Abstract
Relaxation in the mammalian ventricle is initiated by Ca2+ removal from the cytosol, which is performed by three main transport systems: sarcoplasmic reticulum Ca2+-ATPase (SR-A), Na+-Ca2+ exchanger (NCX) and the so-called slow mechanisms (sarcolemmal Ca2+-ATPase and mitochondrial Ca2+ uptake). To estimate the relative contribution of each system to twitch relaxation, SR Ca2+ accumulation must be selectively inhibited, usually by the application of high caffeine concentrations. However, caffeine has been reported to often cause changes in membrane potential due to NCX-generated inward current, which compromises the reliability of its use. In the present study, we estimated integrated Ca2+ fluxes carried by SR-A, NCX and slow mechanisms during twitch relaxation, and compared the results when using caffeine application (Cf-NT) and an electrically evoked twitch after inhibition of SR-A with thapsigargin (TG-TW). Ca2+ transients were measured in 20 isolated adult rat ventricular myocytes with indo-1. For transients in which one or more transporters were inhibited, Ca2+ fluxes were estimated from the measured free Ca2+ concentration and myocardial Ca2+ buffering characteristics. NCX-mediated integrated Ca2+ flux was significantly higher with TG-TW than with Cf-NT (12 vs 7 µM), whereas SR-dependent flux was lower with TG-TW (77 vs 81 µM). The relative participations of NCX (12.5 vs 8% with TG-TW and Cf-NT, respectively) and SR-A (85 vs 89.5% with TG-TW and Cf-NT, respectively) in total relaxation-associated Ca2+ flux were also significantly different. We thus propose TG-TW as a reliable alternative to estimate NCX contribution to twitch relaxation in this kind of analysis.
Myocardium; Relaxation; Calcium fluxes; Sodium-calcium exchanger; Sarcoplasmic reticulum calcium ATPase; Caffeine
Braz J Med Biol Res, December 2003, Volume 36(12) 1717-1723 (Short Communication)
Inhibition of the sarcoplasmic reticulum Ca 2+ pump with thapsigargin to estimate the contribution of Na + -Ca 2+ exchange to ventricular myocyte relaxation
R.A. Bassani1 and J.W.M. Bassani1,2
1Centro de Engenharia Biomédica and 2Departamento de Engenharia Biomédica, Faculdade de Engenharia Elétrica e de Computação, Universidade Estadual de Campinas, Campinas, SP, Brasil
Text
References
Correspondence and Footnotes Correspondence and Footnotes Correspondence and Footnotes
Abstract
Relaxation in the mammalian ventricle is initiated by Ca2+ removal from the cytosol, which is performed by three main transport systems: sarcoplasmic reticulum Ca2+-ATPase (SR-A), Na+-Ca2+ exchanger (NCX) and the so-called slow mechanisms (sarcolemmal Ca2+-ATPase and mitochondrial Ca2+ uptake). To estimate the relative contribution of each system to twitch relaxation, SR Ca2+ accumulation must be selectively inhibited, usually by the application of high caffeine concentrations. However, caffeine has been reported to often cause changes in membrane potential due to NCX-generated inward current, which compromises the reliability of its use. In the present study, we estimated integrated Ca2+ fluxes carried by SR-A, NCX and slow mechanisms during twitch relaxation, and compared the results when using caffeine application (Cf-NT) and an electrically evoked twitch after inhibition of SR-A with thapsigargin (TG-TW). Ca2+ transients were measured in 20 isolated adult rat ventricular myocytes with indo-1. For transients in which one or more transporters were inhibited, Ca2+ fluxes were estimated from the measured free Ca2+ concentration and myocardial Ca2+ buffering characteristics. NCX-mediated integrated Ca2+ flux was significantly higher with TG-TW than with Cf-NT (12 vs 7 µM), whereas SR-dependent flux was lower with TG-TW (77 vs 81 µM). The relative participations of NCX (12.5 vs 8% with TG-TW and Cf-NT, respectively) and SR-A (85 vs 89.5% with TG-TW and Cf-NT, respectively) in total relaxation-associated Ca2+ flux were also significantly different. We thus propose TG-TW as a reliable alternative to estimate NCX contribution to twitch relaxation in this kind of analysis.
