Abstract
The widespread use of ³H and 14C in research has generated a large volume of waste mixed with scintillation liquid, requiring an effective control and appropriate storage of liquid radioactive waste. In the present study, we compared the efficacy of three commercially available scintillation liquids, Optiphase HiSafe 3, Ultima-Gold™ AB (biodegradable) and Insta-Gel-XF (non-biodegradable), in terms of [14C]-glucose and [³H]-thymidine counting efficiency. We also analyzed the effect of the relative amount of water (1.6 to 50%), radioisotope concentration (0.1 to 100 nCi/ml), pH (2 to 10) and color of the solutions (samples containing 0.1 to 1.0 mg/ml of Trypan blue) on the counting efficiency in the presence of these scintillation liquids. There were few significant differences in the efficiency of 14C and ³H counting obtained with biodegradable or non-biodegradable scintillation liquids. However, there was an 83 and 94% reduction in the efficiency of 14C and ³H counting, respectively, in samples colored with 1 mg/ml Trypan blue, but not with 0.1 mg/ml, independent of the scintillation liquid used. Considering the low cost of biodegradable scintillation cocktails and their efficacy, these results show that traditional hazardous scintillation fluids may be replaced with the new safe biodegradable fluids without impairment of ³H and 14C counting efficiency. The use of biodegradable scintillation cocktails minimizes both human and environmental exposure to hazardous solvents. In addition, some biodegradable scintillation liquids can be 40% less expensive than the traditional hazardous cocktails.
Biodegradable chemicals; Scintillation liquids; Liquid waste; Color quenching; Scintillation counting efficiency; Tritium and 14C counting
Braz J Med Biol Res, December 2003, Volume 36(12) 1733-1739
Comparison of the efficacy of biodegradable and non-biodegradable scintillation liquids on the counting of tritium- and [ 14 C]-labeled compounds
R.B. Medeiros1, R.O. Godinho2 and M.F.S.S. Mattos1
1Unidade de Proteção Radiológica and 2Departamento de Farmacologia, Escola Paulista de Medicina, Universidade Federal de São Paulo, São Paulo, SP, Brasil
References
Correspondence and Footnotes Correspondence and Footnotes Correspondence and Footnotes
Abstract
The widespread use of 3H and 14C in research has generated a large volume of waste mixed with scintillation liquid, requiring an effective control and appropriate storage of liquid radioactive waste. In the present study, we compared the efficacy of three commercially available scintillation liquids, Optiphase HiSafe 3, Ultima-Gold AB (biodegradable) and Insta-Gel-XF (non-biodegradable), in terms of [14C]-glucose and [3H]-thymidine counting efficiency. We also analyzed the effect of the relative amount of water (1.6 to 50%), radioisotope concentration (0.1 to 100 nCi/ml), pH (2 to 10) and color of the solutions (samples containing 0.1 to 1.0 mg/ml of Trypan blue) on the counting efficiency in the presence of these scintillation liquids. There were few significant differences in the efficiency of 14C and 3H counting obtained with biodegradable or non-biodegradable scintillation liquids. However, there was an 83 and 94% reduction in the efficiency of 14C and 3H counting, respectively, in samples colored with 1 mg/ml Trypan blue, but not with 0.1 mg/ml, independent of the scintillation liquid used. Considering the low cost of biodegradable scintillation cocktails and their efficacy, these results show that traditional hazardous scintillation fluids may be replaced with the new safe biodegradable fluids without impairment of 3H and 14C counting efficiency. The use of biodegradable scintillation cocktails minimizes both human and environmental exposure to hazardous solvents. In addition, some biodegradable scintillation liquids can be 40% less expensive than the traditional hazardous cocktails.
Key words: Biodegradable chemicals, Scintillation liquids, Liquid waste, Color quenching, Scintillation counting efficiency, Tritium and 14C counting
Introduction
Radioisotopes commonly used in biomedical research possess low energy and a short range of air or fluid penetration. Therefore, they require direct contact with the scintillation medium and special technology for efficient indirect detection of radioactivity (1). Among these radioisotopes, tritium (3H) and radioactive carbon (14C) have many applications in cell biology, pharmacology and clinical research. The widespread use of these isotopes in research requires effective control of the liquid, solid and biological radioactive waste that results from techniques such as radioimmunoassay or radioligand binding assays. Most of these experiments generate a large volume of 3H and 14C waste in scintillation liquid.
