Abstract
Steroid hormones have been implicated in the modulation of TSH secretion; however, there are few and controversial data regarding the effect of progesterone (Pg) on TSH secretion. Medroxyprogesterone acetate (MPA) is a synthetic alpha-hydroxyprogesterone analog that has been extensively employed in therapeutics for its Pg-like actions, but that also has some glucocorticoid and androgen activity. Both hormones have been shown to interfere with TSH secretion. The objective of the present study was to investigate the effects of MPA or Pg administration to ovariectomized (OVX) rats on in vivo and in vitro TSH release and pituitary TSH content. The treatment of adult OVX rats with MPA (0.25 mg/100 g body weight, sc, daily for 9 days) induced a significant (P<0.05) increase in the pituitary TSH content, which was not observed when the same treatment was used with a 10 times higher MPA dose or with Pg doses similar to those of MPA. Serum TSH was similar for all groups. MPA administered to OVX rats at the lower dose also had a stimulatory effect on the in vitro basal and TRH-induced TSH release. The in vitro basal and TRH-stimulated TSH release was not significantly affected by Pg treatment. Conversely, MPA had no effect on old OVX rats. However, in these old rats, ovariectomy alone significantly reduced (P<0.05) basal and TRH-stimulated TSH release in vitro, as well as pituitary TSH content. The results suggest that in adult, but not in old OVX rats, MPA but not Pg has a stimulatory effect on TSH stores and on the response to TRH in vitro.
medroxyprogesterone acetate; progesterone; thyrotropin; aging; ovariectomy; thyrotropin-releasing hormone
Braz J Med Biol Res, September 2000, Volume 33(9) 1111-1118
Effect of medroxyprogesterone acetate on thyrotropin secretion in adult and old female rats
R.M. Moreira1, P.P. Borges2, P.C. Lisboa1,F.H. Curty1, E.G. Moura2 and C.C. Pazos-Moura1
1Laboratório de Fisiologia Endócrina, Instituto de Biofísica Carlos Chagas Filho, Universidade Federal do Rio de Janeiro, Rio de Janeiro, RJ, Brasil
2Departamento de Ciências Fisiológicas, Universidade do Estado do Rio de Janeiro, Rio de Janeiro, RJ, Brasil
References
Correspondence and Footnotes Correspondence and Footnotes Correspondence and Footnotes
Abstract
Steroid hormones have been implicated in the modulation of TSH secretion; however, there are few and controversial data regarding the effect of progesterone (Pg) on TSH secretion. Medroxyprogesterone acetate (MPA) is a synthetic a-hydroxyprogesterone analog that has been extensively employed in therapeutics for its Pg-like actions, but that also has some glucocorticoid and androgen activity. Both hormones have been shown to interfere with TSH secretion. The objective of the present study was to investigate the effects of MPA or Pg administration to ovariectomized (OVX) rats on in vivo and in vitro TSH release and pituitary TSH content. The treatment of adult OVX rats with MPA (0.25 mg/100 g body weight, sc, daily for 9 days) induced a significant (P<0.05) increase in the pituitary TSH content, which was not observed when the same treatment was used with a 10 times higher MPA dose or with Pg doses similar to those of MPA. Serum TSH was similar for all groups. MPA administered to OVX rats at the lower dose also had a stimulatory effect on the in vitro basal and TRH-induced TSH release. The in vitro basal and TRH-stimulated TSH release was not significantly affected by Pg treatment. Conversely, MPA had no effect on old OVX rats. However, in these old rats, ovariectomy alone significantly reduced (P<0.05) basal and TRH-stimulated TSH release in vitro, as well as pituitary TSH content. The results suggest that in adult, but not in old OVX rats, MPA but not Pg has a stimulatory effect on TSH stores and on the response to TRH in vitro.