Key words: Myocardium, Relaxation, Calcium fluxes, Sodium-calcium exchanger, Sarcoplasmic reticulum calcium ATPase, Caffeine
Cytosolic free Ca2+ concentration ([Ca2+]i) is the key factor that determines the degree of myofilament activation, and thus contraction, in striated muscle cells. In cardiac myocytes, electric activation promotes Ca2+ influx, which triggers Ca2+ release from the sarcoplasmic reticulum (SR), resulting in increased [Ca2+]i and a subsequent contraction. On the other hand, the very increase in [Ca2+]i enhances Ca2+ transport via several pathways, which results in cytosolic Ca2+ removal and allows relaxation to develop (1,2). In mammalian ventricular myocytes, the transporters that contribute more significantly to relaxation are the SR Ca2+-ATPase (SR-A, which pumps Ca2+ from the cytosol to the SR lumen) and the Na+-Ca2+ exchanger (NCX, which, operating in the direct mode, extrudes Ca2+ from the cell). Other transporters, such as the sarcolemmal Ca2+-ATPase and the mitochondrial Ca2+ uniporter, show a very small participation in twitch relaxation in this cell type (1-5).
To determine the relative contribution of the different Ca2+ transporters to relaxation of intact myocytes, we estimated the relaxation-associated Ca2+ flux mediated by each transport system during the decline phase of the cytosolic Ca2+ transient (3). For this purpose it is necessary to selectively and additively inhibit these transporters. For instance, during an electrically evoked twitch, all relaxation-promoting transporters are functional. To block the SR-dependent component of relaxation, it is possible to evoke Ca2+ transients by rapid application of caffeine to quiescent cells. Because caffeine at millimolar concentrations promotes SR Ca2+ channel opening (6), not only does it release Ca2+ from the SR, but also prevents significant Ca2+ accumulation in the organelle, which is equivalent to inhibition of the SR-A. In this case, relaxation would rely only on NCX and the so-called slow systems (i.e., sarcolemmal Ca2+-ATPase and the mitochondrial Ca2+ uniporter). To further inhibit NCX, caffeine-evoked Ca2+ transients may be obtained in the absence of extracellular Na+ and Ca2+, with only the slow systems being left uninhibited. From the [Ca2+]i values during each type of specific transient and the time-course of the [Ca2+]i decline, as well as from data on passive cell Ca2+ buffering, it is possible to estimate empirical kinetic parameters for each transporter. These parameters are used to estimate the transporter-mediated Ca2+ fluxes during relaxation and the relative contribution of each transporter to cytosolic Ca2+ removal. This approach has been employed to investigate the interplay of the different Ca2+ transport pathways during relaxation of myocytes from animals of different species (3,4) and in different developmental stages (7), in transgenic animals (8), and in experimental models of cardiovascular disease (9).
As already stated, NCX-dependent Ca2+ fluxes are estimated from Ca2+ transients evoked by caffeine. However, the use of caffeine to inhibit SR Ca2+ accumulation may present problems because: a) both velocity and direction of Ca2+ transport by NCX are dependent on membrane potential (Vm) (10). Thus, it is possible that the kinetics of Ca2+ efflux via NCX is different during caffeine application, when the cell is supposedly at electrical rest, and during a twitch, when a physiological action potential takes place. b) It has been shown that caffeine application may change Vm and even evoke action potentials in quiescent ventricular myocytes (11,12), an effect attributed to the depolarizing current generated by electrogenic Ca2+ efflux via NCX (12). This current (and thus, depolarization) is usually large (13) because caffeine appears to release the entire SR Ca2+ content (4). Although in preliminary experiments, we have detected Vm changes in response to caffeine in only a small number of cells, caffeine-evoked action potentials, when present, show an unusually prolonged time-course and are often accompanied by secondary peaks in the caffeine-evoked Ca2+ transient and contracture (Bassani RA, unpublished results). A solution for this problem might be applying caffeine to cells voltage-clamped at the diastolic Vm (11). However, this condition would still not reproduce the action potential developed during a control twitch. Moreover, voltage-clamp is an invasive method which may disturb the composition of the intracellular medium, although the perforated whole-cell patch-clamp approach may represent a less aggressive alternative of membrane voltage control.
