the causative agent of brucellosis, has been the subject of intensive research. We have described an 18-kDa cytoplasmic protein of Brucella abortus and shown the potential usefulness of this protein as an antigen for the serologic diagnosis of brucellosis. The amino acid sequence of the protein showed a low but significant homology with that of lumazine synthases. Lumazine is an intermediate product in bacterial riboflavin biosynthesis. The recombinant form of the 18-kDa protein (expressed in E. coli) folds like the native Brucella protein and has lumazine-synthase enzymatic activity. Three-dimensional analysis by X-ray crystallography of the homolog Bacillus subtilis lumazine synthase has revealed that the enzyme forms an icosahedral capsid. Recombinant lumazine synthase from B. abortus was crystallized, diffracted X rays to 2.7-Å resolution at room temperature, and the structure successfully solved by molecular replacement procedures. The macromolecular assembly of the enzyme differs from that of the enzyme from B. subtilis. The Brucella enzyme remains pentameric (90 kDa) in its crystallographic form. Nonetheless, the active sites of the two enzymes are virtually identical at the structural level, indicating that inhibitors of these enzymes could be viable pharmaceuticals across a broad species range. We describe the structural reasons for the differences in their quaternary arrangement and also discuss the potential use of this protein as a target for the development of acellular vaccines.
Brucella lumazine synthase; x-ray structure; immunogenicity; brucellosis detection; Bacillus subtilis lumazine synthase
Braz J Med Biol Res, July 2000, Volume 33(7) 741-747
Structural, functional and immunological studies on a polymeric bacterial protein
P.C. Baldi1, C.A. Velikovsky1, B.C. Braden2, G.H. Giambartolomei1, C.A. Fossati1 and F.A. Goldbaum3
1Instituto de Estudios de la Inmunidad Humoral, Facultad de Farmacia y Bioquímica, Universidad de Buenos Aires, Buenos Aires, Argentina
2Department of Natural Sciences, Bowie State University, Bowie, MD, USA
3Instituto de Investigaciones Bioquímicas (Fundación Campomar, IIBBA-CONICET, FCEN-UBA), Buenos Aires, Argentina
Text
References
Correspondence and Footnotes Correspondence and Footnotes Correspondence and Footnotes
Abstract
The characterization of proteins from Brucella spp, the causative agent of brucellosis, has been the subject of intensive research. We have described an 18-kDa cytoplasmic protein of Brucella abortus and shown the potential usefulness of this protein as an antigen for the serologic diagnosis of brucellosis. The amino acid sequence of the protein showed a low but significant homology with that of lumazine synthases. Lumazine is an intermediate product in bacterial riboflavin biosynthesis. The recombinant form of the 18-kDa protein (expressed in E. coli) folds like the native Brucella protein and has lumazine-synthase enzymatic activity. Three-dimensional analysis by X-ray crystallography of the homolog Bacillus subtilis lumazine synthase has revealed that the enzyme forms an icosahedral capsid. Recombinant lumazine synthase from B. abortus was crystallized, diffracted X rays to 2.7-Å resolution at room temperature, and the structure successfully solved by molecular replacement procedures. The macromolecular assembly of the enzyme differs from that of the enzyme from B. subtilis. The Brucella enzyme remains pentameric (90 kDa) in its crystallographic form. Nonetheless, the active sites of the two enzymes are virtually identical at the structural level, indicating that inhibitors of these enzymes could be viable pharmaceuticals across a broad species range. We describe the structural reasons for the differences in their quaternary arrangement and also discuss the potential use of this protein as a target for the development of acellular vaccines.
Key words: Brucella lumazine synthase, x-ray structure, immunogenicity, brucellosis detection, Bacillus subtilis lumazine synthase
Introduction
The sequence, structure and function of protein components of microorganisms represent a field of increasing interest to both microbiologists and immunologists. The characterization of proteins from pathogenic bacteria can help to understand the interaction between the bacterium and the host and to study the humoral and cellular immune responses elicited during the infection. Moreover, proteins can be useful as specific antigens for the serologic diagnosis of bacterial infections and as specific targets for rational drug design of chemotherapeutic agents.
