Abstract
Twelve female ponies were examined daily for 30 days and classified as ovulating (OV; N = 6; 197 ± 6 kg) or prepubertal (PP; N = 6; 196 ± 9 kg). Follicles were detected by ultrasound and gonadotropins quantified by radioimmunoassay. The mean diameter of the largest follicles was significantly larger in OV (38 ± 1 mm) than in PP (26 ± 2 mm) but there was no difference between groups in the size of the second largest follicle. There were more small follicles (<24 mm) in the PP than in the OV group, but PP fillies had a smaller number of follicles >29 mm than the OV fillies. Follicle-stimulating hormone (FSH) levels did not differ between groups but PP fillies had lower luteinizing hormone (LH) peak (8 ± 1 ng/ml) and basal (4 ± 0.5 ng/ml) levels, lower peak magnitude (2 ± 0.2 ng/ml) and period average (5 ± 0.6 ng/ml) than OV fillies (32 ± 4.5, 8 ± 1.2, 17.1 ± 6, and 15 ± 2.3 ng/ml, respectively). The PP group, in contrast to the OV group, showed no relationship between FSH surge and follicle wave emergence. We conclude that an LH concentration higher than 8 ng/ml is needed for follicle growth to a preovulatory size. Wave emergence and FSH secretion seem to be independent events, probably due to an inhibitory neural system in these PP animals. PP fillies may provide a physiological model for the study of follicle wave emergence which apparently does not depend on gonadotropin levels.
Follicles; FSH; LH and prepubertal fillies
Braz J Med Biol Res, June 2004, Volume 37(6) 913-922
Follicle profile and plasma gonadotropin concentration in pubertal female ponies
G.P. Nogueira
Departamento de Apoio, Produção e Saúde Animal, Faculdade de Medicina Veterinária, Universidade Estadual Paulista, Araçatuba, SP, Brasil
 References
References
 Correspondence and Footnotes
Correspondence and Footnotes
Correspondence and Footnotes
Correspondence and Footnotes
Correspondence and Footnotes
Correspondence and Footnotes
Abstract
Twelve female ponies were examined daily for 30 days and classified as ovulating (OV; N = 6; 197 ± 6 kg) or prepubertal (PP; N = 6; 196 ± 9 kg). Follicles were detected by ultrasound and gonadotropins quantified by radioimmunoassay. The mean diameter of the largest follicles was significantly larger in OV (38 ± 1 mm) than in PP (26 ± 2 mm) but there was no difference between groups in the size of the second largest follicle. There were more small follicles (<24 mm) in the PP than in the OV group, but PP fillies had a smaller number of follicles >29 mm than the OV fillies. Follicle-stimulating hormone (FSH) levels did not differ between groups but PP fillies had lower luteinizing hormone (LH) peak (8 ± 1 ng/ml) and basal (4 ± 0.5 ng/ml) levels, lower peak magnitude (2 ± 0.2 ng/ml) and period average (5 ± 0.6 ng/ml) than OV fillies (32 ± 4.5, 8 ± 1.2, 17.1 ± 6, and 15 ± 2.3 ng/ml, respectively). The PP group, in contrast to the OV group, showed no relationship between FSH surge and follicle wave emergence. We conclude that an LH concentration higher than 8 ng/ml is needed for follicle growth to a preovulatory size. Wave emergence and FSH secretion seem to be independent events, probably due to an inhibitory neural system in these PP animals. PP fillies may provide a physiological model for the study of follicle wave emergence which apparently does not depend on gonadotropin levels.
Key words: Follicles, FSH, LH and prepubertal fillies
Introduction
Puberty is the transitional period between sexual immaturity and maturity, after which animals reach the ability to reproduce for the preservation of the species. Each species has its own time course of puberty according to its life span (1). Puberty in fillies is generally considered to be at approximately 12 to 15 months if the animals were born early in the breeding season. Fillies born late may reach sexual maturation during the second spring of life (2). Ponies born during the previous summer and early autumn tend to have fewer ovulations and shorter breeding seasons than those born during the spring, whereas those born late in August and September do not ovulate during their first breeding season (3).