In the present report, we propose an alternative to the use of caffeine to estimate NCX-mediated fluxes, which is using Ca2+ transients obtained during electrically stimulated twitches after SR pump inhibition with thapsigargin (TG). This procedure was compared to caffeine application to the same set of rat ventricular myocytes.
Ventricular myocytes were enzymatically isolated according to Bassani et al. (3) from adult male Wistar rats (10-14 weeks old). Cells were used within 8 h from the time of isolation. Myocytes were plated onto a perfusion chamber (developed at the Centro de Engenharia Biomédica/UNICAMP) coated with a collagen solution and allowed to settle. The chamber was placed on the stage of a microscope equipped for epifluorescence measurement. Cells were perfused with
modified Tyrode's solution (NT; see below for composition) at 23 ± 1ºC, and field-stimulated at 0.5 Hz (biphasic voltage pulses with amplitude 1.2 x threshold, 3-ms duration).
For [Ca2+]i measurement, cells were incubated with the indicator indo-1 (5 µM, acetoxymethyl ester; Molecular Probes, Eugene, OR, USA) for 15 min. Experiments were started after indo-1 had been washed out for 30 min. Indo-1 was excited at 360 nm and emission was collected at 405 and 485 nm and corrected at each wavelength for the background fluorescence recorded in an empty microscopic field of the same size. The ratio of emitted fluorescence at 405 and 485 nm (R) was converted to [Ca2+] (14), as [Ca2+] = Kd × ß [(R - Rmin)/(Rmax - R)], where Rmin and Rmax (R in minimal and saturating [Ca2+], respectively) were determined experimentally (3), ß (ratio of emission at 485 nm at minimal and saturating [Ca2+]) was determined according to Gomes et al. (15), and the in vivo Kd (apparent dissociation constant for indo-1) value of 0.844 µM (16) was used.
After cells were stimulated for 5 min, Ca2+ transients were recorded during three successive electrically evoked twitches for averaging. Electrical stimulation was then interrupted and the perfusate was rapidly switched to NT containing 10 mM caffeine. After caffeine washout, electrical stimulation was resumed for 5 min for replenishment of the SR Ca2+ stores, and then interrupted again. Cells were perfused with Na+- and Ca2+-free (0Na,0Ca) solution for 15-20 s, after which the perfusate was switched to the same solution containing 10 mM caffeine. After most of the [Ca2+]i decline had taken place, NT was switched on.
Cells were treated with 5 µM TG (Calbiochem, San Diego, CA, USA) for 5 min to irreversibly inhibit the SR-A (17,18). Twitches were evoked at 0.1 Hz because [Ca2+]i decline is very slow after TG treatment, and lower stimulation frequencies allow greater variation in [Ca2+]i during a twitch, as well as complete decline of [Ca2+]i to the resting level before the next stimulus (17). All cells were tested for residual SR Ca2+ uptake by caffeine application in 0Na,0Ca solution following electrical stimulation. If a TG-treated cell showed any [Ca2+]i increase in response to caffeine, it was discarded due to incomplete inhibition of the SR-A.
NT had the following composition: 140 mM NaCl, 6 mM KCl, 1 mM MgCl2, 1 mM CaCl2, 10 mM glucose and 5 mM HEPES, pH 7.4 at 23ºC with NaOH. In 0Na,0Ca solution, choline chloride and EGTA replaced NaCl and CaCl2, respectively. TG was dissolved in DMSO, and the concentration of the solvent applied to the cells was <0.1%.