The characterization of proteins from Brucella spp, the causative agent of brucellosis, has been the subject of intensive research, but only a few proteins have been characterized at the structural and functional level. Human and animal brucellosis still constitutes an important health problem in many developing countries. The disease is caused by different species of Brucella which can be distinguished by their preferential host and also by the type of lipopolysaccharide (LPS) present on their surface. Species expressing smooth LPS include B. abortus, B. suis and B. melitensis, which infect mainly cows, pigs and goats and sheep, respectively. Species expressing rough LPS include B. canis and B. ovis, which infect mainly dogs and sheep, respectively. Serology has proven to be an important tool for the diagnosis, prognosis and management of this infection. However, the most widely used tests (mainly agglutination tests) rely on the detection of antibodies directed at Brucella LPS, in spite of several studies showing the diagnostic drawbacks associated with the measurement of this response (1,2). Because of the existence of shared epitopes between the LPS from Brucella and that from another Gram-negative bacterium, false-positives hinder the serologic diagnosis of brucellosis. In addition, the development of high anti-LPS titers in vaccinated animals makes it difficult to differentiate vaccinated from infected cattle by means of agglutination assays (3).
Diagnostic usefulness of Brucella cytoplasmic proteins
In view of the diagnostic problems mentioned above, the main goal of our group has been to obtain Brucella cytoplasmic proteins free from LPS and to test their diagnostic usefulness by ELISA. Our first approach was to prepare an anti-LPS monoclonal antibody (termed BC68) which was coupled to a gel matrix to obtain an immunosorbent. The cytoplasmic fraction of B. abortus was passed through this immunosorbent so that the LPS was retained by the attached antibody while the proteins eluted. This complex mixture of LPS-free cytosolic proteins (CP) was used as antigen in an indirect ELISA to test the reactivity of sera from different hosts. We have found serum reactivity to CP in brucellosis patients (4) and also in cows (5), sheep, goats, pigs and dogs (6) infected with different Brucella species, which suggests that the internal antigens are common to all the Brucella species regardless of the type of LPS. Anti-CP antibodies proved to be useful for differentiating active from inactive human brucellosis (4). In addition, a serologic follow-up performed on patients with different outcomes of the disease showed that the kinetics of the antibody response to proteins is correlated with the clinical outcome of patients (4).
The positive results obtained with the CP antigen prompted us to investigate the potential diagnostic usefulness of particular cytoplasmic proteins of Brucella. We were able to obtain a monoclonal antibody (termed BI24) directed at an 18-kDa cytoplasmic protein of B. abortus which was later shown to be present in all the Brucella species tested (7). The monoclonal antibody (mAb) was used to develop an antigen capture ELISA in which BI24 is adsorbed to the plate and the CP antigen, which contains the 18-kDa protein, is dispensed later. Once the protein is captured by the mAb, the remaining antigens present in CP are removed by washing. In humans, the determination of antibodies against the 18-kDa antigen also permits to differentiate active from inactive brucellosis (7). In a follow-up of 24 patients suffering from acute brucellosis, the titers and the kinetics of the antibody response to the 18-kDa protein were very similar to those against CP (Baldi PC, Velikovsky CA, Giambartolomei GH and Fossati CA, unpublished data). In most of these patients IgM antibodies to the 18-kDa protein were detected very early after the onset of symptoms, later followed by the appearance of IgG antibodies against this antigen (Figure 1). In cattle, detection of antibodies to the 18-kDa protein proved useful to distinguish between animals vaccinated with strain 19 of B. abortus and pregnant heifers experimentally infected with a virulent strain of B. abortus (5). While the latter had high levels of antibodies against the 18-kDa protein, the vaccinated cattle developed only a low and transient antibody response that was no longer detected at 90 days post-vaccination (a time when anti-LPS antibodies were still detected at high levels). The 18-kDa protein and the CP antigen were also useful for the diagnosis of canine brucellosis (6,8). The antibody reactivity to cytoplasmic proteins of Brucella was investigated by ELISA in sera from 30 dogs having confirmed or suspected brucellosis. Antibodies to the 18-kDa protein were found in 26 animals which were also positive for anti-CP antibodies and for anti-LPS antibodies (the latter detected by the slide agglutination test). The overall correlation between the two anti-protein ELISAs reached 93.3% (8).