For an ideal liquid scintillation detector, the amount of light generated must be proportional to the energy transferred and the detection should be perfectly linear. However, an energy transfer loss frequently occurs as a result of absorption of light by solid materials, chromogenic interposition, solution turbidity or pH changes in the scintillation fluid (2,3).
The process of radioactive detection involves scintillation fluids composed of aromatic solvents that increase the efficiency of energy transfer to the organic fluorine compound, improving the detection of light emission. Table 1 lists commercially available scintillation cocktails and their characteristics based on technical charts provided by the manufacturers. The primary and most extensively used scintillation fluid contains 2,5-diphenyloxazole, and is known as PPO. In fact, secondary compounds such as 1,4-bis(5-phenyl-2-oxazolyl)-benzene are also included in the scintillation fluid in order to absorb the light emitted by the PPO, properly distribute the light and enhance the detection process (4). Toluene, xylene and dioxane are also widely used for this purpose (5). These inflammable and extremely toxic solvents represent a risk to laboratory activities, which is aggravated by the inappropriate storage of large amounts of hazardous liquids in the work place, since these liquids cannot simply be dumped into the public sewage system and their incineration is too costly (5). Finally, the liquid associated with radioisotopes is also considered radioactive waste that must be processed according to radioprotection laws (6).
Comparison of the efficacy of biodegradable and non-biodegradable scintillation liquids on the counting of tritium- and [14C]-labelled compounds. R.B. Medeiros, R.O. Godinho and M.F.S.S. Mattos. Brazilian Journal of Medical and Biological Research, 36 (12): 1733, 2003.
The Radiological Protection Unit of UNIFESP/Sociedade Paulista para o Desenvolvimento da Medicina/Hospital São Paulo (UNIFESP/SPDM/HSP) recommends standardized procedures that fulfill current legislation and minimize safety problems in research laboratories (available at http://protecaoradiologica.unifesp.br). Scintillation fluids with special formulations are now commercially available and have been considered safe because of their reduced aromatic content and biodegradability. However, the traditional use of well-established non-biodegradable scintillation liquids and the resistance to change in laboratory routines hamper the acceptance of biodegradable scintillation liquids as solution for some of the safety problems and for the costly incineration of toxic laboratory wastes.
Therefore, we assessed the efficiency of biodegradable aqueous scintillation liquids and compared them to the liquids conventionally used in our laboratories for which the only disposal option is incineration. The aim of the present study was to determine, on the basis of counting efficiency, appropriate conditions for the use of biodegradable liquids. In addition, we propose to bring to the attention of the scientific community the advantages of biodegradable scintillation liquids over non-biodegradable liquids, because there is guaranteed adequate disposal of biodegradable radioactive waste according to Comissão Nacional de Energia Nuclear (CNEN) rules for radioprotection and environmental conservation.
Material and Methods
The influence of the relative amount of aqueous solution, radioisotope concentration, pH and solution color on the detection of radioactivity emitted by [3H]-thymidine or [14C]-glucose was determined for several commercially available scintillation fluids: Insta-Gel-XF, Ultima-Gold AB (Packard Biosciences B.V. Chemical Operations, Ulgersmaweg, Groningen, Netherlands) and Optiphase HiSafe 3 (Perkin Elmer Life Science/Wallac Oy, Mustionkatu, Turku, Finland). All experiments were carried out in quadruplicate. The results are reported as mean ± SEM radioactivity measured as disintegration per minute. Statistical differences among groups were determined by analysis of variance (ANOVA) followed by the Newman-Keuls test, with the level of significance set at P < 0.05. When necessary, a linear regression analysis was performed.
Effect of the relative amount of water on counting efficiency
In order to analyze the influence of the relative amount of water solution on counting efficiency, 3700 Bq (100 nCi) [14C]-glucose or 0.037 MBq (1.0 µCi) [3H]-thymidine was diluted in a final volume of 6 ml containing 0.1 to 3 ml of water and the various scintillation liquids. The aqueous solutions corresponded to 1.6, 8.3, 16.7, 25, 33.3 and 50% of the final volume. The vials were vortexed and the radioactivity was determined with a scintillation counter.