The basic procedure for estimation of Ca2+ fluxes was similar to that described by Bassani et al. (3). The four types of Ca2+ transients, i.e., control twitches, contractures in response to caffeine in Na+-containing (Cf-NT) and Na+-free medium (Cf-00), as well as twitches after TG treatment (TG-TW), were obtained in 20 myocytes. Cf-NT Ca2+ transients showing non-monotonic Ca2+ decline were discarded. In each cell, diastolic [Ca2+]i was considered as the average value recorded immediately before each transient was evoked. A single exponential function was adjusted to the decline phase of each Ca2+ transient for estimation of the amplitude (D[Ca2+]i, i.e., peak minus diastolic [Ca2+]i) and the rate constant of [Ca2+]i decline (K) of the transient. To avoid interference of the signal noise in further calculations, the same function and the estimated parameters were used to generate a "synthetic" [Ca2+]i decline phase of the transient (i.e., the same exponential function that best fitted the [Ca2+]i decay phase of the respective transient).
The total flux of Ca2+ removal from the cytosol during relaxation (Jtot) was assumed to be:
Jtot = JSR + JNCX + Jslow - JL
where JSR, JNCX and Jslow are the Ca2+ fluxes during relaxation carried by SR-A, NCX and the slow transporters, respectively. JL represents the "leakage" Ca2+ flux from the SR and the extracellular medium to the cytosol, and was neglected in the present calculations for the sake of simplicity (3).
To estimate Jtot during relaxation, it is necessary to convert the free [Ca2+] signal (i.e., [Ca2+]i) to total [Ca2+] ([Ca2+]T, i.e., the sum of the free and bound [Ca2+]) as follows:
[Ca2+]T = [Ca2+]i + {Bmax-en × [Ca2+]i/(Kd-en + [Ca2+]i)} + {Bmax-in × [Ca2+]i/(Kd-in + [Ca2+]i)}
where Bmax-en is the maximal concentration of high-affinity, passive Ca2+ binding sites and Kd-en is the apparent dissociation constant of Ca2+ at these sites determined in permeabilized adult rat ventricular myocytes (300 and 0.53 µM, respectively; Ref. 19); Bmax-in and Kd-in are the parameters related to indo-1 (considered as 50 and 0.844 µM, respectively).
The relationship between the time derivative of [Ca2+]T over the decline of the transient (J) and the respective [Ca2+]i values was used to estimate the kinetic parameters of a given transporter x, as follows:
Jx = Vmax/{1 + (Km/[Ca2+]i )n}
where Vmax is the maximal transport velocity, Km is the [Ca2+]i at which velocity is 50% of Vmax, and n is the Hill coefficient.
Thus, if this relationship is applied to the [Ca2+]i decline during Cf-00 (in which both SR Ca2+ accumulation and NCX are inhibited, thus Jtot = Jslow), it is possible to calculate the kinetic parameters for the lumped slow systems. Afterward, using the estimated parameters, this relationship may be used for the transient at Cf-NT or TG-TW (where Jtot = JNCX + Jslow), which allows calculation of NCX parameters. Finally, using the same relationship for the twitch transient and the parameters already estimated, it is possible to estimate the SR-A parameters. It should be stressed that these parameters are empirical and do not necessarily correspond to those determinable directly in subcellular preparations. However, because this kinetic approach takes into account the relationship between transport velocity and [Ca2+]i, it is not necessary to work with Ca2+ transients of similar amplitudes.
By applying the calculated kinetic parameters for each transport system to the twitch [Ca2+]i signal, individual Ca2+ fluxes were determined during twitch relaxation. The Ca2+ flux via each transporter, as well as the sum of all fluxes (Jtot), were integrated over one second after the peak of the twitch Ca2+ transient, and the percent contribution of each individual flux to Jtot was calculated. In this study, for each cell, NCX kinetic parameters were estimated using both Cf-NT and TG-TW, and each of these estimates was used to determine SR-A parameters and flux.
Fluxes are expressed as µM (i.e., µmol Ca2+/liter of cytosol). Data are presented as means ± SEM, or accompanied by the 95% confidence interval (95%CI). Comparison of data was performed by one-way analysis of variance for paired samples (followed, when necessary, by the Bonferroni test) or t-test for paired samples. Statistical significance was considered to occur when P £ 0.05. Percentages were transformed to arc sin Öp prior to statistical comparisons; afterward, means and 95%CI limits were converted back to percentage. Prism (version 2.0, GraphPad Software, San Diego, CA, USA) was used for curve fitting and statistical analysis.
