Abstract
T-type Ca2+ channels are important for cell signaling by a variety of cells. We report here the electrophysiological and molecular characteristics of the whole-cell Ca2+ current in GH3 clonal pituitary cells. The current inactivation at 0 mV was described by a single exponential function with a time constant of 18.32 ± 1.87 ms (N = 16). The I-V relationship measured with Ca2+ as a charge carrier was shifted to the left when we applied a conditioning pre-pulse of up to -120 mV, indicating that a low voltage-activated current may be present in GH3 cells. Transient currents were first activated at -50 mV and peaked around -20 mV. The half-maximal voltage activation and the slope factors for the two conditions are -35.02 ± 2.4 and 6.7 ± 0.3 mV (pre-pulse of -120 mV, N = 15), and -27.0 ± 0.97 and 7.5 ± 0.7 mV (pre-pulse of -40 mV, N = 9). The 8-mV shift in the activation mid-point was statistically significant (P < 0.05). The tail currents decayed bi-exponentially suggesting two different T-type Ca2+ channel populations. RT-PCR revealed the presence of a1G (CaV3.1) and a1I (CaV3.3) T-type Ca2+ channel mRNA transcripts.
Calcium channel; T-type; Electrophysiology; RT-PCR; GH3 cells
Braz J Med Biol Res, June 2004, Volume 37(6) 929-935
Ca V 3.1 and Ca V 3.3 account for T-type Ca 2+ current in GH3 cells
M.A. Mudado1, A.L. Rodrigues1, V.F. Prado2, P.S.L. Beirão1 and J.S. Cruz1
Laboratórios de 1Membranas Excitáveis and 2Neurobiologia Molecular, Departamento de Bioquímica e Imunologia, Instituto de Ciências Biológicas, Universidade Federal de Minas Gerais, Belo Horizonte, MG, Brasil
 References
References
 Correspondence and Footnotes
Correspondence and Footnotes
Correspondence and Footnotes
Correspondence and Footnotes
Correspondence and Footnotes
Correspondence and Footnotes
Abstract
T-type Ca2+ channels are important for cell signaling by a variety of cells. We report here the electrophysiological and molecular characteristics of the whole-cell Ca2+ current in GH3 clonal pituitary cells. The current inactivation at 0 mV was described by a single exponential function with a time constant of 18.32 ± 1.87 ms (N = 16). The I-V relationship measured with Ca2+ as a charge carrier was shifted to the left when we applied a conditioning pre-pulse of up to -120 mV, indicating that a low voltage-activated current may be present in GH3 cells. Transient currents were first activated at -50 mV and peaked around -20 mV. The half-maximal voltage activation and the slope factors for the two conditions are -35.02 ± 2.4 and 6.7 ± 0.3 mV (pre-pulse of -120 mV, N = 15), and -27.0 ± 0.97 and 7.5 ± 0.7 mV (pre-pulse of -40 mV, N = 9). The 8-mV shift in the activation mid-point was statistically significant (P < 0.05). The tail currents decayed bi-exponentially suggesting two different T-type Ca2+ channel populations. RT-PCR revealed the presence of a1G (CaV3.1) and a1I (CaV3.3) T-type Ca2+ channel mRNA transcripts.
Key words: Calcium channel, T-type, Electrophysiology, RT-PCR, GH3 cells
Introduction
The entry of calcium into cells through voltage-gated Ca2+ channels is responsible for a number of intracellular signaling events. Molecular cloning studies have characterized at least 10 genes encoding six different Ca2+ channels (1). Much of the effort devoted to gathering information to elucidate their mechanisms of action is based on the use of heterologous expression systems to avoid contaminating currents. The T-type Ca2+ channel subfamily consists of three genes called a1G, a1H, and a1I (2,3). Many reports have characterized both the biophysical and pharmacological features of each member of this subfamily (4-7).
