Abstract
Angiotensin-(1-7) (Ang-(1-7)) increased osmotic water permeability in the isolated toad skin, a tissue with functional properties similar to those of the distal mammalian nephron. Concentrations of 0.1 to 10 µM were effective, with a peak at 20 min. This effect was similar in magnitude to that of frog skin angiotensin II (Ang II) and oxytocin but lower than that of human Ang II and arginine-vasotocin. The AT2 angiotensin receptor antagonist PD 123319 (1.0 µM) fully inhibited the response to 0.1 µM Ang-(1-7) but had no effect on the response to Ang II at the same concentration. The specific receptor antagonist of Ang-(1-7), A-779, was ineffective in blocking the response to Ang-(1-7) and to frog skin Ang II. The AT1 receptor subtype antagonist losartan, which blocked the response to frog skin Ang II, was ineffective in blocking the response to Ang-(1-7). The present results support the view of an antidiuretic action of Ang-(1-7) in the mammalian nephron.
angiotensin-(1-7); toad skin; osmotic water permeability; receptor subtypes
Braz J Med Biol Res, September 2000, Volume 33(9) 1099-1104
Angiotensin-(1-7) increases osmotic water permeability in isolated toad skin
J.C. Santos, S. Jerez, M. Peral de Bruno and A. Coviello
Facultad de Ciencias Naturales e Instituto Miguel Lillo, INSIBIO and Fundación INELCO, Tucumán, Argentina
Correspondence and Footnotes Correspondence and Footnotes Correspondence and Footnotes
Abstract
Angiotensin-(1-7) (Ang-(1-7)) increased osmotic water permeability in the isolated toad skin, a tissue with functional properties similar to those of the distal mammalian nephron. Concentrations of 0.1 to 10 µM were effective, with a peak at 20 min. This effect was similar in magnitude to that of frog skin angiotensin II (Ang II) and oxytocin but lower than that of human Ang II and arginine-vasotocin. The AT2 angiotensin receptor antagonist PD 123319 (1.0 µM) fully inhibited the response to 0.1 µM Ang-(1-7) but had no effect on the response to Ang II at the same concentration. The specific receptor antagonist of Ang-(1-7), A-779, was ineffective in blocking the response to Ang-(1-7) and to frog skin Ang II. The AT1 receptor subtype antagonist losartan, which blocked the response to frog skin Ang II, was ineffective in blocking the response to Ang-(1-7). The present results support the view of an antidiuretic action of Ang-(1-7) in the mammalian nephron.
Key words: angiotensin-(1-7), toad skin, osmotic water permeability, receptor subtypes
Introduction
Angiotensin-(1-7) (Ang-(1-7)) is a biologically active peptide of the renin-angiotensin system produced from angiotensin I by an independent pathway of the angiotensin-converting enzyme with cellular functions that differ from angiotensin II (Ang II) (1,2). Several renal actions of Ang-(1-7) have been reported, indicating that the peptide has a role in hydromineral balance. In water-loaded rats it produces marked antidiuresis (3) by a direct effect on inner medullary collecting ducts (4). This effect is not blocked by the V2 receptor antagonist (4) but is blocked by the specific antagonist A-779 (5,6) and losartan (7). Ang-(1-7) may play a role during dehydration and hemorrhage in rats (8). In the rat proximal straight tubules a biphasic effect on fluid absorption blocked by losartan has been reported (9), as well as an inhibitory effect on sodium fluxes in proximal tubule cells (10). On the other hand, diuretic and natriuretic effects in vivo (11) and in the isolated rat kidney (12) have been found which may involve PGI2 release (13).
Amphibian skin is a tissue with functional properties similar to those of the distal mammalian nephron (14). In isolated toad skin Ang II increases osmotic water permeability (Posm) (15) through a common receptor with antidiuretic hormone (16-19). Confirming this effect, a primary antidiuretic action of Ang II was demonstrated in the dog kidney (20). In order to obtain further information about the antidiuretic effect of Ang-(1-7) we measured Posm in isolated toad skin and compared its response to that obtained with other Ang II and neurohypophyseal peptides (oxytocin and arginine-vasotocin).