Identity of the 18-kDa protein
The 18-kDa protein was purified by affinity chromatography using the mAb BI24 and the sequence of three internal peptides was determined (7). Later, Hemmen et al. (9) cloned a gene encoding for a 17-kDa Brucella protein whose deduced amino acid sequence showed homology with the internal peptides previously described by us, suggesting that the two proteins were identical. These investigators found this protein to be useful for diagnosing ovine and bovine brucellosis. Others identified a gene encoding a homologue of the 17-kDa Brucella protein in Rhodococcus spp. In turn, database searching revealed that the sequence of both the Brucella and the Rhodococcus proteins had a low but significant homology with that of lumazine synthases involved in bacterial riboflavin biosynthesis (10). To further characterize the Brucella protein and to determine whether it is a lumazine synthase we decided to produce it in recombinant form based on the published nucleotide sequence.
Recombinant expression of the Brucella 18-kDa protein
The 18-kDa protein from Brucella abortus was successfully expressed in the pET vector as inclusion bodies in BL21(DE3) cells. Attempts to obtain soluble expression of the protein were unsuccessful. In the absence of dithiothreitol (DTT) no refolded material was obtained. The refolded protein was purified by anion exchange chromatography under reducing conditions. The appropriate folding of the recombinant 18-kDa protein was confirmed by testing the reactivity of sera from human and animal brucellosis against both the native and the recombinant protein. Although the purified 18-kDa recombinant protein was soluble in solutions containing 1 mM DTT, it aggregated in the absence of a reducing agent. Since the protein contains a single cysteine, this behavior was thought to reflect the formation of intermolecular associations by means of disulfide bridges. This hypothesis was confirmed later when the aggregation in non-reducing media was prevented by treatment with iodoacetamide under reducing conditions. Gel-exclusion chromatography revealed that the iodoacetamide-treated protein has a molecular weight of about 90 kDa, indicating the presence of a pentamer of the 18-kDa protein.
In immunoblotting assays, the recombinant protein was recognized by the mAb BI24 previously used by us to characterize the 18-kDa protein, confirming the identity between the protein described by us (7) and that cloned by Hemmen et al. (9).
Functional studies on the Brucella 18-kDa protein
E. coli cells containing the plasmid encoding the 18-kDa protein were grown and induced to express recombinant protein. For the determination of 6,7-dimethyl-8-ribityllumazine synthase activity, the appropriate reaction mixture was prepared and the cell extract from recombinant bacteria was added. Aliquots were taken at regular intervals and the reaction was stopped by the addition of trichloroacetic acid. The concentration of 6,7-dimethyl-8-ribityllumazine was determined by high performance liquid chromatography coupled with fluorometric monitoring. The 6,7-dimethyl-8-ribityllumazine synthase activity found in the cell extract was approximately 6-fold higher than the activity displayed by control bacteria, clearly indicating that the recombinant Brucella 18-kDa protein is an enzyme with lumazine synthase activity (11).
Crystallographic structure of Brucella lumazine synthase
Crystallographic studies were undertaken to further characterize the 18-kDa protein. Recombinant lumazine synthase from Brucella abortus was crystallized at room temperature by the hanging-drop vapor-diffusion method, yielding crystals that diffracted to 2.7-Å resolution at 300o K. Data were collected at the LNLS protein facility in Campinas, SP, Brazil.
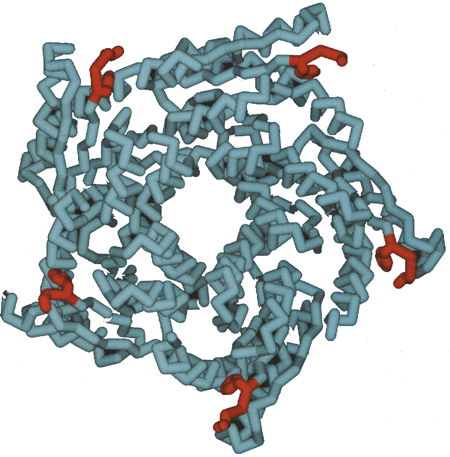
The structure of the Brucella protein was successfully solved by molecular replacement procedures using the lumazine synthase subunits from the Bacillus subtilis structure modeled as poly-alanines. The tertiary structure of the monomeric protein and the pentameric assembly of the monomers closely resemble those found in the B. subtilis lumazine synthase (12). The most striking difference between the structures of lumazine synthase from Brucella and B. subtilis is the non-icosahedral nature of the Brucella enzyme assembly (13).
The pentameric assemblies of the enzymes from B. subtilis normal Brucella abortus indicate no large-scale difference in structure (Figure 2).