Follicular development occurs in waves in adult ponies, with the emergence of the wave coinciding with a surge in follicle-stimulating hormone (FSH) (4). A similar association between wave emergence and FSH surge (2) also occurs during the transitional period that precedes the onset of the ovulatory season in adults. In fillies, the association between gonadotropin changes and puberty has been described (3), but the association with follicular waves has not been studied.
In adults, major waves cause the differential development of a dominant follicle to 30 mm (5) in parallel to regression of the smaller subordinate follicles (4). Minor waves also develop in which dominant follicles do not differentiate (5) and the largest follicle reaches only 19 to 28 mm during the wave. The emergence of minor as well as major waves is associated with a surge in FSH concentrations (6). Surges in circulating FSH and luteinizing hormone (LH) concentrations are more synchronous during the luteal phase than during the follicular phase in mares (7,8).
In a previous study with prepubertal (PP) fillies aged 2-10 months, follicular waves and FSH surges were not temporally associated and the waves did not partition into dominant and subordinate follicles (9). The objective of the present study was to compare, during the peripubertal period, the follicular and gonadotropin characteristics of yearling fillies which did and did not ovulate by the end of spring of the year following their birth. Special consideration was given to temporal associations between changing FSH concentrations, emergence of waves and follicle diameter in fillies around the peripubertal period.
Material and Methods
Animals and ultrasound scanning
Twelve pony fillies born in April-July of the previous year (month unknown for each individual) and weighing 170-222 kg at the end of the experiment were used. The fillies were kept in an outdoor paddock and were maintained on alfalfa/grass hay with free access to water and trace-mineralized salt. The ultrasound scanner was equipped with a 5-MHz linear-array transrectal transducer (Tokyo Keiki LS 300; Products Group International, Lyons, CO, USA) for the examination of ovaries and uterus, performed as described previously (10). On June 22, the fillies were divided into two groups: those that had ovulated or appeared to be approaching ovulation (ovulatory or post-pubertal group, OV; N = 6) and those that had not (non-ovulatory or PP group; N = 6). The separation into groups was based on the presence or absence of a corpus luteum or a preovulatory-sized follicle (>30 mm). The experimental period of 30 days extended from June 23 to July 22 and the research was conducted in Cross Plains, WI, USA.
Follicles
On each day of the 30-day period, the three largest follicles per ovary were measured with the electronic calipers of the ultrasound. The others (>10 mm) were counted and their diameter was estimated using the centimeter scale displayed on the side of the ultrasound image. Follicles were grouped into diameter categories of 10-14, 15-19, 20-24, 25-29, and >30 mm. No attempt was made to maintain day-to-day identity of follicles except for follicles that attained 25 mm.
Follicular waves were detected and characterized according to a mathematical model described elsewhere (11). Briefly, each yearling was evaluated according to the following procedures: 1) constructing profiles of the diameters of each of the 3 largest follicles per ovary without considering day-to-day identification of follicles, but with maintenance of the distinction between left and right ovaries; 2) dividing the follicles into large and small categories using the largest diameter reached by the second largest follicle (large-category follicle) and the remaining subordinate follicles (small-category follicles); 3) using the large category to profile the diameters of individual large follicles, these values were plotted graphically in the figures; 4) using the 6 largest follicles per yearling in the small-follicle category to detect significant waves of follicular activity.
A significant (P < 0.05) increase followed by a significant decrease in mean diameter of the 6 largest small-category follicles per yearling, based on the Tukey multiple range test, was used to identify a follicular wave. If the placement of the nadir was not clear from the means because of fluctuations, the decision was supported by inspection of the diameter profile of the 3 largest follicles per ovary and by paired t-tests. The maximal diameter of the largest follicle and the second largest follicle and the difference in maximal diameter were also determined for each animal.