Material and Methods
Toads (Bufo arenarum) hydrated overnight were used. After pithing, the ventral pelvic skin was dissected and divided into two symmetrical parts which were mounted (dermal surface outward) over the bottom of plastic tubes (surface area 1.77 cm2) and immersed in a bath of 10 ml of aerated Ringer solution. The inner epidermal surface was bathed with the same solution diluted 1/5 in distilled water and Posm was measured gravimetrically by weighing the tube on a scale (Mettler H10) to the nearest 0.1 mg. After an equilibration period of 1 h in which the solutions were changed twice, one or two control periods of 20 min were allowed to elapse, followed by an experimental one in which the Posm response to the hormone added to the dermal bath was measured. In some experiments, an antagonist was added to the paired half-skin 10 min before the addition of the hormone (Ang II) to both halves. In another experiment the preparation was weighed during several periods of 20 min in order to determine the time course of the response. Results are reported as mg cm-2 h-1 and the hormonal effect as the mean and standard error (SEM) of the difference between the experimental period and the previous control one. The Ringer solution contained 90 mM NaCl, 2 mM KCl, 1 mM MgSO4, 1 mM CaCl2, 25 mM Tris-HCl buffer, and 5 mM glucose, pH 7.4, 220 mOsmol/kg H2O.
Drugs
Ang-(1-7) (Asp-Arg-Val-Tyr-Ile-His-Pro) was synthesized by Dr. Clara Peña, Departamento de Química Biológica, Facultad de Farmacia y Bioquímica, Universidad Nacional de Buenos Aires, Argentina, and also obtained from Bachem (Torrance, CA, USA); frog skin Ang II (Ala-Pro-Gly [Ile3,Val5] angiotensin II), human Ang II (Asp-Arg-Val-Tyr-Ile-His-Pro-Phe), oxytocin and arginine-vasotocin were purchased from Sigma Chemical Co. (St. Louis, MO, USA); losartan (DuP 753) was a gift from Dr. Ronald D. Smith (DuPont Merck, Wilmington, DE, USA); PD 123319 (P-186) was obtained from Research Biochemicals Int. (Natick, MA, USA); A-779 (Asp-Arg-Val-Tyr-Ile-His-D-Ala) was a gift from Professor Robson A.S. Santos (Belo Horizonte, MG, Brazil).
Statistical analysis
Results are reported as the mean ± SEM and the Student t-test for paired samples was used for statistical analysis. When more than two means were compared, one-way ANOVA was performed, followed by the Newman-Keuls test. The level of significance was set at P<0.05.

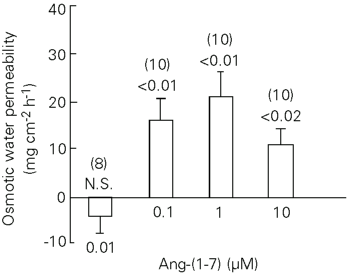
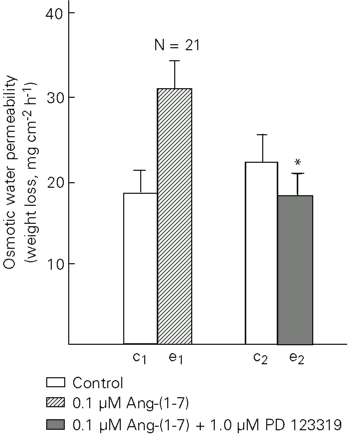
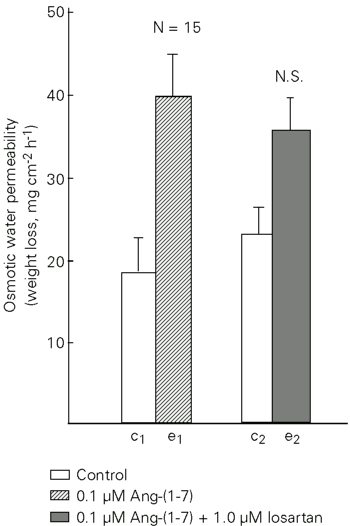
Ang-(1-7) significantly increased Posm over basal flow at concentrations of 0.1 to 10 µM (Figure 1). The higher concentration (10 µM) elicited a lower response that was not statistically different from 1 µM. Ang-(1-7) obtained from two different sources did not differ in the response elicited (data not shown). Figure 2 comparatively shows the response to different angiotensins and neurohypophyseal hormones (oxytocin and arginine-vasotocin). Arginine-vasotocin, the antidiuretic neurohypophyseal hormone of Amphibia, was by far more potent in increasing Posm. The time course of the response to Ang-(1-7) showed a peak at 20 min after addition of the hormone to the dermal bathing solution (Figure 3A,B). The response of the skins varied widely. Figure 3B shows the response of a highly reactive skin. A-779 (1 µM), a specific antagonist of Ang-(1-7), had no effect on the response of toad skin to 0.1 µM Ang-(1-7) (Figure 4) and to 0.1 µM frog skin Ang II (Figure 5). However, the Posm response to Ang-(1-7) was completely blocked by the AT2 antagonist PD 123319 (Figure 6). The AT1 antagonist losartan (Figure 7) was unable to block the response to Ang-(1-7). The antagonists used (A-779, PD 123319 and losartan) had no effect on basal osmotic water permeability (data not shown).