As the active site of lumazine synthase is formed by the interface between two adjacent monomers of the pentameric assembly, the Brucella enzyme maintains the active site of the B. subtilis enzyme. When the lumazine substrate analog (5-nitroso-6-ribitylamino-2,4(1H,3H) pyrimidindione) from the 2.4-Å capsid structure of B. subtilis (12) is modeled into the Brucella enzyme binding site, it is clear that the main-chain atoms responsible for recognition and binding of the substrate are topologically well preserved. The only structural difference in the recognition site is the substitution of a tryptophan residue in the B. abortus protein for a phenylalanine in the B. subtilis protein. As the function of this side chain apparently consists of substrate binding to the substrate pyrimidine ring via stacking interactions, the Phe and Trp side chains appear to be functionally equivalent.
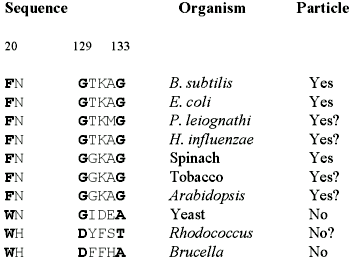
We further analyzed the structural reasons for divergence in macromolecular assembly between pentameric and icosahedral enzymes. The C-terminal a-helix of the B. subtilis protein begins at residue 121 for nearly two turns but deviates into a 5-residue loop before continuing to the C-terminus as an a-helix. This 5-residue kink (GTKAG) contributes a number of contacts to a neighboring pentamer. On the other hand, the C-terminal a-helix in the B. abortus structure begins at residue 122 and is a continuous helix, unable to form this potential capsid-stabilizing loop.
On the basis of sequence alignment, Mörtl et al. (14) suggested that a four-residue insertion in the yeast Saccharomyces cerevisiae lumazine synthase compared to B. subtilis protein was the basis for the lack of capsid assembly for the yeast enzyme. These residues, however, are located just after the kink in the B. subtilis structure and would not necessarily disrupt the C-terminal a-helix. Glycine residues initiate and terminate the kink in the B. subtilis structure. Moreover, a consensus sequence of GT(or G)KAG occurs in all of the lumazine synthase molecules which form icosahedral 60-mer capsids (Figure 3), namely B. subtilis (12), E. coli (14) and spinach (15). Lumazine synthases which have been identified as pentameric, B. abortus (herein) and yeast (14,16) do not contain the glycine residues that originate and terminate the kink. Glycine exhibits a broader range of conformational stability than amino acids with ß-carbons which in this case may destabilize the a-helical conformation, allowing the helix to bend. While the C-terminal a-helix of the B. subtilis enzyme is bent as a result of this kink, the helix in the B. abortus enzyme is relatively straight and represents yet another reason for the lack of capsid formation as a result of steric contacts with a potential pentamer neighbor.
Concluding remarks
Previous reports have shown the potential usefulness of Brucella lumazine synthase in the serologic diagnosis of human and animal brucellosis (5,7,9,17). Interestingly, the determination of the antibody response to this protein yields results equivalent to those obtained with a complex mixture of cytoplasmic proteins of Brucella (5,7,8).
The expression system and refolding procedures allowed us to obtain the protein as a soluble pentamer, with antigenic properties similar to those of the native Brucella protein. The enzymatic activity displayed by the purified recombinant Brucella abortus lumazine synthase protein confirms that the biological activity of this protein is that of lumazine synthase.
As stated by Mörtl et al. (14), bacteria are devoid of an uptake system for riboflavin. They are therefore dependent on internal riboflavin synthesis and should be vulnerable to inhibitors of riboflavin synthesis. Since this enzyme is not present in mammals, knowledge about its three-dimensional structure could serve as a basis for the rational design of enzyme inhibitors with therapeutic activity.
We determined the three-dimensional structure of Brucella lumazine synthase and compared this new structure with that of the analogue B. subtilis enzyme complexed with an analog substrate. The protein structure of B. subtilis revealed that the enzyme forms a particle with a molecular weight close to 1,000 kDa (12). This particle comprises 60 ß subunit monomers (lumazine synthase) arranged in 12 pentamers, forming an icosahedral capsid. It contains also 3 a subunits (riboflavin synthase) enclosed inside the capsid. The entire structure is called lumazine synthase-riboflavin synthase complex. In contrast, the lumazine synthase of E. coli is not physically associated with another enzyme of the riboflavin pathway (14).