GH3 cells are clonal somatomammotropic cells that have been used to study the mechanisms underlying prolactin and growth hormone secretion. As is the case for other pituitary cells, hormone secretion by GH3 cells is regulated by free intracellular Ca2+ concentration, which is controlled by Ca2+ influx through voltage-dependent Ca2+ channels. Under normal conditions, two types of Ca2+ currents can be demonstrated in GH3 cells: L-type, high voltage-activated and T-type, low voltage-activated Ca2+ currents (8). Whereas L-type Ca2+ channels have been extensively studied, the absence of a pharmacological agent specific for T-type Ca2+ channels has hampered the study of their physiological role. As a consequence, few reports are available concerning T-type Ca2+ currents in GH3 cells. In a recent paper (9) the functional expression of T-type Ca2+ channels in one sub-clonal line (GH3/B6) related to GH3 cells was reported. These investigators were able to isolate a very small and infrequent (~10% of the cells) Ca2+ current, which they considered to be similar to T-type Ca2+ currents. They also reported the presence of only an a1G transcript. These two cell lines differ in some aspects, with GH3/B6 expressing more prolactin than GH3 cells. The molecular basis for the differences between these pituitary cell lines has not been determined. We then asked the following questions: What is the profile of T-type Ca2+ channel expression for GH3 cells? Can we measure T-type Ca2+ currents reliably? In order to answer these questions we used electrophysiological and RT-PCR techniques to characterize the T-type channel a1 subunits in neuroendocrine GH3 cells.
Material and Methods
RNA extraction and RT-PCR
Total RNA was extracted from GH3 cells using TRIZOL (Gibco BRL, Gaithersburg, MD, USA) according to manufacturer's instructions, and treated with deoxyribonuclease I (Gibco BRL). RT-PCR was carried out using the access RT-PCR introductory kit from Promega (Madison, WI, USA). The primers used (10) were a1G (5' CAG GAG ACG AAA CCT TGA 3' and 5' GAC GAG GAT AAG ACG TCT 3'), a1I (5' CAG GAT CCG GAA CTT GTT 3' and 5' GAT GAG GAC CAG AGC TCA 3'), and ß-actin (5' GTT CCG ATG CCC CGA GGA TCT 3' and 5' GCA TTT GCG GTG CAC GAT GGA 3'). Each tube contained 10 µl avian myeloblastosis virus (AMV)/Tfl 5X buffer, 10 mM dNTP, 25 µM each primer, 0.1 u/µl AMV reverse transcriptase (RT), 0.1 u/µl Tfl DNA polymerase, 0.15 µg/µl DNA-free RNA, and 29 µl nucleotide-free water to a final volume of 50 µl. Tubes were warmed to 48ºC for 45 min to allow reverse transcription and immediately submitted to amplification steps consisting of 2 min of denaturation at 94ºC followed by 40 cycles (30 s at 94ºC, 1 min at 55ºC, 2 min at 65ºC). PCR products were analyzed in a 0.8% agarose gel stained with ethidium bromide. For some unknown reason, primer used to visualize the a1H (CaV3.2) isoform did not work properly and we could not determine with certainty if GH3 cells expressed or not the CaV3.2 T-type Ca2+ channels.
Cell culture and electrophysiology
GH3 cells acquired from ATCC (Rockville, MD, USA) were cultured and maintained as described elsewhere (11,12). The standard pipette solution contained 100 mM CsCl, 10 mM EGTA, 2 mM MgCl2, and 40 mM HEPES, pH adjusted to 7.2 with CsOH. In some experiments we used fluoride-rich internal solutions that contained 80 mM CsF, 20 mM CsCl, 2 mM MgCl2, 10 mM EGTA, and 40 mM HEPES, pH 7.2 with CsOH to accelerate the run-down of high voltage-activated Ca2+ currents (13). The external solution contained 130 mM CsCl, 5 mM CaCl2, 0.5 mM MgCl2, 10 mM HEPES, and 5 mM glucose, pH adjusted to 7.4 with CsOH. In some experiments we used 10 µM nicardipine to block the L-type Ca2+ current but unfortunately we found that this concentration also inhibited the T-type Ca2+ current. Thereafter, we did not use nicardipine. All experiments were carried out at room temperature (22-25o). To analyze the voltage dependence for activation a modified Boltzmann equation was used to fit the experimental points:
I/Imax = (1 + A)/{1 + exp[(V1/2 - Vm)/ k]} + C, where I is the current amplitude, Imax is the maximal current, V1/2 is the activation midpoint, Vm is the membrane voltage, k is the slope factor, and A and C are the offsets. Inactivation and deactivation kinetics were fitted to single or double exponentials.