Results

Ang-(1-7) significantly increased toad skin Posm at concentrations of 0.1 to 10 µM with a peak at 20 min similar to that observed with Ang II (15). There was a wide variability in the response between animals with non-responsive skins and others showing a clear response (Figure 3B). Human Ang II was more potent than Ang-(1-7), whose effect was similar to that of frog skin Ang II, an angiotensin peptide found in the skin of the frog Crinia georgiana (21). Arginine-vasotocin, the naturally neurohypophyseal peptide existing in Amphibia, had the highest effect. Doses of 10 µM Ang-(1-7) elicited responses of lower magnitude, although without being statistically significant. This may be attributed to the release of inhibitory substances such as prostaglandins at these concentrations since PGE1 inhibited the effect of Ang II on toad skin Posm (22). No effect of Ang-(1-7) on Posm was found in the isolated toad bladder (data not shown).
The response of toad skin to Ang II is mediated by an AT1 receptor subtype since the effect of Ang II on Posm is blocked by losartan but not by PD 123319 (17). The fact that antidiuretic hormone antagonists blocked the stimulating effect of Ang II on Posm (18) and that losartan blocked the Posm response to antidiuretic hormone in toad skin (19) supports the view of a common receptor for Ang II and antidiuretic hormone in toad skin. In the present experiments, we unexpectedly obtained a complete inhibition of the Posm response to Ang-(1-7) by PD 123319 whereas A-779, considered to be a specific antagonist of Ang-(1-7) (5,6), had no effect. Losartan was also unable to block the response to Ang-(1-7). A-779 completely blocked the antidiuretic effect of Ang-(1-7) on water-loaded rats, suggesting the existence of a non-AT1 angiotensin receptor that is recognized by losartan (7). Our results using PD 123319 showed that Ang-(1-7) increased Posm mainly through an AT2 receptor which in this case was not recognized by losartan, an AT1 receptor subtype antagonist. Our data concerning toad skin did not show a specific receptor for Ang-(1-7), in contrast to the ventrolateral medulla of rats (23,24), porcine coronary artery rings (25) and bovine aortic endothelial cells (26).
Blockade of the Ang-(1-7) receptor by PD 123319 and losartan has been described in studies on the release of [3H] norepinephrine from rat atria (27), suggesting that Ang-(1-7) may act on receptor subtypes 1 and 2. Also, in dog coronary arteries the release of nitrites by Ang-(1-7) was reduced by losartan and PD 123319 (28), as also was the relaxing effect on preconstricted pig coronary arteries in the presence of bradykinin (29). In C6 glioma cells the effect of Ang-(1-7) on prostaglandin synthesis was mediated by subtype 1 angiotensin receptors (30).
Ang II may be converted to Ang-(1-7) by carboxypeptidases and prolylcarboxypeptidase (31). If this were the case in our preparation, PD 123319, the specific antagonist of the AT2 receptor subtype which blocks the effect of Ang-(1-7), would also block at least partially the effect of Ang II on osmotic water permeability in toad skin, but this inhibition was not observed.