Blood sampling and hormone assay
Blood was collected daily by jugular venipuncture into heparinized tubes and refrigerated before being centrifuged for plasma separation. Plasma was then placed in polyethylene vials for cold storage (-20ºC) until the time for assay. Circulating concentrations of FSH (12) and LH (13) were determined using radioimmunoassays previously validated for this species. The intra-assay and inter-assay coefficients of variation and the sensitivity were 10.9%, 13.2% and 0.1 ng/ml for FSH and 5.9%, 9.4% and 0.8 ng/ml for LH, respectively, as determined in two assays each for FSH and LH. A technique developed to study episodic fluctuations in circulating hormones (14) was used to detect peak concentrations of FSH and LH in individual yearlings. This procedure had been used to detect FSH and LH surges in blood samples collected daily from monkeys during the menstrual cycle (15). Briefly, the program determines threshold concentrations of FSH or LH based on the within-day variability of the assay results between duplicate samples for each yearling. Concentrations higher than the threshold values were detected and identified as peak hormone concentrations. The FSH or LH surge in an individual yearling was defined by a progressive increase and decrease in concentrations higher than 1 ng/ml that encompassed a peak concentration (nadir-to-peak-to-nadir). The frequency of occurrence of individual FSH and LH surges, the number of FSH and LH surges per yearling and the magnitude of the surge were analyzed.
Statistical analysis
Analysis of variance (ANOVA) was used for sequential data to identify follicular waves in the mean values of small follicles, followed by the Tukey multiple range test. Main effects of group and day and group-by-day interaction were determined and when a significant main effect or interaction was detected, the Tukey multiple comparison test was used to determine the mean difference between groups within days and the paired t-test was used to determine the main difference between days within groups. Daily changes in hormone concentration and follicle profile centered on the largest follicle diameter for the PP fillies were compared among groups of yearlings until the last day on which the data from all fillies in each group could be included. ANOVA was used to determine a group effect for single-point measurements. To determine whether simultaneous peaks of both LH and FSH were occurring at random or not, the observed number of times that both surges occurred together was compared to the expected number if each surge occurred independently of the other. The expected frequencies of both hormone surges occurring on the same day were calculated from the observed occurrence of surges of each hormone during the 30-day period. For example, if the observed frequency of surges was 5/30 (17%) for LH and 8/30 (27%) for FSH the expected frequency of both surges occurring together would be 4.6% (17 x 27%). This expectancy calculation assumes that a surge occurs independently of the other (16). The observed values were compared to the expected values by chi-square analysis. Proportional data were examined by chi-square analysis to determine group effects. Data are reported as means ± SEM unless otherwise indicated. Significance was indicated by a probability of P < 0.05.
Results
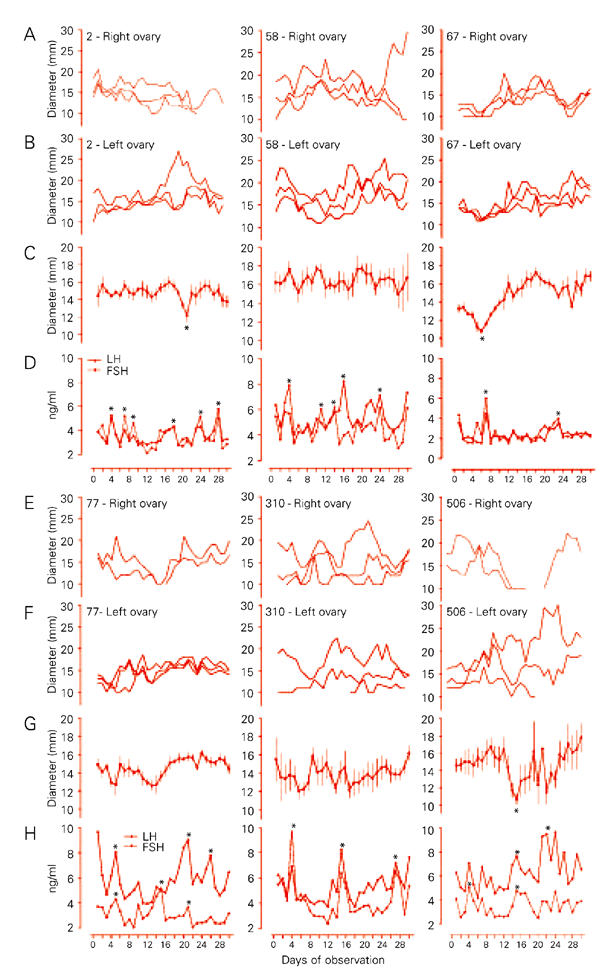
From the first ultrasound scan it was possible to divide the fillies into two categories, i.e., six fillies with larger follicles (³30 mm) or a corpus luteum, called OV, and 6 fillies with smaller follicles and without a corpus luteum, called PP. This first observation was consistent throughout the 30-day period when 6 fillies ovulated and 6 did not. The weight at the end of the experiment did not differ between groups (197 ± 6, 188-225, and 196 ± 9, 170-225 kg, respectively). The diameter of the largest follicle was wider for OV than for PP fillies (P = 0.001), but there was no significant difference in the diameter of the second largest follicle. There were more small follicles in PP than OV fillies. The mean ± SEM values for the follicle profile of OV and PP fillies are shown in Table 1. Most of the follicular waves did not partition into dominant and subordinate follicles (Figure 1).