An EPC-9 patch-clamp amplifier and pulse software were used to measure whole-cell Ca2+ currents. Capacitive currents were compensated electronically and a P/4 protocol (14) was used for linear leak and residual capacitance subtraction. Ca2+ currents were low pass filtered at 2.5 kHz and sampled at 10 or 25 kHz. Pipettes were pulled from glass capillaries (Perfecta, São Paulo, SP, Brazil) and had resistances of 2-4 MW when filled with pipette solution. The holding potential used to measure ICa was -80 mV and a pre-pulse to -120 mV for 500 ms was applied immediately prior to the test pulses unless otherwise described. Cell membrane capacitances, used to normalize current amplitudes and thus minimize variance caused by differences in cell size, were measured automatically using a built-in routine in the Pulse software. Series resistance was compensated by 30 to 60%. Patch-clamp experiments were performed on 35-mm Petri dishes using an inverted microscope (Olympus, New Hide Park, NY, USA) with a 40X phase contrast objective. The bath was perfused continuously at 1-2 ml/min throughout the experiment. Solutions were gravity fed to the input ports of a solenoid valve mounted close to the bath, which was used to choose between one of two solutions. Further data were processed with Excel (Microsoft, Redmond, WA, USA) and SigmaPlot (SPSS Inc., Chicago, IL, USA).
Data are reported as means ± SEM and were analyzed statistically by the Student t-test, with the level of significance set at P < 0.05. Exponential fits were calculated by the method of Levenberg-Marquardt. The F-test for comparing two models such as single or double exponential fits was used.
Results
The whole-cell Ca2+ currents displayed in Figure 1 were evoked by a pair of 120-ms square pulses to 0 mV, with a 10-ms interval to assure that T-type channels would not fully recover from inactivation. A rapidly decaying inward current followed by a sustained component was recorded in response to the first pulse (see Figure 1A). Since T-type Ca2+ channels recover from inactivation slowly (~300 ms is a typical value; 1) the second Ca2+ current elicited in response to a step depolarization after the 10-ms interval at -80 mV showed only the sustained component. The digital subtraction of the second current record from the first revealed a fast inactivating inward Ca2+ current (Figure 1B). The current inactivation at 0 mV was described by a single exponential function with a time constant of 18.32 ± 1.87 ms (N = 16).
Figure 2A shows a family of current traces recorded in response to a voltage-clamp protocol. A conditioning step to -120 mV was applied for 500 ms to recruit T-type Ca2+ channels from inactivation, and then the voltage was increased to various membrane potentials from -60 to -10 mV. When the cell was depolarized to potentials more positive than -60 mV a transient inward current appeared at the onset of the test pulse followed by a sustained component that showed very slow decay.
Figure 2B illustrates sample current recordings elicited by voltage steps from -50 to -10 mV. Importantly, a 500-ms conditioning pulse to -40 mV was applied to inactivate T-type Ca2+ channels. All recorded currents decayed more slowly. Figure 2C and D shows the peak current normalized by the cell capacitance and plotted as a function of membrane voltage. Composite data indicate that the current density obtained from -120 mV (Figure 2C) was higher than that obtained by subtracting current traces between the two different holding potentials (Figure 2D, open circles). The continuous line (Figure 2D) represents the I-V relationship as plotted in Figure 2C. As indicated, the difference-Ca2+ current began to appear at -60 mV and peaked at about -30 mV (Figure 2D, open circles). Our results revealed the presence of a low voltage-activated Ca2+ current in GH3 cells which is consistent with reported data (13).
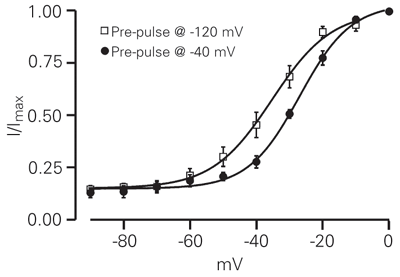
To characterize the activation properties we generated normalized current-voltage curves (Figure 3). The half-maximal voltage activation (V1/2) and the slope factors (k) for the two conditions are -35.02 ± 2.4 and 6.7 ± 0.3 mV (Figure 3, pre-pulse of -120 mV, squares, N = 15), and -27.0 ± 0.97 and 7.5 ± 0.7 mV (Figure 3, pre-pulse of -40 mV, circles, N = 9). The 8-mV shift in the activation mid-point was statistically significant (P < 0.05).