Effect of the concentration of radiolabeled compound on counting efficiency
For the determination of radioactivity as a function of radioisotope concentration, 0.1, 1, 10, and 100 nCi [3H]-thymidine or [14C]-glucose diluted in 1 ml of water were added to vials containing 5 ml Insta-Gel-XF, Optiphase HiSafe 3 or Ultima-Gold AB scintillation fluids. The vials were vortexed and radioactivity was determined with a scintillation counter.
Effect of solution pH on counting efficiency
In this set of experiments, the influence of pH was determined by diluting 100 nCi [3H]-thymidine or [14C]-glucose in 1 ml of phosphate-buffered saline, pH 7.0, glycine-HCl buffer, pH 2.0, or borate buffer, pH 10.0. Subsequently, 5 ml of the scintillation liquids Insta-Gel-XF, Optiphase HiSafe 3 and Ultima-Gold AB were added, vials were vortexed and radioactivity was determined.
Effect of sample color on counting efficiency
The assays were carried out using 100 nCi [3H]-thymidine or [14C]-glucose diluted in 1 ml of water in the presence or absence of 0.1 or 1 mg/ml Trypan blue. Next, 5 ml of the scintillation liquids Insta-Gel-XF, Optiphase HiSafe 3 and Ultima-Gold AB were added to the vials.
Results
Effect of aqueous solution volume on 14 C and 3 H counting efficiency
As shown in Figure 1A, when the proportion of water varied from 0 to 33% of total volume, the efficiency of [14C]-glucose counting was not significantly changed, regardless of the scintillation fluid used. However, when the samples were diluted in 3.0 ml of water, which corresponds to 50% of the final volume, there was a significant reduction (55%) in counting efficiency with the Optiphase HiSafe 3 scintillation liquid.
The increase in the relative amount of water in the samples induced a linear reduction in the efficiency of [3H]-thymidine counting, with similar slopes for all scintillation liquids used, as shown in Figure 1B.
Effect of aqueous solution volume on the efficiency of [14C]-glucose (A) and [3H]-thymidine (B) counting. Each point indicates the mean ± SEM (N = 4). (The error bars are too small to be visible). *P < 0.05 compared to the Insta-Gel-XF group (ANOVA followed by Newman-Keuls test). The linear reduction in the efficiency of [3H]-thymidine counting (solid lines in panel B) was similar for all scintillation liquids used. DPM = disintegration per minute.
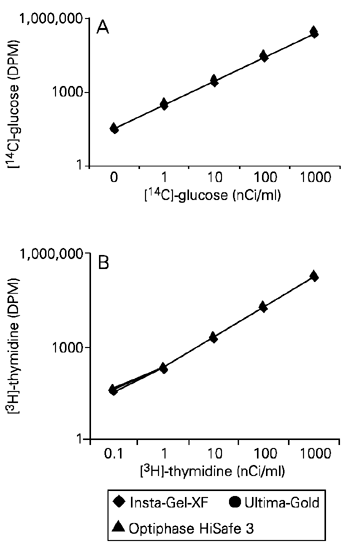
Effect of concentration of radioactive samples on 14 C and 3 H counting efficiency
Regardless of the scintillation liquid used, the radioactivity detected was proportional to the [14C]-glucose and [3H]-thymidine concentration used, as shown in Figure 2A and B, respectively.
Effect of concentration of radioactive samples on the efficiency of [14C]-glucose (A) and [3H]-thymidine (B) counting. Each point indicates the mean ± SEM (N = 4). (The error bars are too small to be visible). DPM = disintegration per minute.
Effect of sample pH on 14 C and 3 H counting efficiency
The changes in solution pH did not influence the efficiency of [14C]-glucose counting. On the other hand, when Optiphase HiSafe 3 and Ultima-Gold AB were used at low pH (2.0), the efficiency of [3H]-thymidine counting was 9 and 20% lower with Ultima-Gold AB and Optiphase HiSafe 3 than that obtained using Insta-Gel-XF (Figure 3A and B).