The results obtained here with toad skin, a tissue with functional properties similar to those of the distal mammalian nephron, support the antidiuretic effect of Ang-(1-7) observed in the rat (3) and the increase in hydraulic conductivity of mammalian inner medullary collecting tubules (4). We demonstrated for the first time the effect of an angiotensin on Posm in isolated toad skin mediated by an AT2 receptor subtype which is not recognized by losartan.
Discussion
References
1. Ferrario CM, Brosnihan KB, Diz DI, Jaiswal N, Khosla MC, Milsted A & Tallant EA (1991). Angiotensin-(1-7): a new hormone of the angiotensin system. Hypertension, 18 (Suppl 5): III-126-III-133.
2. Santos RAS & Campagnole-Santos MJ (1994). Central and peripheral actions of angiotensin-(1-7). Brazilian Journal of Medical and Biological Research, 27: 1033-1047.
3. Santos RAS & Baracho NCV (1992). Angiotensin-(1-7) is a potent antidiuretic peptide in rats. Brazilian Journal of Medical and Biological Research, 25: 651-654.
4. Santos RAS, Simões e Silva AC, Magaldi AJ, Khosla MC, Cesar KR, Passaglio KT & Baracho NCV (1996). Evidence for a physiological role of angiotensin-(1-7) in the control of hydroelectrolyte balance. Hypertension, 27: 875-884.
5. Santos RAS, Campagnole-Santos MJ, Baracho NCV, Fontes MAP, Silva LCS, Neves LAA, Oliveira DR, Caligiorne SM, Rodrigues ARV, Gropen Jr C, Carvalho WS, Simões e Silva AC & Khosla MC (1994). Characterization of a new angiotensin antagonist selective for angiotensin-(1-7): evidence that the actions of angiotensin-(1-7) are mediated by specific angiotensin receptors. Brain Research Bulletin, 35: 293-298.
6. Ambuhl P, Felix D & Khosla MC (1994). [7-D-Ala]-angiotensin-(1-7): selective antagonism of angiotensin-(1-7) in the rat paraventricular nucleus. Brain Research Bulletin, 35: 289-291.
7. Baracho NCV, Simões-e-Silva AC, Khosla MC & Santos RAS (1998). Effect of selective angiotensin antagonists on the antidiuresis produced by angiotensin-(1-7) in water-loaded rats. Brazilian Journal of Medical and Biological Research, 31: 1221-1227.
8. Botelho LM, Block CH, Khosla MC & Santos RAS (1994). Plasma angiotensin (1-7) immunoreactivity is increased by salt load, water deprivation and hemorrhage. Peptides, 15: 723-729.
9. Garcia NH & Garvin JL (1994). Angiotensin (1-7) has a biphasic effect on fluid absorption in the proximal straight tubule. Journal of the American Society of Nephrology, 5: 1133-1138.
10. Andreatta van Leyen S, Romero MF, Khosla MC & Douglas JG (1993). Modulation of phospholipase A2 activity and sodium transport by angiotensin-(1-7). Kidney International, 44: 932-936.
11. Handa RK, Ferrario CM & Strandhoy JW (1996). Renal actions of angiotensin-(1-7): in vivo and in vitro studies. American Journal of Physiology, 270: F141-F147.
12. Delli Pizzi AM, Hilchey SD & Bell-Quilley CP (1994). Natriuretic action of angiotensin-(1-7). British Journal of Pharmacology, 111: 1-3.
13. Hilchey SD & Bell-Quilley CP (1995). Association between the natriuretic action of angiotensin-(1-7) and selective stimulation of renal prostaglandin I2 release. Hypertension, 25: 1238-1244.
14. Dicker SE (1970). The skin and bladder of amphibians as models for the mammalian nephron. Hormones, 1: 352-363.
15. Coviello A & Brauckmann ES (1973). Hydrosmotic effect of angiotensin II: isolated toad skin. Acta Physiologica Latinoamericana, 23: 18-25.
16. Coviello A, Orce G & Causarano J (1974). Effect of a competitive antagonist (8-Leu-angiotensin II) of angiotensin II on sodium and water transport in toad skin. Acta Physiologica Latinoamericana, 24: 409-413.