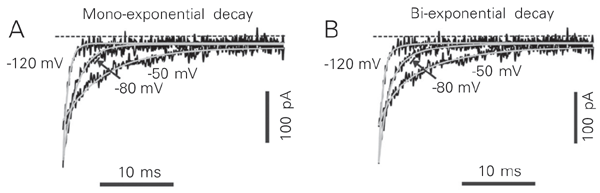
A distinguishing feature of native T-type Ca2+ channels is their slow deactivation kinetics (1). The rate of deactivation was measured as the rate of decay of the tail currents at different repolarizing membrane potentials (Figure 4 and Table 1). Somewhat surprisingly, the tail currents were best fitted by a double exponential, an unexpected finding since deactivation kinetics described for T-type Ca2+ current should follow a mono-exponential decay (10). However, there are recent reports suggesting that CaV3.3 (a1I) channels close in a biexponential manner (15-17). It has been well established that GH3 cells contain two types of voltage-dependent Ca2+ channels (L- and T-type channels) (8,18). The voltage dependence of deactivation was examined in a potential range (-120 to -60 mV) to avoid any interference by L-type Ca2+ channels (18). It is possible that the protocol used to measure tail currents was revealing L-type Ca2+ channel deactivation along with T-type deactivation. This could account, at least in part, for the fast component of the tail currents. Another explanation is that GH3 cells express more than one T-type Ca2+ channel and, as suggested above, the CaV3.3 (a1I) channel actually deactivates faster than the other T-type channels (19). Prompted by a study describing the acceleration of L-type Ca2+ channels run-down by substituting fluoride for chloride in the pipette solution (13), we performed a similar analysis in which the L-type Ca2+ current was presumably abolished, allowing us to record a "pure" T-type Ca2+ current. Figure 4B shows a family of tail currents recorded from a GH3 cell. The tail current decay was well described by the sum of two exponentials (see also Table 1). The fitted time constants are shown in Figure 4D. Figure 5 illustrates the tail currents from Figure 4B on a faster time scale. Over the range of voltages at which channels deactivate significantly, the tail currents were better described by the sum of two exponentials (Figure 5B). Although only 30 ms is shown in Figure 5, the currents were fitted over the entire 100-ms pulse duration. To our surprise, the fast and slow time constants measured in fluoride-rich internal solution showed some differences when compared with those measured in chloride-rich internal solutions.
CaV 3.1 and CaV 3.3 account for T-type Ca2+ current in GH3 cells. M.A. Mudado, A.L. Rodrigues, V.F. Prado, P.S.L. Beirão and J.S. Cruz. Brazilian Journal of Medical and Biological Research, 37 (6): 929, 2004.
These findings are consistent with the idea of two isoforms being expressed in GH3 cells with different deactivation kinetics. An alternative view would be that the GH3 cells do express only one type of low voltage-activated channel, which deactivates bi-exponentially in a way similar to the CaV3.3 (a1I) channel.
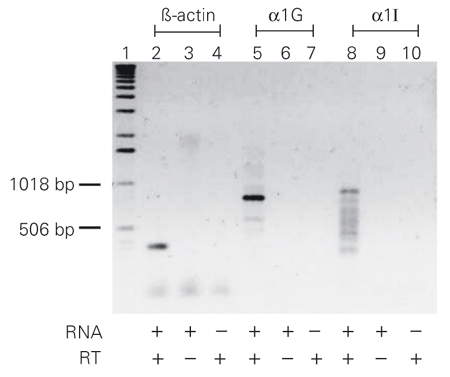
As our time constants of deactivation were not sufficiently accurate to indicate which subunits are expressed in GH3 cells, we carried out an RT-PCR with primers specific for a1G (Figure 6, lanes 5 to 7) and a1I (Figure 6, lanes 8 to 10). The total extracted RNA was treated with DNAse to ensure that no genomic DNA was present when the reaction was performed. Lanes 5 and 8 show transcripts with molecular weights of ~800 bp and ~870 bp that are in agreement with previous sequencing data for the a1G and a1I subunits (10). We also used primers specific for ß-actin as a positive control (Figure 6, lanes 2 to 4).
The presence of transcripts for a1G and a1I is additional evidence that the GH3 cells are probably expressing these two functional subunits. This argument is supported by the presence of two distinct deactivation time constants for the Ca2+ currents when fluoride was used in the internal medium.
Whole-cell inward Ca2+ currents evoked by a double-pulse protocol in GH3 neuroendocrine cells. A, Representative traces in response to step depolarization to 0 mV for 120 ms from a holding potential of -80 mV. The first current trace corresponds to the activation of both T-type (transient) and L-type Ca2+ (sustained) currents. The second trace was recorded after a brief time at -80 mV (10 ms) and then a return to 0 mV. The recorded current trace represents only L-type Ca2+ current (sustained) because T-type channels were inactivated. B, Current trace obtained by digitally subtracting the current evoked after the pre-pulse from that before the pre-pulse as in A.