Key words: medroxyprogesterone acetate, progesterone, thyrotropin, aging, ovariectomy, thyrotropin-releasing hormone
Introduction
Although thyroid hormones and TRH are the main regulators of serum TSH, steroid hormones have been shown to modulate TSH secretion. Previous reports indicated that, physiologically, estrogen has a stimulatory effect on TRH-stimulated TSH release (1,2). Progesterone (Pg) has been reported to have no effect (3) or to stimulate (4) TSH secretion, although there are very few reports on this subject. Testosterone seems to be a stimulatory factor of TSH secretion in castrated male rats (5). Many reports suggest that an excess of glucocorticoids decreases TSH release (6), although their physiological role in TSH regulation is still unclear. Medroxyprogesterone acetate (MPA) is a synthetic a-hydroxyprogesterone analog commonly employed in Pg replacement therapy as well as in the treatment of precocious puberty and for contraception (7,8), situations in which the aim is to suppress gonadotropin secretion (9). Most MPA effects are mediated via Pg receptors; however, because of its structural homology with other steroids, MPA exhibits limited binding to androgen and glucocorticoid receptors, and therefore also has androgen- and glucocorticoid-like activities (10-13), Therefore, since medroxyprogesterone might activate steroid receptors that have been implicated in the regulation of TSH secretion, we investigated whether medroxyprogesterone modifies the in vivo and in vitro TSH release from the glands of ovariectomized (OVX) adult rats. We also investigated if aging interferes with TSH responses to MPA. Furthermore, since there is little and controversial information regarding Pg modulation of TSH secretion, we also evaluated the effects of Pg on TSH release and on the response to TRH in vitro.
Material and Methods
Female rats were bred in our animal facilities and housed under controlled conditions of temperature (24 ± 1oC) and light (12-h light starting at 7 a.m.).
Experiment I: Effect of MPA and Pg treatment of adult OVX rats on serum and pituitary TSH
Adult rats showed a regular 4-5-day estrous cycle monitored by vaginal cytology at least for two weeks before starting the experiments. Groups of rats weighing 160-175 g were ovariectomized and one group subjected to surgical stress (sham operated - normal) was used as control. The OVX groups were injected subcutaneously (sc) with 0.25 or 2.5 mg/100 g body weight of MPA (Depo-Provera, Rhodia Pharma, São Paulo, SP, Brazil), daily for 9 days. One group of OVX rats received vehicle (saline) instead of MPA and was used as control. The normal group was treated with vehicle (saline). The estrous cycles of sham-operated rats were monitored by daily morning vaginal smears. In another set of experiments, OVX rats received 0.25 or 2.5 mg Pg/100 g body weight, sc, daily for 9 days. In all experiments, rats were sacrificed 24 h after the last injection and three weeks after ovariectomy. After sacrifice by decapitation, blood was collected from the trunk and serum was obtained and stored at -20oC until the time for TSH assay. The anterior pituitaries were dissected out and homogenized in 500 µl of cold phosphosaline buffer, pH 7.6, and stored at
-20oC for TSH determination.
Experiment II: Effect of MPA and Pg treatment of adult OVX rats on basal and TRH-stimulated TSH release in vitro
Rats were treated as described above with MPA or Pg (Sigma Chemical Co., St. Louis, MO, USA) administered sc at the dose of 0.25 mg 100 g body weight-1 day-1 for 9 days. On the day of sacrifice the pituitaries were quickly dissected out, the anterior pituitary was separated from the posterior pituitary and transected with a longitudinal midline cut. Each anterior hemipituitary was immediately transferred to a tube containing 1 ml of Krebs-Ringer bicarbonate medium, pH 7.4, and incubated at 37oC in an atmosphere of 95% O2/5% CO2 in a Dubnoff metabolic shaker. After a 30-min pre-incubation period, the medium was removed and the hemipituitaries were resuspended in 1 ml of fresh medium. At the end of 1-h incubation, an aliquot was removed for measurement of basal TSH and TRH (Sigma) was added to a final concentration of 50 nM in all groups. The incubation was continued for 30 min to determine the TSH released in response to TRH. Data are reported as DTSH, calculated by the difference in medium TSH after TRH and basal TSH. Each hemipituitary was homogenized in phosphate-buffered saline, pH 7.6, for measurement of intrapituitary TSH.