17. Peral de Bruno M & Coviello A (1996). Effects of angiotensin II antagonists on the contractile and hydrosmotic effect of AT II and AT III in the toad (Bufo arenarum). Journal of Comparative Physiology, B, 165: 565-570.
18. Peral de Bruno M, Romano L & Coviello A (1996). Interaction between antagonists of angiotensin II and antidiuretic hormone in isolated toad tissues. Comparative Biochemistry and Physiology, 113A: 307-314.
19. Peral de Bruno M, Romano L, Jerez S & Coviello A (1997). El Losartan inhibe el efecto de la hormona antidiurética en un modelo funcional de nefrón distal. Revista Argentina de Cardiología, 65 (Suppl III): 35-38.
20. Levens NR, Peach NJ, Darracott Vaughan ED & Carey RM (1981). Demonstration of a primary antidiuretic action of angiotensin II: effects of intrarenal converting enzyme inhibition in the conscious dog. Endocrinology, 108: 318-330.
21. Proto MC, Coviello A, Khosla MC & Bumpus FM (1983). Effects of frog-skin angiotensin II in amphibians. Hypertension, 5 (Suppl V): V101-V104.
22. Coviello A, Brauckmann ES, Budeguer de Atenor MS, Apud JA & Causarano J (1975). Hydrosmotic effect of angiotensin II in the toad skin: role of cyclic AMP. Acta Physiologica Latinoamericana, 25: 379-386.
23. Fontes MA, Silva LC, Campagnole-Santos MJ, Khosla MC, Guertzenstein PG & Santos RAS (1994). Evidence that angiotensin-(1-7) plays a role in the central control of blood pressure at the ventro-lateral medulla acting through specific receptors. Brain Research, 665: 175-180.
24. Lima DX, Fontes MA, Oliveira RC, Campagnole-Santos MJ, Khosla MC & Santos RAS (1996). Pressor action of angiotensin I at the ventrolateral medulla: effect of selective angiotensin blockade. Immunopharmacology, 33: 305-307.
25. Porsti I, Bara AT, Busse R & Hecker M (1994). Release of nitric oxide by angiotensin-(1-7) from porcine coronary endothelium: implications for a novel angiotensin receptor. British Journal of Pharmacology, 111: 652-654.
26. Tallant EA, Lu X, Weiss RB, Chappell MC & Ferrario CM (1997). Bovine aortic endothelial cells contain an angiotensin-(1-7) receptor. Hypertension, 29: 388-393.
27. Gironacci MM, Adler Graschinsky E, Peña C & Enero MA (1994). Effects of angiotensin II and angiotensin-(1-7) on the release of [3H] norepinephrine from rat atria. Hypertension, 24: 457-460.
28. Seyedi N, Xu X, Nasjletti A & Hintze TH (1995). Coronary kinin generation mediates nitric oxide release after angiotensin receptor stimulation. Hypertension, 26: 164-170.
29. Gorelik G, Carbini LA & Scicli AG (1998). Angiotensin 1-7 induces bradykinin-mediated relaxation in porcine coronary artery. Journal of Pharmacology and Experimental Therapeutics, 286: 403-410.
30. Jaiswal N, Diz DI, Tallant EA, Khosla MC & Ferrario CM (1991). Characterization of angiotensin receptors mediating prostaglandin synthesis in C6 glioma cells. American Journal of Physiology, 260: R1000-R1006.
31. Ferrario CM, Chappell MC, Dean RH & Iyer SN (1998). Novel angiotensin peptides regulate blood pressure, endothelial function, and natriuresis. Journal of the American Society of Nephrology, 9: 1716-1722.
Acknowledgments
We thank Dr. Clara Peña, Departamento de Química Biológica, Facultad de Farmacia y Bioquímica, Universidad Nacional de Buenos Aires, Argentina, for providing Ang-(1-7), Dr. Ronald D. Smith, DuPont Merck, Wilmington, DE, USA, for losartan, and Professor Robson A.S. Santos, Laboratório de Hipertensão, Departamento de Fisiologia e Biofísica, Instituto de Ciências Biológicas, Universidade Federal de Minas Gerais, Belo Horizonte, MG, Brazil, for providing the specific Ang-(1-7) antagonist A-779.