Follicle profile and plasma gonadotropin concentration in pubertal female ponies. G.P. Nogueira. Brazilian Journal of Medical and Biological Research, 37 (6): 913, 2004.
Ovulating fillies had more circulating LH (highest value, lowest value, basal levels, average throughout the 30-day period) than PP fillies. Circulating FSH did not differ between groups. LH and FSH pulses occurred together more times than expected by chance in PP fillies. The gonadotropin end points during the 30-day period are shown in Table 2. Individual follicle profile, the average for the mathematical model and daily gonadotropin levels for the PP group are shown in Figure 1. Gonadotropin concentration was normalized according to the largest follicle diameter in the PP group and to the corresponding diameter in the OV fillies (Figure 2) to determine the interference of endocrine profile with follicle growth. At the time when the largest follicle stopped growing in PP fillies, LH level was lower than in OV fillies (P > 0.05) but there was no difference in FSH concentration. The diameters of the largest and second largest follicle were centered on the day of the main FSH surge during the 30-day period to determine the relationship between FSH secretion and wave emergence (Figure 3). PP fillies showed no variation in follicle diameter (average of the 6 largest ones) after the FSH peak, whereas OV fillies showed a significant increase in follicle diameter. There was no apparent relationship between gonadotropin secretion and follicle wave emergence in PP fillies.
Follicle profile and plasma gonadotropin concentration in pubertal female ponies. G.P. Nogueira. Brazilian Journal of Medical and Biological Research, 37 (6): 913, 2004.
Follicular data for each of the 6 prepubertal fillies that did not ovulate during the 30-day observation period. Panels A, B, E, F: diameter profiles of the 3 largest follicles in the right and left ovary without considering day-to-day identity. Panels C, G: day-to-day diameter profile of follicles in the large category and mean profile of the 6 largest follicles per filly after excluding the follicles in the large category. Panels D, H: LH and FSH profile during the 30-day period. Asterisks indicate differences between days. The numbers at the top of each panel indicate the number of the animal. FSH = follicle-stimulating hormone; LH = luteinizing hormone. P < 0.05 compared to the other days (paired t-test).
Follicle and gonadotropin profile normalized to the day of largest diameter for the prepubertal fillies (26 mm = day zero) and to the day of the corresponding follicle diameter for the ovulating fillies. Data are reported as means ± SEM for 6 animals in each group. FSH = follicle-stimulating hormone; LH = luteinizing hormone; OV = ovulating fillies; PP = prepubertal fillies. LH level differed (P < 0.01) between OV and PP only on day 0 (ANOVA repeated measures and Tukey test).
Largest (filled circles) and 2nd largest (open circles) follicle diameter centered on the higher value of FSH (filled lozenges). PP = prepubertal (left graph) and OV = ovulating (right graph) fillies. Data are reported as means ± SEM for 6 animals in each group. Differences between days are indicated by asterisks (ANOVA repeated measures and Tukey test).
Discussion
In PP fillies, follicle diameter increased to a maximum of 26 mm, after which it regressed without reaching a preovulatory size (>30 mm). The main characteristic of the endocrine profile at the time of follicle regression in PP fillies was lower circulating LH concentrations. Although low, LH levels did not interfere with the growth of the second largest follicle. In PP fillies there was no relationship between FSH secretion and wave emergence and the partition of dominant and subordinate follicles during a follicular wave was not evident. In cattle and sheep, the gonadal block is governed by the gonadostatic mechanism but in horses (3,9), as in primates (1), the PP hiatus is of central origin.
