Abstract
Actin-based motor protein requirements and nitric oxide (NO) production are important features of macrophage activity during phagocytosis or microbicidal processes. Different classes of myosins contribute directly or indirectly to phagocytosis by providing mechanical force for phagosome closure or organelle movement. Recent data have shown the presence of myosins IC, II, V and IXb in phagosomes of bone marrow-derived murine macrophages. In our investigation we demonstrated the presence of different classes of myosins in J774 macrophages. We also analyzed the effect of gamma interferon (IFN-gamma), with or without calcium ionophore or cytochalasin B, on myosins as well as on inducible nitric oxide synthase (iNOS) expression and NO production. Myosins IC, II, Va, VI and IXb were identified in J774 macrophages. There was an increase of myosin V expression in IFN-gamma-treated cells. iNOS expression was increased by IFN-gamma treatment, while calcium ionophore and cytochalasin B had a negative influence on both myosin and iNOS expression, which was decreased. The increases in NO synthesis were reflected by increased iNOS expression. Macrophages activated by IFN-gamma released significant amounts of NO when compared to control groups. In contrast, NO production by calcium ionophore- and cytochalasin B-treated cells was similar to that of control cells. These results suggest that IFN-gamma is involved in macrophage activation by stimulating protein production to permit both phagocytosis and microbicidal activity.
myosins; macrophages; IFN-gamma; iNOS
Braz J Med Biol Res, February 2001, Volume 34(2) 221-226 (Short Communication)
Myosin V and iNOS expression is enhanced in J774 murine macrophages treated with IFN- g
D.S. Reis1, M.A. Souza1, J.R. Mineo1 and F.S. Espindola2
1Departamento de Imunologia, Microbiologia e Parasitologia, and 2Instituto de Genética e Bioquímica, Universidade Federal de Uberlândia, Uberlândia, MG, Brasil
Text
References
Correspondence and Footnotes Correspondence and Footnotes Correspondence and Footnotes
Abstract
Actin-based motor protein requirements and nitric oxide (NO) production are important features of macrophage activity during phagocytosis or microbicidal processes. Different classes of myosins contribute directly or indirectly to phagocytosis by providing mechanical force for phagosome closure or organelle movement. Recent data have shown the presence of myosins IC, II, V and IXb in phagosomes of bone marrow-derived murine macrophages. In our investigation we demonstrated the presence of different classes of myosins in J774 macrophages. We also analyzed the effect of gamma interferon (IFN-g), with or without calcium ionophore or cytochalasin B, on myosins as well as on inducible nitric oxide synthase (iNOS) expression and NO production. Myosins IC, II, Va, VI and IXb were identified in J774 macrophages. There was an increase of myosin V expression in IFN-g-treated cells. iNOS expression was increased by IFN-g treatment, while calcium ionophore and cytochalasin B had a negative influence on both myosin and iNOS expression, which was decreased. The increases in NO synthesis were reflected by increased iNOS expression. Macrophages activated by IFN-g released significant amounts of NO when compared to control groups. In contrast, NO production by calcium ionophore- and cytochalasin B-treated cells was similar to that of control cells. These results suggest that IFN-g is involved in macrophage activation by stimulating protein production to permit both phagocytosis and microbicidal activity.
Key words: myosins, macrophages, IFN-g, iNOS
Activated macrophages are important effector cells in inflammatory processes and in host defense against microorganisms. Elimination of foreign agents by these cells involves phagocytosis and generation of reactive oxygen species such as nitric oxide (NO). NO is an inflammatory mediator directly related to cell activation (1) that contributes to the death or inhibition of different pathogens, as well as tumor cells. NO is derived from a catalytic transformation of L-arginine to citrulline by nitric oxide synthases (NOS). There are three major isoforms of NOS: the constitutively expressed neuronal NOS (nNOS), the endothelially expressed NOS (eNOS) and the inducible NOS (iNOS) (2). Both nNOS and eNOS are regulated by the calcium/calmodulin complex and iNOS is a Ca2+-independent enzyme (3,4). However, recent reports have suggested that calcium regulates iNOS expression and NO synthesis in macrophages (1,5,6). Although studies have been done in an attempt to address the role of calcium in NO synthesis, this relation remains unclear. Calcium ionophore A23187, a mobile ion carrier that transports divalent cations such as Ca2+ and Mg2+, reduces NO release and iNOS expression by chondrocytes treated with IL-1 (7). However, it can be used to increase NO synthesis in gamma interferon (IFN-g)-treated murine peritoneal macrophages, providing evidence that calcium is linked to induction of NO synthesis in macrophages (6).