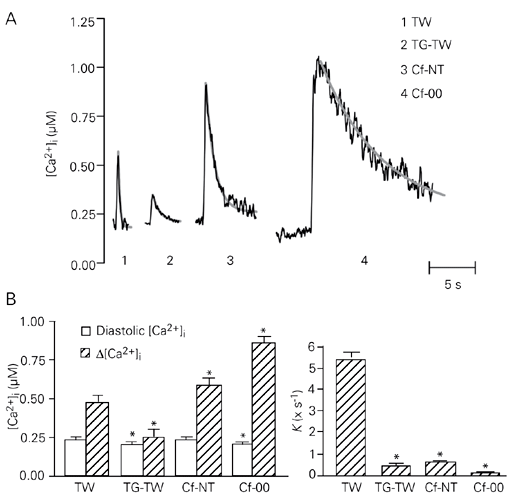
Figure 1A shows typical Ca2+ transients obtained in an isolated rat ventricular myocyte during control twitches, Cf-NT, TG-TW and Cf-00, and Figure 1B depicts mean values of diastolic [Ca2+]i, D[Ca2+]i and K. Diastolic [Ca2+]i depended on the type of contraction (P < 0.001) and was lower for transients obtained after a 10- to 20-s rest (e.g., Cf-00 and TG-TW) than after a 2-s rest (Cf-NT and control twitches, P < 0.05). D[Ca2+]i also depended on the kind of transient (P < 0.001): caffeine-evoked transients were significantly higher than control twitches (P < 0.05), while TG-TW was lower than control twitches (P < 0.001). This is expected because the amount of Ca2+ released from the SR by caffeine is at least twice as large than that released during a twitch (17). Also, impairment of SR function by TG generally decreases Ca2+ transient amplitude in the mammalian ventricle (17,18), although, in the present experiments TG-TW amplitude was not so small because of the lower stimulation frequency (17). K was highly variable among types of contraction (P < 0.001), but comparable in Cf-NT and TG-TW (P > 0.05), during which SR Ca2+ accumulation is inhibited.
The total Ca2+ flux (integrated over 1 s after the twitch [Ca2+]i peak) was slightly, but significantly (P = 0.0072) higher when TG-TW was used to estimate NCX-dependent Ca2+ flux (Table 1). These values are close to those previously described for adult rat myocytes during a twitch (3,7). The use of TG-TW resulted in a higher estimate of the NCX-dependent integrated Ca2+ flux
(P = 0.0314) and a lower estimate of SR-A-dependent Ca2+ flux (P = 0.0491) compared to the use of Cf-NT (Table 1). The integrated flux carried by the slow transporters was determined independently of NCX and amounted to 2.30 ± 0.22 µM.
Inhibition of the sarcoplasmic reticulum Ca2+ pump with thapsigargin to estimate the contribution of Na+-Ca2+ exchange to ventricular myocyte relaxation. R.A. Bassani and J.W.M. Bassani. Brazilian Journal of Medical and Biological Research, 36 (12): 1717, 2003.
As a result of the differences in individual Ca2+ fluxes, the relative contribution of each Ca2+ transport system to twitch relaxation showed statistically significant differences depending on whether TG-TW or Cf-NT was used to estimate NCX participation. The contribution of the slow systems was not significantly affected (2.6% of the total integrated flux with both TG-TW and Cf-NT). However, estimated NCX participation was greater with TG-TW than that with Cf-NT (P = 0.0174). Conversely, SR-A contribution was lower with TG-TW than with Cf-NT (P = 0.0169), as shown in Table 1. The relative contributions of NCX and SR-A to twitch relaxation obtained in the present study with Cf-NT were comparable to those previously described using the same approach in adult rat ventricular myocytes (3,7).