In the present experiment six fillies did not cycle over the 30-day experimental period (June-July). Fillies born during the first half of the year and well-nourished can be expected to reach puberty by spring of the next year (2,17). The phenomenon of puberty retardation when fillies are exposed prematurely to long photoperiods may account for the failure of fillies born in late summer to ovulate during the following summer. Such fillies would be exposed to increasing day length earlier and this may preclude ovulation at 12 months of age, delaying puberty until the following spring when they would be about 18 months old. Thus, a light-controlled puberty retardation phenomenon may have evolved in late born fillies so that puberty does not occur during the following year (17).
The largest follicle diameter during the 30-day period was larger in the OV group than in the PP group, but the diameter of the second largest follicle did not differ between groups. The daily difference between the largest and second largest follicles was higher for OV than PP, as also was the difference between the day of the largest follicle diameter minus the diameter of the second largest follicle (among the 30 days; Table 1). Follicles grew to a certain diameter throughout the PP period, after which they regressed as a consequence of the hormonal milieu during the PP period.
The PP fillies had more follicles smaller than 24 mm but fewer follicles larger than 24 mm. The higher number of small follicles for the PP group may be a consequence of the lack of interaction between follicles and hypothalamus-pituitary axis. Since the largest follicle grew beyond dominance diameter it should have been able to exert dominance over other follicles (18), but this was not the case.
A distinct follicle could be observed in fillies 2 and 506 that apparently exerted dominance since the diameter of other follicles diminished during this period (Figure 1). The reduction in diameter of the subordinate follicles appeared as a significant decrease in the average for the follicles. This event was not observed in the other fillies during the 30-day period, a fact possibly reflecting the sexual maturation of these fillies. There was a broad variation in the apparent follicular waves of the PP group, and in addition daily FSH concentrations fluctuated widely, as indicated by irregular levels (Figure 1). This seems to be similar to the minor follicle waves observed in adults, in which the largest follicle reaches only 19 to 28 mm and does not differentiate into the dominant follicle during the wave (5) but in which, in contrast to these fillies, an FSH surge occurs prior to the wave emergence.
FSH varied throughout the experimental period but its levels were similar in the two groups, whereas LH levels were apparently suppressed in PP fillies. The central inhibition occurring in PP fillies seems to be similar to that observed in primates, in which the activity of the hypothalamic-pituitary axis is arrested in late infancy, gonadotropin secretion declines to low levels characteristic of PP primates, and the juvenile period of gonadal quiescence is initiated. The gonadostatic hypothesis, whereby an enhanced sensitivity of the gonadostat must be the major determinant underlying the diminished secretion of gonadotropin, is not applicable to higher primates (19) or horses (3).
Centralizing the hormone and gonadotropin profile to the largest follicle diameter in the PP group proved to be illustrative about the mechanism involved in the inhibition of follicle increase up to a certain diameter (Figure 2). The largest follicle grew to 26 ± 2 mm in the PP group and then regressed, while in the OV fillies it grew until ovulation (38.3 ± 1 mm). At the time when the largest follicle started to regress in the PP group, FSH showed the same concentration in both groups (OV fillies: 3.34 ± 0.5 ng/ml, PP: 2.67 ± 0.5 ng/ml), whereas LH levels were higher in OV fillies (9.56 ± 1.7 ng/ml) compared to PP (5.01 ± 0.9 ng/ml). As shown for adults (20), the most important gonadotropin that governs follicle growth after deviation, around 22 mm, is LH. A decline in FSH concentration occurred in OV fillies from day -7 to day 0 as the largest follicle was growing (Figure 2). Low FSH has been postulated to play a role in follicle deviation (7); however, this decline in FSH was not observed in the PP fillies (Figure 2), probably due to lack of LH (20). The lack of circulating LH interfered with the development of the largest follicle but not of the second largest follicle. The reduced LH concentration in the PP fillies was observed throughout the 30-day period. The maximum diameter of the second largest follicle in PP fillies was not different from this endpoint in OV fillies. A low LH concentration did not reduce the growth rate of the second largest follicle during the study period because the size attained by the 2nd largest follicle (19 ± 1 mm) was not enough for the LH requirement that occurs after the deviation diameter.