Experiment III: Effect of MPA treatment of old OVX rats on basal and TRH-stimulated TSH release in vitro
Healthy 23-month-old rats that had lost estrous cyclicity, as evaluated by vaginal smears, were OVX or sham operated (normal) and then treated sc with MPA (0.25 mg 100 g body weight-1 day-1 for 9 days) or saline following the same protocol as described before. The in vitro experiment was performed following the same procedures as described previously.
Radioimmunoassay
TSH concentrations in serum, homogenates and incubation medium were determined by radioimmunoassay using reagents supplied by the National Institute of Diabetes, Digestive and Kidney Diseases and expressed in terms of the preparation reference provided (RP2). Serum Pg was measured using a commercial kit (Coat-a-Count, Diagnostic Products Co., Los Angeles, CA, USA). For TSH, the within-assay variation was 7.9%, the coefficient of variation between assays was 6.7% and the minimum assay sensitivity was 0.52 ng/ml.
Statistical analysis
Data are reported as mean ± SEM. One-way analysis of variance (ANOVA) followed by the Newman-Keuls multiple comparison test was applied to the data, with the level of significance set at P<0.05.

Experiment I: Effect of MPA and Pg treatment of adult OVX rats on serum and pituitary TSH
As shown in Figure 1, TSH content was increased approximately 2-fold in pituitaries of OVX rats treated sc with 0.25 mg MPA 100 g body weight-1 day-1 for 9 days, compared to both OVX control and normal sham-operated rats. However, the OVX group treated with a 10 times higher dose of MPA (2.5 mg/100 g body weight) showed a TSH content similar to that of the normal and OVX groups. MPA did not modify serum TSH at either dose used (Figure 1). Progesterone treatment, regardless of the dose employed, did not change pituitary or serum TSH (Figure 2). Serum Pg concentration of the OVX group treated with 0.25 mg Pg was similar to that of normal rats at different phases of the estrous cycle, while serum levels were significantly higher than normal when 2.5 mg Pg was used (proestrus: 10.9 ± 1.2, estrus: 9.6 ± 1.0, diestrus I: 12.0 ± 1.1, diestrus II: 14.3 ± 2.6, OVX + 0.25 mg Pg: 7.9 ± 2.1, OVX + 2.5 mg Pg: 50.1 ± 14.2 ng/ml).
Experiment II: Effect of MPA and Pg treatment of young adult OVX rats on the basal and TRH-stimulated TSH release in vitro
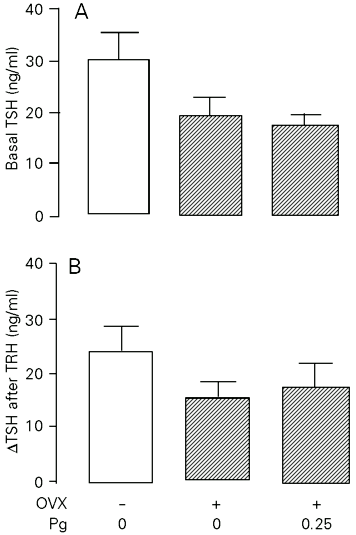
The data from the in vitro study with MPA and Pg treatment of young adult rats are summarized in Figures 3 and 4, respectively. In young adult rats, OVX alone did not change significantly the basal TSH release from isolated hemipituitaries. However, the OVX group receiving MPA (0.25 mg/100 g body weight for 9 days) showed an increase (P<0.05) in basal TSH release compared to normal and OVX saline-treated groups (Figure 3, panel A). The TRH-stimulated TSH release from the glands of OVX saline-injected rats was lower (P<0.05) than that of the normal group (Figure 3, panel B). However, the treatment of OVX rats with MPA induced an increment in the TRH response of the OVX group to levels comparable to those of the normal group. The TSH content of the incubated glands did not differ significantly among groups (Figure 3, panel C). Treatment of OVX rats with Pg had no significant effect on basal or TRH-stimulated TSH secretion in vitro (Figure 4). However, as observed in experiment I, treatment with MPA or Pg did not significantly change serum TSH (data not shown).