The present results thus show that using TG-TW instead of Cf-NT to selectively inhibit SR Ca2+ accumulation significantly increased the estimated contribution of NCX to cytosolic Ca2+ clearance associated with twitch relaxation. In a previous study we had also compared Cf-NT and TG-TW and observed, as seen in the present analysis, that the time course of [Ca2+]i decline was similar in both types of transients; however, Ca2+ fluxes were not determined in that study (3). Moreover, in the previous analyses (3,4,7-9), Ca2+ fluxes and the relative contributions of Ca2+ transporters to relaxation were estimated with average Ca2+ transient data, which yielded a single value for a given cell population. In the present study, we analyzed data from individual cells, which not only permitted us to estimate the variability within the cell population, but also permitted us to submit the data to statistical comparison.
Using Cf-NT to selectively inhibit SR Ca2+ accumulation is less costly and much easier, from an experimental point of view, than using TG-TW. However, the use of TG-TW presents some advantages, such as: a) differently from caffeine, TG directly inhibits SR-A; b) during TG-TW, the Ca2+ transient is triggered by an action potential waveform, although it has been shown that TG treatment decreases the action potential duration in rat ventricular myocytes (18); c) because the TG-TW transient has much lower amplitude than that obtained with Cf-NT, the NCX-mediated inward current and the likelihood of undesirable changes in Vm are considerably smaller. On the other hand, the marked effect of SR inhibition in depressing the Ca2+ transient amplitude might pose a problem with TG-TW because of the difficulty of extracting reliable data from a low-amplitude signal. This can be partly overcome by decreasing the stimulation frequency, as done in the present study. However, it is important that one be certain that full inhibition of SR-A is obtained with TG treatment because incomplete blockade greatly affects the results and renders them unreliable.
Although the mean difference in estimated NCX-mediated Ca2+ flux and NCX relative contribution to the [Ca2+]i decline during twitch relaxation reached as much as 50% when data obtained with Cf-NT and TG-TW were compared, only in 9 out of 20 cells were these values more than 20% higher with TG-TW. This suggests that caffeine might have caused Vm changes in only part of the cell population studied. Despite the present results, caffeine might still be considered a valuable tool in this kind of experiment, as long as its limitations are acknowledged. Also, care should be exercised when using Cf-NT for this type of analysis under conditions that favor cell Ca2+ overload (12) and/or reduce resting Vm stability, such as down-regulation of the inward rectifying K+ current (IK1) that may occur in some kinds of heart disease (20), which are likely to facilitate the undesirable effects of caffeine on Vm.
Ca2+ transients during inhibition of Ca2+ transporters. A, Typical Ca2+ transients recorded from an adult rat ventricular myocyte during electrical stimulation at 0.5 Hz under control conditions (TW) and at 0.1 Hz after treatment with thapsigargin (TG-TW), and in quiescence after application of 10 mM caffeine in the presence (Cf-NT) and absence (Cf-00) of extracellular Na+ and Ca2+. The gray curves superimposed on the traces represent single exponential functions fitted to the phase of [Ca2+]i decline of each transient (R2 > 0.97 in all cases). B, Values of diastolic [Ca2+]i and Ca2+ transient amplitude (D[Ca2+]i, left panel) and rate constant of [Ca2+]i decline (right panel) for all four types of transient. *P < 0.05 compared to TW (one-way ANOVA for paired samples, followed by the Bonferroni test).
Acknowledgments
We are grateful to Ms. Elizângela S. Oliveira for excellent technical support.
Address for correspondence: R.A. Bassani, Centro de Engenharia Biomédica, UNICAMP, Caixa Postal 6040, 13084-971 Campinas, SP, Brasil. Fax: +55-19-3289-3346. E-mail: rosana@ceb.unicamp.br
Research supported by FAPESP (No. 95/0355-3). Received March 11, 2003. Accepted August 19, 2003.
- 1. Bers DM, Bassani JWM & Bassani RA (1996). Na/Ca exchange and Ca fluxes during contraction and relaxation in mammalian ventricular muscle. Annals of the New York Academy of Sciences, 779: 430-442.
- 2. Bers DM (2001). Excitation-Contraction Coupling and Cardiac Contractile Force 2nd edn. Kluwer Press, Dordrecht, The Netherlands.