Comparison with the structure of Brucella lumazine synthase has revealed the mechanism of divergent macromolecular assembly while maintaining a conserved binding site structure. It appears that the formation of the substrate binding site and the assembly of the enzyme are related. While the Brucella and yeast enzyme share a pentameric assembly they also feature a Trp residue at the binding site (Figure 3), the bulk of the side chain compensating for a more open site than found in the Bacillus enzyme. The capsid-forming lumazine synthases (from E. coli, B. subtilis and spinach) are sequence homologous in the C-terminal a-helix and contain a phenylalanine interacting with the substrate. As a consequence, the lumazine synthase from Photobacterium leiognathi, Haemophilus influenzae, Actinobacillus pleuropneuminiae, Photobacterium phosphoreum, tobacco and Arabidopsis, all sharing the GT(or G)KAG sequence within the C-terminal helix and a phenylalanine at the substrate binding site, are therefore potential capsid structures, whereas the enzyme from Rhodococcus (DYFST in the C-terminal helix and Trp in the substrate binding site), like the Brucella and yeast enzymes, will be pentameric in its active form. Conservation of the main features of the active site among species would indicate that inhibitors of these enzymes could be used as viable pharmaceuticals across a broad species range.
Recently, Persson et al. (18) determined the three-dimensional structures of the capsid-forming lumazine synthase from spinach and of the pentameric enzyme from the fungus Magnaporte grisea. They found a different structural reason for macromolecular divergence, indicating the heterogeneity of the phylogenetic evolution of this enzyme.
Even though Brucella abortus lumazine synthase does not form the icosahedral particle enzyme, its polymeric nature could explain, at least in part, the immunogenic nature of this protein. Interestingly, as in the B. subtilis protein, the 8-10 amino-terminal residues of the Brucella protein are not well defined in their crystallographic structure. The lack of defined electron density for these residues suggests that this portion of the protein is not essential for the overall folding of the pentamer. In principle, these amino-terminal residues could be changed by peptides pertaining to other immunogenic Brucella proteins (Figure 4). Construction and expression of chimeric proteins using the pentameric structure of this Brucella protein as a carrier and presenting different peptides to the immune system in a pentavalent manner and within an immunogenic context could be the basis for the development of acellular vaccines. Experimental tests of this hypothesis are needed in order to ascertain the usefulness of Brucella lumazine synthase as an immunogenic carrier protein.
Address for correspondence: F.A. Goldbaum, Instituto de Investigaciones Bioquímicas, Fundación Campomar, Av. Patricias Argentinas, 435, Buenos Aires 1405, Argentina. Fax: +54-114-865-2246. E-mail: goldbaum@iib.uba.ar
Presented at the XIV Annual Meeting of the Federação de Sociedades de Biologia Experimental, Caxambu, MG, Brasil, August 25-28, 1999. Research supported by grant BID 802/OC-AR-PICT 00084 from Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT) and by grant PIP 0764/98 from Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET). B.C. Braden was supported by NIH grant 1R15AI44790-01and National Aeronautics and Space Administration grant NCC5-232 (Model Institutes for Excellence). Received January 7, 2000. Accepted March 10, 2000.
- 1. Alton GG, Jones LM, Angus RD & Verger JM (1988). Techniques for the Brucellosis Laboratory Institut National de la Recherche Agronomique, Paris.
- 2. Diaz R & Moriyón I (1989). Laboratory techniques in the diagnosis of human brucellosis. In: Young EJ & Corbel MJ (Editors), Brucellosis: Clinical and Laboratory Aspects CRC Press Inc., Boca Raton.
- 3. Nicoletti P, Jones LM & Berman DT (1978). Comparison of the subcutaneous and conjunctival route of vaccination with Brucella abortus strain 19 vaccine in adult cattle. Journal of the American Veterinary Medical Association, 173: 1450-1456.
- 4. Goldbaum FA, Rubbi CP, Wallach JC, Miguel SE, Baldi PC & Fossati CA (1992). Differentiation between active and inactive human brucellosis by measuring antiprotein humoral immune responses. Journal of Clinical Microbiology, 30: 604-607.
- 5. Baldi PC, Giambartolomei GH, Goldbaum FA, Abdón LF, Velikovsky CA, Kittelberger R & Fossati CA (1996). Humoral immune response against LPS and cytoplasmic proteins of Brucella in cattle vaccinated with Brucella abortus S19 or experimentally infected with Yersinia enterocolitica 0:9. Clinical and Diagnostic Laboratory Immunology, 3: 472-476.