- In vitro basal (panel A) and TRH-stimulated (panel B) TSH release from hemipituitaries of young adult (3 months old) sham-operated or ovariectomized (OVX) rats treated with medroxyprogesterone acetate (MPA, 0.25 mg/100 g body weight, sc, for 9 days) or saline. After incubation, pituitary TSH content was measured (panel C). Number of hemipituitaries per group: 6-8; *P<0.05 vs normal and OVX; **P<0.05 vs normal and OVX + MPA (ANOVA followed by Newman-Keuls test). DTSH values were calculated as the difference between medium TSH and basal TSH after TRH.
- In vitro basal (panel A) and TRH-stimulated (panel B) TSH release from hemipituitaries of young adult (3 months old) sham-operated or ovariectomized (OVX) rats treated with progesterone (Pg, 0.25 mg/100 g body weight, sc, for 9 days) or saline. Number of hemipituitaries per group: 9-13. DTSH values were calculated as the difference between medium TSH and basal TSH after TRH.
Experiment III: Effect of MPA treatment of old OVX rats on basal and TRH-stimulated TSH release in vitro
These results are presented in Figure 5. In old rats, basal and TRH-stimulated TSH release from glands of saline- or MPA-treated OVX groups were significantly (P<0.05) reduced compared to the sham-operated old group. Pituitary TSH content (P<0.05) of the saline-treated OVX old rats was also significantly lower than that of sham-operated old rats. Serum TSH did not differ significantly among the old groups (sham = 0.49 ± 0.08, OVX = 0.75 ± 0.07, OVX + MPA = 0.76 ± 0.1 ng/ml).
- In vitro basal (panel A) and TRH-stimulated (panel B) TSH release from hemipituitaries of 23-month-old sham-operated or ovariectomized (OVX) rats treated with medroxyprogesterone acetate (MPA, 0.25 mg/100 g body weight, sc, for 9 days) or saline. After incubation, pituitary TSH content was measured (panel C). Number of hemipituitaries per group: 8-9; *P<0.05 vs normal (ANOVA followed by Newman-Keuls test). DTSH values were calculated as the difference between medium TSH and basal TSH after TRH.
Results

In this study we demonstrate that MPA treatment increased the pituitary TSH content of adult OVX rats treated sc for 9 days. This stimulatory effect depended on the dose used, being observed only at the lower dose (0.25 mg/100 g body weight). The in vitro assay also showed a stimulatory effect of MPA treatment on basal and TRH-induced TSH release from the pituitaries of adult OVX rats. We (14) and others (3,15,16) had shown that the decreased TSH response to TRH of OVX rats was restored by estrogen treatment. We also had reported before (5) a stimulatory effect of testosterone given chronically to castrated male rats on in vitro basal and TRH-stimulated TSH release. Here, we found that a synthetic progestin promoted the same effect.
However, the mechanism by which MPA exerts its effect on TSH is uncertain. It is possible that the effect of MPA was not through the activation of Pg receptors since there was no effect of the same treatment with Pg on TSH content or on basal and TRH-stimulated TSH secretion in vitro (Figures 2 and 4). Although early reports (3) did not show any effect of Pg on TSH release in response to TRH injected into OVX rats, Tsai et al. (4) recently showed that a 3-day treatment of rats with Pg led to an increased TSH and prolactin response to TRH in vitro. Additionally, these investigators demonstrated a stimulatory effect of Pg added to the incubation medium of isolated pituitaries from OVX rats, which was associated with a parallel increase in cAMP production. These results suggest that Pg might act directly on the pituitary gland, modulating TRH actions on TSH and prolactin secretion. However, in the present study, Pg did not change pituitary TSH content or in vitro TSH secretion. The disagreement on the results might be related to the use of different protocols, especially the duration of treatment.