Several studies have reported the effect of different treatments on the regulation of macrophage-like cells by measurement of NO release or NOS expression (1,3,5,6,8,9). Cytokines such as IFN-g are able to induce the expression of NOS (3), and thus stimulate the microbicidal activity of macrophages. Moreover, treatment with IFN-g in combination with lipopolysaccharide (LPS) results in high levels of NO production by macrophages (6,10,11), including the J774 cell line (3,11). There is, however, a regulatory mechanism controlling this activation. Treatment with IL-13 before the stimulus with IFN-g plus LPS was found to inhibit the release of NO when compared to stimulation with IFN-g plus LPS without IL-13, suggesting that the activation of NOS by IFN-g and LPS is controlled by downregulating cytokines (12). Production of NO by macrophages is also inhibited by treatment with cytochalasins, drugs that inhibit actin filament polymerization (12).
In addition to having a microbicidal activity, macrophages also destroy pathogens after engulfment and enclosure in phagosomes. To perform their phagocytic function, macrophages depend on a motility that is conferred by the dynamic reorganization of the cytoskeleton and the involvement of various motor proteins based on microtubules or actin filaments. Actin-based motor proteins are called myosins and hydrolyze ATP to produce mechanical force (13). More than 13 structurally distinct classes have been identified in many cell types (14). Most of them play important roles in cell motility, vesicle transport, membrane traffic, and phagocytosis. Myosin II has been well characterized as providing support in contractile functions of actin filaments in the cells and may aid vesicle budding from the trans-Golgi networks. Myosins V and VI are implicated in organelle movement and vesicle transport in various organisms. Myosin I participates in endocytic and exocytic membrane traffic (14). In addition to these functions, there is strong evidence that myosins contribute to phagocytosis. In a recent study on erythrocytes phagocytosed by murine bone marrow-derived macrophages, four distinct classes of myosins were identified (myosins IC, II, V and IXb) and located in phagosomes. In addition, myosin IC has been implicated in contractile activity during phagosome formation (15). Therefore, it is possible that myosin production is increased after activation to satisfy the protein requirement during cell motility.
The main objective of the present study was to analyze the effect of IFN-g in the presence or absence of calcium ionophore or cytochalasin B on myosins and iNOS expression and NO release.
J774 murine macrophages were obtained from the American Type Culture Collection (Rockville, MD, USA) and were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% heat-inactivated FBS, 2 mM L-glutamine, 100 µg/ml penicillin and 100 U/ml streptomycin, in a humidified 37oC/5% CO2 incubator, for 10 days in 25-cm2 flasks. On day 11, cells were added to 96-well plates at a concentration of 5 x 105 cells/200 µl (9) in the same medium.
After 24 h, the culture medium of each well was replaced with 200 µl of fresh medium containing one of the following preparations: a) 5% conditioned medium from L1210 mouse leukemia cells (Rio de Janeiro Cell Bank, Rio de Janeiro, RJ, Brazil) transfected to produce murine IFN-g; the concentration of IFN-g in the conditioned medium corresponded to 2 U/ml when compared to a standard curve of murine recombinant IFN-g, b) 10 µM cytochalasin B (Sigma Chemical Co., St. Louis, MO, USA), c) 5 µM calcium ionophore (Sigma), d) IFN-g plus cytochalasin B or IFN-g plus calcium ionophore, or e) medium alone, followed by incubation for 24 h at 37oC/5% CO2. For NO measurement, aliquots of 50 µl from wells of each of the above treatments, as well as a blank and standards with different concentrations of Na2NO3, were mixed with an equal volume (50 µl) of Greiss reagent (6). NO production was measured at 570 nm using a microplate reader (Titertek Multiskan Plus; Flow Laboratories International A.S., Lugano, Switzerland). DMEM medium was used as blank and diluent to reduce interference.