A deviation mechanism was not apparently activated in most PP fillies either because of low LH concentration or because of the central origin of the puberal hiatus. These results indicate that LH was needed for continued growth of the largest follicle after 25 mm. A temporal indication for a role of LH in the continued growth of the largest follicle after the beginning of deviation is that a transient elevation in LH concentration usually encompasses the day of deviation in mares (4,20). FSH levels were similar in the OV and PP groups but PP fillies had lower LH values for most of the end points, except for the number of surges and number of days between surges (Table 2).
There was a greater association between the LH and FSH surges, and the number of surges occurring in association was larger than that expected to occur by chance in the PP but not in the OV fillies (Table 2). In pregnant mares there seems to be a relationship between periodic FSH fluctuations (frequency and magnitude) and the pattern and type of follicular wave. Wave characteristics ranged from rhythmical occurrence with a distinct dominant follicle to sporadic occurrence of non-prominent waves without dominance (6). Without central interference from the follicles during central gonadotropin secretion suppression, either before puberty (3,9) or during the luteal phase (21) the fluctuations of LH and FSH seem to occur in a synchronous manner, with a higher degree of coupling between surges.
Normalizing FSH surge with follicle diameter average allowed us to reach the conclusion that there is no relationship between FSH surge and follicle wave emergence in non-ovulating fillies (Figure 3). Prior to puberty, the follicles grew and regressed in an apparent wave fashion but never attained the preovulatory size. This behavior is similar to the minor waves that occur in mares, without the development of a dominant follicle (5,6). Gonadotropin release in the adult is regulated by negative feedback of the gonadal hormones on LH and FSH secretion that may be exerted either directly on the gonadotropes to suppress their responsiveness to GnRH stimulation or indirectly at a suprapituitary level to modulate the frequency and/or amplitude of the GnRH pulse generator (22). In the immature filly, low LH levels do not provide the necessary stimulus for complete follicular development after the deviation diameter, and therefore the sustained rise in estradiol cannot be produced to activate the preovulatory surge mechanism.
Thus, it is possible to conclude that PP fillies can be used as a model for the study of intraovarian factors involved in follicle growth since they have a milieu with low LH levels and no apparent relationship between FSH level and wave emergence.
Acknowledgments
We are grateful to the National Hormone and Pituitary Program (NIDDK, Dr. A.F. Parlow) for providing equine FSH and LH (eFSH and eLH) RIA materials, and to O.J. Ginther for providing the animals and hormone measurements.
Address for correspondence: G.P. Nogueira, Departamento de Apoio, Produção e Saúde Animal, UNESP, 16050-680 Araçatuba, SP, Brasil. Fax: +55-18-3622-6487. E-mail: gpn@fmva.unesp.br
Presented at the Annual Conference of the International Embryo Transfer Society in Foz do Iguaçu, PR, Brazil in 2002, and reported in abstract form (Theriogenology, 57: 617, 2002). Research supported by Equiservice Publishing and by EquiCulture, Inc., Cross Plains, WI, USA. G.P. Nogueira was the recipient of a FAPESP fellowship (No. 98-00287-6) and UNESP. Received June 9, 2003. Accepted January 27, 2004.
- 1. Therasawa E & Fernandez DL (2001). Neurobiological mechanisms of the onset of puberty in primates. Endocrine Reviews, 22: 111-151.
- 2. Ginther OJ (1992). Reproductive Biology of the Mare, Basic and Applied Aspects 2nd edn. Equiservices Publishing, Cross Plains, WI, USA.
- 3. Wesson JA & Ginther OJ (1981). Puberty in the female pony: reproductive behavior, ovulation, and plasma gonadotropin concentration. Biology of Reproduction, 24: 977-986.