However, there are other points to be considered. The half-lives of MPA and Pg are different. MPA injected into muscle has a long-acting activity because it stays in the circulation for months, due to the slow release from the injection site (17). However, in the present study, we used subcutaneous administration which probably favored rapid absorption since the clearance of this aqueous solution should be rapid (17). Nevertheless, the elimination half-life of Pg is very short (5 min) compared to that of MPA (24 h) (18), and therefore it is possible that the concentration of Pg achieved in the circulation during the experiment, although much higher than the physiological values in rats receiving the higher dose, was not sufficient to activate Pg receptors at the same intensity as observed with the lower dose of MPA. In any case, the effects observed seem not to be dose dependent, since they were not observed with the higher dose of MPA.
Another important consideration concerns the status of Pg receptors in OVX rats. Pg receptors exert a dual control of estrogen and Pg, which act together to regulate the cellular concentration of Pg receptors. Estrogen increases the Pg receptor mRNA levels and protein synthesis (19) in most tissues, although a recent report (20) showed a depressive effect of estrogen on Pg receptor content in rat uterine epithelial cells. On the other hand, Pg induces a decrease in the Pg receptor protein concentration and mRNA levels (21) and consequently reduces the effects of the hormone after prolonged treatment, although this is not true for all tissues, with the reverse effect also having been reported (22). In the pituitary gland, the regulation of Pg receptors seems to show similarities to as well as differences from that of the uterus. Recently (23), it was demonstrated that ovariectomy reduced mRNA levels for both isoforms, A and B, of the Pg receptor in the pituitary gland, and that estrogen treatment increased the levels of these mRNAs, but Pg had no effect. Therefore, in our experiments, Pg receptors may have been reduced at the thyrotrope level in rats receiving MPA or Pg, although this hypothesis needs experimental confirmation.
Several studies have provided evidence that MPA has androgen receptor agonist activity (24,25) and in addition it had been demonstrated that MPA interacts with cytosol androgen receptors in the rat hypothalamus and anterior pituitary (26). The present report, together with our previous observation that testosterone can also stimulate basal and TRH-induced TSH secretion (5), raises the possibility that the stimulatory effect of MPA on TSH might be mediated trough androgen receptors. In any case, the mechanism underlying the effects of MPA on TSH secretion needs to be further investigated.
Since serum TSH was not changed by MPA treatment we suggest that in vivo counterregulatory mechanisms maintained the basal TSH secretion in the normal range. However, the decreased TSH storage might impair the compensatory rise of TSH necessary, for example, to correct primary hypothyroidism.
However, contrary to the effect observed in young adult rats, the same MPA dose had no influence on the in vitro basal or TRH-stimulated TSH release from pituitaries of old OVX rats or on their pituitary TSH content. These results suggest that aging is associated with alterations in the pituitary responsiveness to the steroid. There have been reports of decreased estrogen receptors in the brain and pituitary of aged female rodents (27,28). The significant decrease in the in vitro TSH release and intrapituitary TSH content caused by ovariectomy alone in old rats suggests that the ovary plays an important role in the regulation of the TSH response to TRH, even in old rats that lost their estrous cyclicity (29,30). This leads us to speculate that ovarian factors other than estrogen and Pg might be important, directly or indirectly, in the maintenance of TSH stores and TSH response to TRH in old rats. It is known that stroma cells continue to produce androgens after the end of folliculogenesis and testosterone has been reported to have stimulatory effects on TSH secretion (3,30). Chen and Walfish (31) showed a lower TSH response to TRH administered to old OVX rats compared to the adult OVX group, although the TRH response was not affected in intact old rats.
In summary, chronic treatment of OVX rats with MPA, but not Pg, increased TSH storage and the response to TRH in vitro, an effect that was not observed in aged OVX rats.
Discussion
Acknowledgments
We are grateful to Ms. Norma de Araújo Faria, Mr. Advaldo Bezerra and Mr. Wagner Bezerra for excellent technical assistance.