J774 monolayers were washed with PBS to eliminate excess FBS and homogenized using a micro-extraction protocol (16). After the addition of 15 µl of ice-cold lysis buffer (50 mM Tris-HCl, 150 mM NaCl, 1% Triton X-100, 0.25% sodium deoxycholate, 1 µg/ml leupeptin, and 1 mM PMSF, pH 8.0), cells were scraped and transferred to Eppendorf tubes and kept on ice for 1 h to complete cell lysis. Aliquots of 5 µl from each lysate were diluted to 50 µl with water for protein estimation by the Bradford method (Bio-Rad Laboratories, Hercules, CA, USA). A mass of 15 µg of total protein from each cell extract was separated by electrophoresis on a 5-22% gradient minigel (SDS-PAGE) under reducing conditions and transferred to nitrocellulose membranes. Nitrocellulose sheets were then incubated with monoclonal antibodies against iNOS (Transduction Laboratories, Lexington, KY, USA) or myosin II (Biomedical Technologies, Stoughton, MA, USA), or with affinity-purified rabbit polyclonal antibodies produced against human myosin IC, chicken brain myosin Va, pig myosin VI and human myosin IXb (a gift from M.S. Mooseker, Yale University). Antibody reactivity was assessed following incubation with horseradish peroxidase-labeled secondary antibodies using a chemiluminescent detection system (ECL; Amersham, Arlington Heights, IL, USA). Densitometric analysis was carried out using the Image Master VSD® videodocumentation system and software (1996, version 2.0; Pharmacia Biotech Inc., San Francisco, CA, USA). Results were analyzed statistically by the Bartlett test, using the Graphpad Instat TM software (Copyright© 1990-1993, v.2.02).
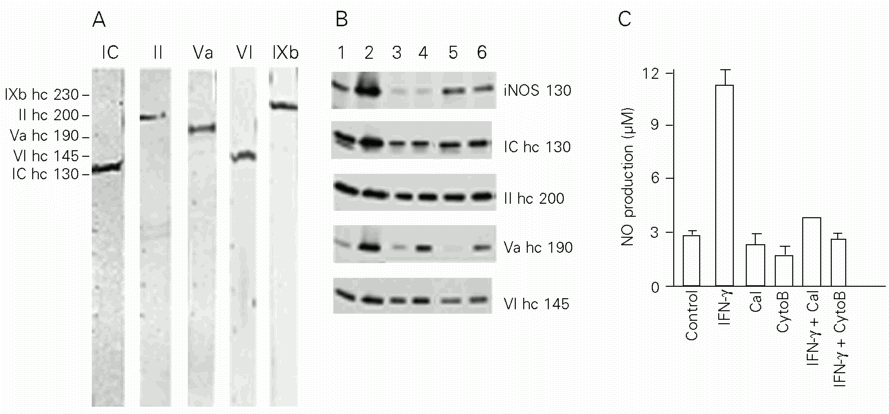
Myosins IC, II, Va, VI and IXb were identified in J774 cells with the respective single 130-, 200-, 190-, 145- and 230-kDa bands being recognized by the cited antibodies using previously described methods (13-15). As shown in Figure 1A, this cell line presents a large number of myosins of different classes. These findings are consistent with a recent study in which myosins IC, II, V and IXb were identified in murine medullar macrophages (15). Our results also demonstrate that a single cell type can present numerous myosins. This agrees with Bement et al. (17), who documented the expression of at least a dozen myosins within a given vertebrate cell type. There was no evidence of degradation products, probably due to the method of preparation of a homogenate from fresh cells and to the use of protease inhibitors.