Address for correspondence: A. Coviello, Fundación INELCO, Av. Mitre 35, 4000 Tucumán, Argentina. Fax: +54-381-421-4497. E-mail: acoviello@tucbbs.com.ar
Research supported by the Consejo Nacional de Investigaciones Científicas y Técnicas, Argentina (P.I.P. 5008) and Program I-106 from the Consejo de Investigaciones de la Universidad Nacional de Tucumán, Argentina. An Abstract of this work was presented to the XVth International Congress of Nephrology, May 2-6, 1999, Buenos Aires, Argentina. Received February 24, 1999. Accepted May 8, 2000.
- 1. Ferrario CM, Brosnihan KB, Diz DI, Jaiswal N, Khosla MC, Milsted A & Tallant EA (1991). Angiotensin-(1-7): a new hormone of the angiotensin system. Hypertension, 18 (Suppl 5): III-126-III-133.
- 2. Santos RAS & Campagnole-Santos MJ (1994). Central and peripheral actions of angiotensin-(1-7). Brazilian Journal of Medical and Biological Research, 27: 1033-1047.
- 3. Santos RAS & Baracho NCV (1992). Angiotensin-(1-7) is a potent antidiuretic peptide in rats. Brazilian Journal of Medical and Biological Research, 25: 651-654.
- 4. Santos RAS, Simőes e Silva AC, Magaldi AJ, Khosla MC, Cesar KR, Passaglio KT & Baracho NCV (1996). Evidence for a physiological role of angiotensin-(1-7) in the control of hydroelectrolyte balance. Hypertension, 27: 875-884.
- 5. Santos RAS, Campagnole-Santos MJ, Baracho NCV, Fontes MAP, Silva LCS, Neves LAA, Oliveira DR, Caligiorne SM, Rodrigues ARV, Gropen Jr C, Carvalho WS, Simőes e Silva AC & Khosla MC (1994). Characterization of a new angiotensin antagonist selective for angiotensin-(1-7): evidence that the actions of angiotensin-(1-7) are mediated by specific angiotensin receptors. Brain Research Bulletin, 35: 293-298.
- 6. Ambuhl P, Felix D & Khosla MC (1994). [7-D-Ala]-angiotensin-(1-7): selective antagonism of angiotensin-(1-7) in the rat paraventricular nucleus. Brain Research Bulletin, 35: 289-291.
- 7. Baracho NCV, Simőes-e-Silva AC, Khosla MC & Santos RAS (1998). Effect of selective angiotensin antagonists on the antidiuresis produced by angiotensin-(1-7) in water-loaded rats. Brazilian Journal of Medical and Biological Research, 31: 1221-1227.
- 8. Botelho LM, Block CH, Khosla MC & Santos RAS (1994). Plasma angiotensin (1-7) immunoreactivity is increased by salt load, water deprivation and hemorrhage. Peptides, 15: 723-729.
- 9. Garcia NH & Garvin JL (1994). Angiotensin (1-7) has a biphasic effect on fluid absorption in the proximal straight tubule. Journal of the American Society of Nephrology, 5: 1133-1138.
- 10. Andreatta van Leyen S, Romero MF, Khosla MC & Douglas JG (1993). Modulation of phospholipase A2 activity and sodium transport by angiotensin-(1-7). Kidney International, 44: 932-936.
- 11. Handa RK, Ferrario CM & Strandhoy JW (1996). Renal actions of angiotensin-(1-7): in vivo and in vitro studies. American Journal of Physiology, 270: F141-F147.
- 12. Delli Pizzi AM, Hilchey SD & Bell-Quilley CP (1994). Natriuretic action of angiotensin-(1-7). British Journal of Pharmacology, 111: 1-3.
- 13. Hilchey SD & Bell-Quilley CP (1995). Association between the natriuretic action of angiotensin-(1-7) and selective stimulation of renal prostaglandin I2 release. Hypertension, 25: 1238-1244.