- 6. Baldi PC, Wanke MM, Loza ME & Fossati CA (1994). Brucella abortus cytoplasmic proteins used as antigens in an ELISA potentially useful for the diagnosis of canine brucellosis. Veterinary Microbiology, 41: 127-134.
- 7. Goldbaum FA, Leoni J, Wallach JC & Fossati CA (1993). Characterization of an 18-kilodalton Brucella cytoplasmic protein which appears to be a serological marker of active infection of both human and bovine brucellosis. Journal of Clinical Microbiology, 31: 2141-2145.
- 8. Baldi PC, Wanke MM, Loza ME, Monachesi N & Fossati CA (1997). Diagnosis of canine brucellosis by detection of IgG antibodies against an 18 kDa cytoplasmic protein of Brucella spp. Veterinary Microbiology, 57: 273-281.
- 9. Hemmen F, Weynants V, Scarcez T, Letesson J-J & Saman E (1995). Cloning and sequence analysis of a newly identified Brucella abortus gene and serological evaluation of the 17-kilodalton antigen that it encodes. Clinical and Diagnostic Laboratory Immunology, 2: 263-267.
- 10. De Mot R, Nagy I, Schoofs G & Vanderleyden J (1996). Identification of a Rhodococcus gene cluster encoding a homolog of the 17-kDa antigen of Brucella and a putative regulatory protein of the AsnC-Lrp family. Current Microbiology, 33: 26-30.
- 11. Goldbaum FA, Velikovsky CA, Baldi PC, Mörtl S, Bacher A & Fossati CA (1999). The 18 kDa cytoplasmic protein of Brucella species - an antigen useful for diagnosis - is a lumazine synthase. Journal of Medical Microbiology, 48: 833-839.
- 12. Ritsert K, Huber R, Turk D, Ladenstein R, Schmidt-Base K & Bacher A (1995). Studies on the lumazine synthase/riboflavin synthase complex of Bacillus subtilis: crystal structure analysis of reconstituted, icosahedral ß-subunit capsids with bound substrate analogue inhibitor at 2.4 Å resolution. Journal of Molecular Biology, 253: 151-167.
- 13. Braden BC, Velikovsky CA, Cauerhff AA, Polikarpov I & Goldbaum FA (2000). Divergence in macromolecular assembly: X-ray crystallographic structure analysis of lumazine synthase from Brucella abortus Journal of Molecular Biology, 297: 1031-1036.
- 14. Mörtl S, Fischer M, Richter G, Tack J, Weinkauf S & Bacher A (1996). Biosynthesis of riboflavin: lumazine synthase of Escherichia coli Journal of Biological Chemistry, 271: 33201-33207.
- 15. Jordan DB, Bacot KO, Carlson TJ, Kessel M & Viitanen PV (1999). Plant riboflavin biosynthesis. Cloning, chloroplast location, expression, purification, and partial characterization of spinach lumazine synthase. Journal of Biological Chemistry, 274: 22114-22121.
- 16. Garcia-Ramirez JJ, Santos MA & Revuelta JL (1995). The Saccharomyces cerevisiae RIB4 gene codes for 6,7-dimethyl-8-ribityllumazine synthase involved in riboflavin biosynthesis. Molecular characterization of the gene and purification of the encoded protein. Journal of Biological Chemistry, 270: 23801-23807.
- 17. Letesson J-J, Tibor A, Van Eynde G, Wansard V, Weynants V, Denoel P & Saman E (1997). Humoral immune responses of Brucella-infected cattle, sheep and goats to eight purified recombinant Brucella proteins in an indirect enzyme-linked immunosorbent assay. Clinical and Diagnostic Laboratory Immunology, 4: 556-564.
- 18. Persson K, Schneider G, Jordan DB, Viitanen PV & Sandalova T (1999). Crystal structure analysis of a pentameric fungal and an icosahedral plant lumazine synthase reveals the structural basis for differences in assembly. Protein Science, 8: 2355-2365.
Correspondence and Footnotes
Publication Dates
-
Publication in this collection
14 June 2000 -
Date of issue
July 2000
History
-
Received
07 Jan 2000 -
Accepted
10 Mar 2000