Immunoblots of iNOS and myosins are shown in Figure 1B and the corresponding protein densitometric analysis is shown in Table 1. While the amount of iNOS was increased in the IFN-g-treated group (66.8%), little expression of this protein was detected (2%) in calcium ionophore- and cytochalasin B-treated cells. iNOS expression was also reduced in groups treated with IFN-g simultaneously with calcium ionophore (~11%) or cytochalasin B (~7%). In myosin immunoblots we observed that myosin V expression was particularly increased in IFN-g-treated cells (61%) when compared to control cells (5%). Calcium ionophore or cytochalasin B had a negative influence on protein expression for most of the myosins. However, myosin V was not found to be inhibited by cytochalasin B. NO production was positively correlated with iNOS expression (Figure 1C). Macrophages activated by IFN-g released significant levels of NO (11.24 ± 1.07 µM) when compared to control groups (2.77 ± 0.48 µM) (P<0.05). In contrast, NO production by calcium ionophore- and cytochalasin B-treated cells (2.23 ± 1.14 µM and 1.68 ± 0.69 µM) was similar to control (P<0.05) (Figure 1C). The production of NO was also decreased by calcium ionophore plus IFN-g treatment (3.83 ± 0.71 µM) when compared to IFN-g-treated groups (P<0.05). The same was observed in groups treated with cytochalasin B plus IFN-g (2.58 ± 0.43 µM) (P<0.01). To confirm that the inhibition of iNOS and myosin expression was really due to calcium ionophore or cytochalasin B treatment and not to decreased viability, a dose-dependent assay was devised. J774 macrophages were incubated with different concentrations of calcium ionophore (1.25, 2.5, 5.0 and 10.0 µM) or cytochalasin B (2.5, 5.0, 10.0 and 20.0 µM). Both treatments resulted in 70-80% cell viability, similar to that of control, confirming the specificity of our results.
Elimination of microorganisms by macrophages depends both on phagocytosis and release of toxic agents, such as reactive oxygen and nitrogen intermediates. The present results suggest that IFN-g is involved in J774 macrophage activation by stimulating either iNOS induction or the production of some myosins to support both processes. This is supported by the observation that myosin expression, mainly myosin V, is increased by IFN-g, as observed for iNOS. Myosin V is one of the myosins implicated in vesicle and organelle transport and its expression in J774 macrophages is sensitive to IFN-g, suggesting that macrophage activation probably increases myosin V production. Furthermore, the cytochalasin B-treated groups showed an inhibitory effect on protein expression for both iNOS and myosins. This can be explained by the fact that the cytoskeleton is involved in signal transduction events.
In conclusion, our data suggest that IFN-g may participate in the expansion of the myosin repertoire involved in cell motility, opening new perspectives for a better understanding of myosin involvement in the mechanisms of pathogen clearance by activated macrophages.
Acknowledgments
We gratefully acknowledge the collaboration of Dr. Mark S. Mooseker (Department of Mol. Cell. Dev. Biology, Yale University, New Haven, CT, USA), Drs. Enilza M. Espreafico and Eliana Valéria Patussi (Departamento de Biologia Celular, Molecular e Bioagentes Patogênicos, Faculdade de Medicina de Ribeirão Preto, USP, Ribeirão Preto, SP, Brazil), and members of the Laboratório de Bioquímica e Biologia Molecular (Instituto de Genética e Bioquímica, Universidade Federal de Uberlândia (UFU), Uberlândia, MG, Brazil) and Laboratório de Imunologia (Departamento de Imunologia, Microbiologia e Parasitologia, UFU, Uberlândia, MG, Brazil). We also thank Jodi Dee Hunt for the English revision of the manuscript.
Address for correspondence: F.S. Espindola, Instituto de Genética e Bioquímica, Universidade Federal de Uberlândia, Rua Acre, Bloco 2E, Sala 39, 38400-982 Uberlândia, MG, Brasil. Fax: +55-34-218-2476. E-mail: foued@ufu.br
Presented at the XV Annual Meeting of the Federação de Sociedades de Biologia Experimental, Caxambu, MG, Brazil, August 23-26, 2000. Research supported by CAPES (D.S. Reis), CNPq (J.R. Mineo), FAPEMIG (F.S. Espindola, No. CBS1781/97), and The Pew Latin American Fellow Program (F.S. Espindola). Received April 12, 2000. Accepted November 30, 2000.