Influence of sample pH on the efficiency of [14C]-glucose (A) and [3H]-thymidine (B) counting. Each point represents the mean ± SEM (N = 4). (The error bars are too small to be visible) *P < 0.05 compared to the Insta-Gel-XF group (ANOVA followed by Newman-Keuls test). DPM = disintegration per minute.
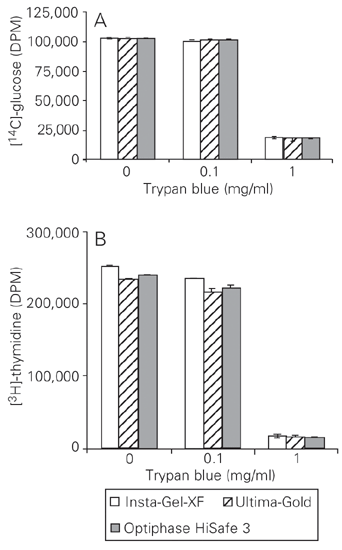
Effect of sample color on 14 C and 3 H counting efficiency
In order to analyze the influence of color quenching on the counting efficiency, [14C]-glucose and [3H]-thymidine were diluted in 1 ml of water containing 0.1 to 1 mg/ml Trypan blue. As shown in Figure 4A, the efficiency of [14C]-glucose radioactive counting was reduced by 83% in the presence of 1 mg/ml but not of 0.1 mg/ml Trypan blue solution, regardless of the scintillation liquid. Similarly, for [3H]-thymidine samples, the reduction at 1.0 mg/ml Trypan was approximately 94% (Figure 4B).
Influence of sample color on the efficiency of [14C]-glucose (A) and [3H]-tritium (B) counting. Each bar indicates the mean ± SEM (N = 4). (The error bars are too small to be visible). DPM = disintegration per minute.
Discussion
The widespread use of radioisotopes in medical and biological research has generated a large volume of solid, liquid and biological wastes that need special treatment for disposal. In order to minimize the production of hazardous radioactive liquid waste we compared the efficacy of non-biodegradable scintillation cocktails containing organic solvents with biodegradable ones. We compared the effectiveness of three commercially available scintillation cocktails on the detection of [14C]-glucose and [3H]-thymidine radioactivity and determined the effect of the most common problems associated with the liquid scintillation counting such as water volume variations, sample pH and color quenching.
The present study showed few significant differences in the efficiency of 14C and 3H counting when using biodegradable scintillation liquids Ultima-Gold AB or Optiphase HiSafe 3 compared to a non-biodegradable one (Insta-Gel-XF), indicating that biodegradable cocktails do not impair the detection of radioactivity when used under appropriate conditions.
Except for the lower efficiency of [3H]-thymidine counting using Ultima-Gold AB at pH 2.0, replacement of Insta-Gel-XF with biodegradable liquids resulted in a similar counting profile in terms of volume of aqueous solution, concentration of radioisotopes and variations in sample color and pH. The reduced counting efficiency for tritium at extremely low pH (2.0) may have been related to the higher turbidity of the scintillation fluid at acidic pH. This result, however, does not impair the use of Ultima-Gold AB, but simply indicates that the sample pH should be corrected before counting.
The most relevant interference with counting efficiency was observed in the colored samples (Figure 4A,B). In general, color quenching results from the attenuation of photons during their passage through the medium (7). The reduction of counting efficiency was observed in samples containing the higher concentration of Trypan blue, 1 mg/ml, regardless of the scintillation liquid used. It is therefore essential to monitor the counting efficiency in colored samples, when a precise determination of radioactive molecules is necessary. In this case, standards of known radioactivity should be used to obtain a correction factor and to correct the color quenching.
Our data also showed that the quenching effect on counting efficiency is also dependent on the type of radioisotopes. The higher susceptibility of 3H compared to 14C might be explained by the lower energy of tritium that reduces the efficiency of energy transfer to the solvent molecules (1).