Address for correspondence: C.C. Pazos-Moura, Laboratório de Fisiologia Endócrina, Instituto de Biofísica Carlos Chagas Filho, CCS, Bloco G, UFRJ, 21949-900 Rio de Janeiro, RJ, Brasil. Fax: +55-21-280-8193. E-mail: cpazosm@biof.ufrj.br
Research supported by CNPq, FINEP, FUJB and FAPERJ. Received October 5, 1999. Accepted June 21, 2000.
- 1. Chen HJ & Walfish PG (1978). Effects of estradiol benzoate on thyroid-pituitary function in female rats. Endocrinology, 103: 1023-1030.
- 2. Kimura N, Arai K, Sahara Y, Suzuki H & Kimura N (1994). Estradiol transcriptionally and posttranscriptionally up-regulates thyrotropin-releasing hormone receptor messenger ribonucleic acid in the rat pituitary cells. Endocrinology, 134: 432-440.
- 3. Watanobe H & Takebe K (1987). Role of postnatal gonadal function in the determination of thyrotropin (TSH) releasing hormone-induced TSH response in adult male and female rats. Endocrinology, 120: 1711-1718.
- 4. Tsai Sc, Lu Cc, Lau Cp, Hwang Gs, Lee Hy, Chen Sl, Huang Sw, Shih Hc, Chen YH, Chiao YC, Wang SW & Wang PS (1996). Progesterone stimulates in vitro release of prolactin and thyrotropin involving cAMP production in rat pituitary. Chinese Journal of Physiology, 39: 245-251.
- 5. Borges PP, Curty FH, Pazos-Moura CC & Moura EG (1998). Effect of testosterone propionate treatment on thyrotropin secretion of young and old rats in vitro Life Sciences, 62: 2035-2043.
- 6. Brabant G, Ocran K, Ranft U, von zur Muhlen A & Hesch RD (1989). Physiological regulation of thyrotropin. Biochimie, 71: 293-301.
- 7. Lee PA (1981). Medroxyprogesterone therapy for sexual precocity in girls. American Journal of Diseases of Children, 135: 443-445.
- 8. Wheeler MD & Styne DM (1991). Drug treatment in precocious puberty. Drug, 41: 717-728.
- 9. Mishell Jr DR, Kharma KM, Thorneycroft IH & Nakamura RM (1972). Estrogenic activity in women receiving an injectable progestogen for contraception. American Journal of Obstetrics and Gynecology, 113: 372-376.
- 10. Pridjian G, Schmit V & Schreiber J (1987). Medroxyprogesterone acetate: receptor binding and correlated effects on steroidogenesis in rat granulosa cells. Journal of Steroid Biochemistry, 26: 313-319.
- 11. Poulin R, Baker D, Poirier D & Labrie F (1989). Androgen and glucocorticoid receptor-mediated inhibition of cell proliferation by medroxyprogesterone acetate in ZR-75-1 human breast cancer cells. Breast Cancer Research and Treatment, 13: 161-172.
- 12. Mathews JH, Abrams CA & Monshim A (1970). Pituitary-adrenal function in the patients receiving medroxyprogesterone acetate for true precocious puberty. Journal of Clinical Endocrinology and Metabolism, 30: 653-658.
- 13. Sadeghi-Nejad A, Kaplan SL & Grumbach MM (1971). The effect of medroxyprogesterone acetate on adrenocortical function in children with precocious puberty. Journal of Pediatrics, 78: 616-624.
- 14. Moreira RM, Lisboa PC, Curty FH & Pazos-Moura CC (1997). Dose-dependent effects of 17-ß estradiol on pituitary thyrotropin content and secretion in vitro Brazilian Journal of Medical and Biological Research, 30: 1129-1134.
- 15. Fisher JS & D'Angelo SA (1978). Stimulatory and inhibitory action of estradiol on TSH secretion. Endocrinology, 88: 687-691.
- 16. Castro-Vazquez A, Ojeda SR, Krulich L & McCann SM (1981). TSH release during the estrous cycle of the rat: Variations in responsiveness to cervicovaginal stimulation. Neuroendocrinology, 33: 193-198.