- 1. Denlinger LC, Fisette PL, Garis KA, Kwon G, Vazquez-Torres A, Simon AD, Nguyen B, Proctor RA, Bertics PJ & Corbett JA (1996). Regulation of inducible nitric oxide synthase expression by macrophage purinoreceptors and calcium. Journal of Biological Chemistry, 271: 337-342.
- 2. Pan J, Burgher KL & Szszepanik GE (1996). Tyrosin phosphorylation of inducible nitric oxide synthase: implications for potential post-translational regulation. Biochemical Journal, 314: 889-894.
- 3. Cunha FQ, Assreuy J, Moncada S & Liew FY (1993). Phagocytosis and induction of nitric oxide synthase in murine macrophages. Immunology, 79: 408-411.
- 4. Yuan T, Vogel HJ, Sutherland C & Walsh MP (1998). Characterization of the Ca2+-dependent and -independent interactions between calmodulin and its binding domain of inducible NOS. FEBS Letters, 431: 210-214.
- 5. Mustafa SB & Olson MS (1999). Effects of calcium channel antagonists on LPS-induced hepatic iNOS expression. American Journal of Physiology, 277: G351-G360.
- 6. Park YC, Jun CD, Kang HS, Kim HD, Kim MD & Chung HT (1996). Role of intracellular calcium as a priming signal for the induction of nitric oxide synthesis in murine peritoneal macrophages. Immunology, 87: 296-302.
- 7. Geng Y & Lotz M (1995). Increased intracellular Ca2+ selectively suppresses IL-1-induced NO production by reducing iNOS mRNA stability. Journal of Cell Biology, 129: 1651-1657.
- 8. Eason S & Martin W (1995). Involvement of tyrosine kinase and protein kinase C in the induction of nitric oxide synthase by lipopolysaccharide and interferon-gamma in J774 macrophages. Archives Internationales de Pharmacodynamie et de Therapie, 330: 225-240.
- 9. Bogdan C, Thüring H, Dlaska M, Röllinghoff M & Weiss G (1997). Mechanism of suppression of macrophage nitric oxide release by IL-13: influence on the macrophage population. Journal of Immunology, 159: 4506-4513.
- 10. Norby SW, Weyhenmeyer JA & Clarkson RB (1997). Stimulation and inhibition of nitric oxide production in macrophages and neural cells as observed by spin trapping. Free Radical Biology and Medicine, 22: 1-9.
- 11. Kim HM, Lee EH, Shin TK, Chung CK & Na NH (1998). Inhibition of the induction of the inducible nitric oxide synthase in murine brain microglial cells by salicylate. Immunology, 95: 389-394.
- 12. Fernandes PD, Araújo HM, Rivero-Moreno V & Assrey J (1996). Depolymerization of macrophage microfilaments prevents induction and inhibits activity of nitric oxide synthase. European Journal of Cell Biology, 71: 356-362.
- 13. Hasson T & Mooseker MS (1996). Vertebrate unconventional myosins. Journal of Biological Chemistry, 271: 16431-16434.
- 14. Mermall V, Post PL & Mooseker MS (1998). Unconventional myosins in cell movement, membrane traffic, and signal transduction. Science, 279: 527-533.
- 15. Swanson JA, Johnson MT, Beningo K, Post P, Mooseker MS & Araki N (1999). A contractile activity that closes phagosomes in macrophages. Journal of Cell Science, 112: 307-316.
- 16. Suneja SK & Potashner SJ (1998). Quantification of a neurotrophin receptor from submilligram quantities of brain tissue using Western blotting. Brain Research Brain Research Protocols, 3: 88-93.
- 17. Bement WM, Hasson T, Wirth JA, Cheney RE & Mooseker MS (1994). Identification and overlapping expression of multiple unconventional myosin genes in vertebrate cell types. Proceedings of the National Academy of Sciences, USA, 91: 6549-6553.
Correspondence and Footnotes
Publication Dates
-
Publication in this collection
09 Feb 2001 -
Date of issue
Feb 2001
History
-
Accepted
30 Nov 2000 -
Received
23 Apr 2000