The fundamental advantage of using biodegradable scintillation cocktails is related to the disposal of radioactive residues. For the non-biodegradable product it is necessary to request the chemical analysis of the waste sample according to the Brazilian Standard Rules and to obtain the industrial waste registration document required by the environmental agency (Companhia de Tecnologia de Saneamento Ambiental, CETESB) (8,9). It is also relevant to take into account the cost of waste disposal, which includes the storage and treatment of toxic residues. On the other hand, non-hazardous aqueous radioactive wastes that are readily soluble in water may be disposed of via the sanitary sewage system if the concentration and maximum disposal radioactivity limits are observed (3.7 x 109 Bq/m3 or 0.1 µCi/ml for 3H, or 7.4 x 108 Bq/m3 or 0.02 µCi/ml for 14C) according to the criteria outlined by CNEN. In fact, if the concentration of radioactive waste is below these limits, the waste is no longer characterized as radioactive but is considered to belong to the chemical group of waste defined as Class I Hazardous based on the Brazilian Standard Rules (ABNT NBR-10004 and 10007).
The present results show that traditional hazardous scintillation fluids can be replaced with safe biodegradable ones. The benefits of using environmentally benign fluids include the reduction of both liquid and solid radioactive waste since the containers of hazardous solvents must also be stored for later incineration. In addition to reducing the overall cost of radioactive waste disposal, the use of biodegradable scintillation cocktails minimizes both human and environmental exposure to hazardous solvents. In addition, some biodegradable scintillation liquids can be 40% less expensive than the traditional hazardous cocktails (see Table 1).
Acknowledgments
We thank the Natural Products Section, Department of Pharmacology, UNIFESP/EPM, for the use of laboratory facilities.
Address for correspondence: R.B. Medeiros, Unidade de Proteção Radiológica, EPM, UNIFESP, Rua Botucatu, 659, 04023-900 São Paulo, SP, Brazil. Fax: +55-11-5539-4208. E-mail: rbitelli.ddi@epm.br
Research supported by the Fundo de Auxílio aos Docentes e Alunos (FADA), UNIFESP/EPM. Publication supported by FAPESP. Received August 29, 2002. Accepted September 3, 2003.
- 1. Bitelli T (1982). Noções de física nuclear. In: Bitelli T (Editor), Dosimetria e Higiene das Radiações Grêmio Politécnico, São Paulo, SP, Brazil, 27-237.
- 2. Carvalho PR (1999). Produtos químicos e seus efeitos. In: Carvalho PR (Editor), Boas Práticas Químicas em Biossegurança Interciência, Rio de Janeiro, RJ, Brazil, 79-85.
- 3. Comissão Nacional de Energia Nuclear, Norma CNEN-NE.6.05 (1985). Gerência de rejeitos. In: Comissão Nacional de Energia Nuclear (Editor), Gerência de Rejeitos Radioativos em Instalações Radiativas, Resolução CNEN 19/85. CNEN, Rio de Janeiro, RJ, Brazil, 7-11.
- 4. Comissão Nacional de Energia Nuclear (1998). Rejeitos radioativos oriundos de atividades de pesquisa. In: Comissão Nacional de Energia Nuclear (Editor), Programa de Gerência de Rejeitos Radioativos em Pesquisa CNEN, Rio de Janeiro, RJ, Brazil, 13-15.
- 5. Meechan PJ (1997). Color quenching in "environmentally friendly" cocktails. Health Physics, 73: 808-813.
- 6. De Vol TA, Brown DD, Leyba JD & Fjeld RA (1996). A comparison of four aqueous-miscible liquid scintillation cocktails with an alpha/beta discriminating Wallac 1415 liquid scintillation counter. Health Physics, 70: 41-46.
- 7. Neame KD (1978). Sources of error in the channels ratio method for efficiency determination in liquid scintillation counting. Analytical Biochemistry, 91: 323-339.
- 8. Associação Brasileira de Normas Técnicas (1995). ABNT. NBR 10004. Resíduos Sólidos - Classificação ABNT, Rio de Janeiro, RJ, Brazil.
- 9. Associação Brasileira de Normas Técnicas (1995). ABNT. NBR 10007. Amostragem de Resíduos - Procedimento ABNT, Rio de Janeiro, RJ, Brazil.
Correspondence and Footnotes
Publication Dates
-
Publication in this collection
17 Nov 2003 -
Date of issue
Dec 2003
History
-
Accepted
03 Sept 2003 -
Received
29 Aug 2002