- 17. Cornette JC, Kirton KT & Duncan GW (1971). Measurement of medroxyprogesterone acetate (Provera) by radioimmunoassay. Journal of Clinical Endocrinology, 33: 459-466.
- 18. Williams CL & Stancel GM (1996). Estrogens and progestins. In: Goodman and Gilman's The Pharmacological Basis of Therapeutics 9th edn. McGraw-Hill, New York.
- 19. Nardulli AM, Greene GL, O'Malley BW & Katzenellenbogen BS (1988). Regulation of progesterone receptor messenger ribonucleic acid and protein levels in MCF-7 cells by estradiol: analysis of estrogen's effect on progesterone receptor synthesis and degradation. Endocrinology, 122: 935-944.
- 20. Parczyk K, Madjno R, Michna H, Nishino Y & Schneider MR (1997). Progesterone receptor repression by estrogens in rat uterine epithelial cells. Journal of Steroid Biochemistry and Molecular Biology, 63: 304-316.
- 21. Nardulli AM & Katzenellenbogen BS (1988). Progesterone receptor regulation in T 47D human breast cancer cells: analysis by density labeling of progesterone receptor synthesis and degradation and their modulation by progestin. Endocrinology, 122: 1532-1540.
- 22. Martel D, Moner MN, Roche D, De Feo VJ & Psychoyos A (1989). Hormonal dependence of the metrial gland: further studies on oestradiol and progesterone receptor levels in the rat. Journal of Endocrinology, 120: 465-472.
- 23. Szabo M, Kilen SM, Nho SJ & Schwartz NB (2000). Progesterone receptor A and B messenger ribonucleic acid levels in the anterior pituitary of rats are regulated by estrogen. Biology of Reproduction, 62: 95-102.
- 24. Couture P, Thériault C, Simard J & Labrie F (1993). Androgen receptor-mediated stimulation of 17ß-hydroxysteroid dehydrogenase activity by dihydrotestosterone and medroxyprogesterone acetate in ZR-75-1 human breast cancer cells. Endocrinology, 132: 179-185.
- 25. Bentel JM, Birrell SN, Pickering MA, Holds DJ, Horsfall DJ & Tilley WD (1999). Androgen receptor agonist activity of the synthetic progestin, medroxyprogesterone acetate, in human breast cancer cells. Molecular and Cellular Endocrinology, 154: 11-20.
- 26. Perez-Palacios G, Chavez B, Vilchis F, Escobar N, Larrea F & Perez AE (1983). Interaction of medroxyprogesterone acetate with cytosol androgen receptors in the rat hypothalamus and pituitary. Journal of Steroid Biochemistry, 19: 1729-1735.
- 27. Haji M, Kato KI, Nawata H & Ibayashi H (1981). Age-related changes in the concentrations of cytosol receptors for sex steroid hormones in the hypothalamus and pituitary gland of the rat. Brain Research, 204: 373-386.
- 28. Steiger RN, Huang HH, Chamberlain DS & Mutes J (1980). Changes in control of gonadotropin secretion in the transition period between regular cycles and constant estrus in ageing female rats. Biology of Reproduction, 22: 595-603.
- 29. Nass TE, La Polt PS, Judd HL & Lu JHK (1984). Alterations in ovarian steroid and gonadotropin secretion preceding the cessation of regular oestrus cycles in ageing female rats. Journal of Endocrinology, 100: 43-50.
- 30. Christianson D, Roti E, Vagenakis AG & Braverman LE (1981). The sex related difference in serum thyrotropin concentration is androgen mediated. Endocrinology, 108: 529-535.
- 31. Chen HJ & Walfish PG (1978). Effects of age and ovarian function on the pituitary-thyroid system in female rats. Journal of Endocrinology, 78: 225-232.
Correspondence and Footnotes
Publication Dates
-
Publication in this collection
01 Sept 2000 -
Date of issue
Sept 2000
History
-
Accepted
21 June 2000 -
Received
05 Oct 1999