- 3. Bassani JWM, Bassani RA & Bers DM (1994). Relaxation in rabbit and rat cardiac cells: species-dependent differences in cellular mechanisms. Journal of Physiology, 476: 279-293.
- 4. Bassani RA, Bassani JWM & Bers DM (1994). Relaxation in ferret ventricular myocytes: unusual interplay among calcium transport systems. Journal of Physiology, 476: 295-308.
- 5. Negretti N, O'Neill SC & Eisner DA (1993). The relative contributions of different intracellular and sarcolemmal systems to relaxation in rat ventricular myocytes. Cardiovascular Research, 27: 1826-1830.
- 6. Rousseau E & Meissner G (1989). Single cardiac sarcoplasmic reticulum Ca2+-release channel: activation by caffeine. American Journal of Physiology, 256: H328-H333.
- 7. Bassani RA & Bassani JWM (2002). Contribution of Ca2+ transporters to relaxation in intact ventricular myocytes from developing rats. American Journal of Physiology, 282: H2406-H2413.
- 8. Li L, Chu G, Kranias EG & Bers DM (1998). Cardiac myocyte calcium transport in phospholamban knockout mouse: relaxation and endogenous CaMKII. American Journal of Physiology, 274: H1335-H1347.
- 9. McCall E, Ginsburg KS, Bassani RA, Shannon TS, Qi M, Samarel AM & Bers DM (1998). Ca flux, contractility, and excitation-contraction coupling in hypertrophic rat ventricular myocytes. American Journal of Physiology, 274: H1348-H1360.
- 10. Blaustein M & Lederer WJ (1999). Sodium/calcium exchange: its physiological implications. Physiological Reviews, 79: 763-854.
- 11. Zaniboni M, Yao A, Barry WH, Musso E & Spitzer K (1998). Complications associated with rapid caffeine application to cardiac myocytes that are not voltage-clamped. Journal of Molecular and Cellular Cardiology, 30: 2229-2235.
- 12. Schlotthauer K & Bers DM (2000). Sarcoplasmic reticulum Ca2+ release causes myocyte depolarization: underlying mechanism and threshold for triggered action potentials. Circulation Research, 87: 774-780.
- 13. Varro A, Negretti N, Hester SB & Eisner DA (1993). An estimate of the calcium content of the sarcoplasmic reticulum in rat ventricular myocytes. Pflügers Archives, 423: 158-160.
- 14. Grynkiewicz C, Poenie M & Tsien RY (1985). A new generation of Ca2+ indicators with greatly improved fluorescence properties. Journal of Biological Chemistry, 260: 3440-3450.
- 15. Gomes PAP, Bassani RA & Bassani JWM (1998). Measuring [Ca2+] with fluorescent indicators: theoretical approach to the ratio method. Cell Calcium, 24: 17-26.
- 16. Bassani JWM, Bassani RA & Bers DM (1995). A method for calibration of indo-1 and resting [Ca]i in intact rabbit cardiac myocytes. Biophysical Journal, 68: 1453-1460.
- 17. Bassani JWM, Bassani RA & Bers DM (1993). Twitch-dependent SR Ca accumulation and release in rabbit ventricular myocytes. American Journal of Physiology, 265: C533-C540.
- 18. Kirby MS, Sagara Y, Gaa S, Inesi G, Lederer WJ & Rogers TB (1991). Thapsigargin inhibits contraction and Ca transient in cardiac cells by specific inhibition of the sarcoplasmic reticulum calcium pump. Journal of Biological Chemistry, 267: 12545-12551.
- 19. Bassani RA, Shannon TS & Bers DM (1998). Passive Ca2+ binding in ventricular myocardium of neonatal and adult rats. Cell Calcium, 23: 433-442.
- 20. Pogwizd SM, Qi M, Yuan W, Samarel AM & Bers DM (1999). Upregulation of Na+/Ca2+ exchange expression and function in an arrhythmogenic rabbit model of heart failure. Circulation Research, 85: 1009-1019.
Correspondence and Footnotes
Publication Dates
-
Publication in this collection
17 Nov 2003 -
Date of issue
Dec 2003
History
-
Accepted
19 Aug 2003 -
Received
11 Mar 2003