- 4. Gastal EL, Gastal MO, Bergfelt DR & Ginther OJ (1997). Role of diameter difference among follicles in selection of a future dominant follicle in mares. Biology of Reproduction, 57: 1320-1327.
- 5. Ginther OJ (1993). Major and minor waves during the equine estrous cycle. Journal of Equine Veterinary Science, 13: 18-25.
- 6. Ginther OJ & Bergfelt DR (1992). Association between FSH concentrations and major and minor follicular waves in pregnant mares. Theriogenology, 38: 817-821.
- 7. Bergfelt DR & Ginther OJ (1993). Synchronous fluctuations of LH and FSH in plasma samples collected daily during the estrous cycle in mares. Theriogenology, 40: 1137-1145.
- 8. Irvine CHG, Turner JE, Alexander SL, Shand N & van Noordt S (1998). Gonadotrophin profiles and dioestrous pulsatile release patterns in mares as determined by collection of jugular blood at 4 h intervals throughout an oestrous cycle. Journal of Reproduction and Fertility, 113: 315-322.
- 9. Nogueira GP & Ginther OJ (2000). Dynamics of follicle populations and gonadotropin concentrations in fillies age two to ten months. Equine Veterinary Journal, 32: 482-488.
- 10. Ginther OJ (1995). Ultrasonic Imaging and Animal Reproduction: Horses Book 2. Equiservices Publishing, Cross Plains, WI, USA.
- 11. Ginther OJ & Bergfelt DR (1992). Ultrasonic characterization of follicular waves in mares without maintaining identity of individual follicles. Journal of Equine Veterinary Science, 12: 349-354.
- 12. Freedman LJ, Garcia MC & Ginther OJ (1979). Influence of photoperiod and ovaries on seasonal reproductive activity in mares. Biology of Reproduction, 20: 567-574.
- 13. Whitmore HL, Wentworth BC & Ginther OJ (1973). Circulating concentrations of luteinizing hormone during estrous cycle of mares as determined by radioimmunoassay. American Journal of Veterinary Research, 34: 631-636.
- 14. Clifton DK & Steiner RA (1983). Cycle detection: A technique for estimating the frequency and amplitude of episodic fluctuations in blood hormones and substrate concentrations. Endocrinology, 112: 1057-1064.
- 15. Matteri RL, Bridson WE, Dierschkle DL, Wegner FH & Durning M (1992). The secretion of bioactive and immunoreactive follicle-stimulating hormone (FSH) and luteinizing hormone (LH) through the menstrual cycle of the rhesus monkey. American Journal of Primatology, 26: 243-257.
- 16. Ginther OJ (1984). Mobility of the twin embryonic vesicle in mares. Theriogenology, 22: 83-95.
- 17. Wesson JA & Ginther OJ (1982). Influence of photoperiod on puberty in the female pony. Journal of Reproduction and Fertility, 32 (Suppl): 269-274.
- 18. Gastal EL, Gastal MO & Ginther OJ (1999). Experimental assumption of dominance by a smaller follicle and associated hormonal changes in mares. Biology of Reproduction, 61: 724-730.
- 19. Levine Ross J, Loriaux DL & Cutler Jr GB (1983). Developmental changes in neuroendocrine regulation of gonadotropin secretion in gonadal dysgenesis. Journal of Clinical Endocrinology and Metabolism, 57: 288-293.
- 20. Gastal EL, Bergfelt DR, Nogueira GP, Gastal MO & Ginther OJ (1999). Role of luteinizing hormone in follicle deviation based on manipulating progesterone concentrations in mares. Biology of Reproduction, 61: 1492-1499.
- 21. Bergfelt DR & Ginther OJ (1992). Relationship between circulating concentrations of FSH and follicular waves during early pregnancy in mares. Equine and Veterinary Science, 12: 274-279.
- 22. Plant TM (1986). A striking sex difference in the gonadotropin response to gonadectomy during infantile development in the rhesus monkey (Macaca mulatta). Endocrinology, 119: 539-545.
Correspondence and Footnotes
Publication Dates
-
Publication in this collection
27 May 2004 -
Date of issue
June 2004
History
-
Received
09 June 2003 -
Accepted
27 Jan 2004