- 14. Dicker SE (1970). The skin and bladder of amphibians as models for the mammalian nephron. Hormones, 1: 352-363.
- 15. Coviello A & Brauckmann ES (1973). Hydrosmotic effect of angiotensin II: isolated toad skin. Acta Physiologica Latinoamericana, 23: 18-25.
- 16. Coviello A, Orce G & Causarano J (1974). Effect of a competitive antagonist (8-Leu-angiotensin II) of angiotensin II on sodium and water transport in toad skin. Acta Physiologica Latinoamericana, 24: 409-413.
- 17. Peral de Bruno M & Coviello A (1996). Effects of angiotensin II antagonists on the contractile and hydrosmotic effect of AT II and AT III in the toad (Bufo arenarum). Journal of Comparative Physiology, B, 165: 565-570.
- 18. Peral de Bruno M, Romano L & Coviello A (1996). Interaction between antagonists of angiotensin II and antidiuretic hormone in isolated toad tissues. Comparative Biochemistry and Physiology, 113A: 307-314.
- 19. Peral de Bruno M, Romano L, Jerez S & Coviello A (1997). El Losartan inhibe el efecto de la hormona antidiurética en un modelo funcional de nefrón distal. Revista Argentina de Cardiología, 65 (Suppl III): 35-38.
- 20. Levens NR, Peach NJ, Darracott Vaughan ED & Carey RM (1981). Demonstration of a primary antidiuretic action of angiotensin II: effects of intrarenal converting enzyme inhibition in the conscious dog. Endocrinology, 108: 318-330.
- 21. Proto MC, Coviello A, Khosla MC & Bumpus FM (1983). Effects of frog-skin angiotensin II in amphibians. Hypertension, 5 (Suppl V): V101-V104.
- 23. Fontes MA, Silva LC, Campagnole-Santos MJ, Khosla MC, Guertzenstein PG & Santos RAS (1994). Evidence that angiotensin-(1-7) plays a role in the central control of blood pressure at the ventro-lateral medulla acting through specific receptors. Brain Research, 665: 175-180.
- 24. Lima DX, Fontes MA, Oliveira RC, Campagnole-Santos MJ, Khosla MC & Santos RAS (1996). Pressor action of angiotensin I at the ventrolateral medulla: effect of selective angiotensin blockade. Immunopharmacology, 33: 305-307.
- 25. Porsti I, Bara AT, Busse R & Hecker M (1994). Release of nitric oxide by angiotensin-(1-7) from porcine coronary endothelium: implications for a novel angiotensin receptor. British Journal of Pharmacology, 111: 652-654.
- 26. Tallant EA, Lu X, Weiss RB, Chappell MC & Ferrario CM (1997). Bovine aortic endothelial cells contain an angiotensin-(1-7) receptor. Hypertension, 29: 388-393.
- 27. Gironacci MM, Adler Graschinsky E, Peńa C & Enero MA (1994). Effects of angiotensin II and angiotensin-(1-7) on the release of [3H] norepinephrine from rat atria. Hypertension, 24: 457-460.
- 28. Seyedi N, Xu X, Nasjletti A & Hintze TH (1995). Coronary kinin generation mediates nitric oxide release after angiotensin receptor stimulation. Hypertension, 26: 164-170.
- 29. Gorelik G, Carbini LA & Scicli AG (1998). Angiotensin 1-7 induces bradykinin-mediated relaxation in porcine coronary artery. Journal of Pharmacology and Experimental Therapeutics, 286: 403-410.
- 30. Jaiswal N, Diz DI, Tallant EA, Khosla MC & Ferrario CM (1991). Characterization of angiotensin receptors mediating prostaglandin synthesis in C6 glioma cells. American Journal of Physiology, 260: R1000-R1006.
- 31. Ferrario CM, Chappell MC, Dean RH & Iyer SN (1998). Novel angiotensin peptides regulate blood pressure, endothelial function, and natriuresis. Journal of the American Society of Nephrology, 9: 1716-1722.
Correspondence and Footnotes
Publication Dates
-
Publication in this collection
01 Sept 2000 -
Date of issue
Sept 2000
History
-
Received
24 Feb 1999 -
Accepted
08 May 2000