Current-voltage relationships (I-V) in GH3 cells. A, Current traces evoked by depolarizations from -60 mV to -20 mV (holding potential -80 mV, pre-pulse -120 mV). B, Current traces elicited by depolarizations from -50 mV to -10 mV (holding potential -80 mV, pre-pulse -40 mV). C, Averaged density current-voltage relationship obtained for GH3 Ca2+ currents (filled circles, N = 15). Currents were normalized to cell capacitance to avoid variability due to cell size. D, Averaged current density-voltage relationships for peak currents obtained using a pre-pulse at -120 mV (continuous line), and after subtracting current traces between the two different holding potentials (Figure 2D, open circles, N = 7). All recordings were done using 5 mM calcium as the charge carrier. Data in panels C and D are reported as means ± SEM.
Voltage-dependent activation of GH3 Ca2+ currents. The peak current at each potential was normalized to the maximum peak current and was averaged for each potential. The symbols indicate composite data obtained from a pre-pulse set at -120 mV (squares, N = 15) and from a pre-pulse at -40 mV (circles, N = 9). Solid lines represent the fitting of Boltzmann equations with half-activation voltages of -35.02 mV (pre-pulse at -120 mV) and -27.04 mV (pre-pulse at -40 mV) and slope factors of 6.65 (pre-pulse at -120 mV) and 7.51 (pre-pulse at -40 mV).
Voltage dependence of deactivation kinetics. A, Representative calcium current tail traces evoked by different repolarizing potentials between -120 and -30 mV. The pipette solution contained a high chloride concentration. B, Representative calcium tail currents. The voltage protocol was the same as in A. The pipette solution had a high concentration of fluoride ion. C, Plot of mean deactivation time constants (t) against repolarization potentials. Data points are the two time constants, which represent the fast (open circles, N = 8) and slow components of the tail (filled circles, N = 8) current decay shown in panel A. Also shown is the weighted t (tw, triangles) that was derived using the equation: tw = A1tfast + A2tslow, where A1 and A2 are the normalized amplitudes of the fast and slow components. D, Deactivation time constants plotted as in C. The fast (open circles, N = 4) and slow (filled circles, N = 4) components are represented and were taken from current traces as shown in panel B. The triangles indicate the weighted time constant.
Exponential fits to tail currents. Records are from Figure 4B on an expanded time scale. The thicker smooth gray lines are fits to a single exponential (A, left) or the sum of two exponentials (B, right).
T-type Ca2+ channel subtypes in rat GH3 cells. RT-PCR performed to identify the presence of mRNA for the a1G and a1I subunits in GH3 cells. The positive control contained primers specific for ß-actin (lane 2). Negative controls did not include RNA or reverse transcriptase (RT) in the tubes (lanes 3, 4, 6, 7, 9 and 10).
Discussion
The use of GH3 as a model cell for testing drugs and toxins and for studying hormone secretion has been well established. The importance of knowing which types of channels are present in these cells is obvious since specific drugs can modulate channels in different ways.
In general, T-type Ca2+ channels display either similar or smaller peak currents upon replacement of Ca2+ with Ba2+ (20). Accordingly, in the present study we measured Ba2+ currents which were found to be identical to the currents measured when Ca2+ was used (data not shown). These results allow us to suggest that GH3 cells may not express the a1H isoform, which has been shown to conduct Ba2+ better than Ca2+ (10).
Close inspection of our I-V data (Figure 2C and D) shows that the 500-ms depolarizing pre-pulse to -40 mV seems not to prevent Ca2+ currents from being activated at relatively low membrane potentials (~-50 mV). As discussed above, this finding suggests the presence of low voltage-activated currents generated by L-type channels belonging to the a1D isotype. Since these channels deactivate faster than T-type channels we wondered if the time constants calculated using chloride-rich internal solutions (Figure 4C) were somehow faster than expected because of the contribution to the decay coming from deactivation of the a1D channels.
We examined the expression of T-type Ca2+ channel subunits in GH3 cells to determine which subunits were present. Our results suggest that transient inward current flows in a combination of a1G and a1I channels. We used RT-PCR with primers specific for T-type Ca2+ channel subunit mRNA and obtained PCR products for a1G and a1I T-type Ca2+ channels (Figure 6). The presence of the a1G subunit in GH3 cells has been previously suggested (1) and is now confirmed by our data. In addition, we report the presence of the a1I subunit as a new finding.
In this report, we identified the molecular nature of T-type Ca2+ channels selectively expressed in the neuroendocrine GH3 cells. The combination of biophysical and molecular biological data led us to conclude that T-type Ca2+ currents in GH3 cells arise from a1G and a1I isoforms. Why do GH3 cells express two distinct T-type Ca2+ channels? Do they make different contributions to the electrical properties of GH3 cells? An exciting possibility is the implication of transient increases in [Ca2+]i afforded by the differential expression profile of T-type Ca2+ channels in cell proliferation control.
Address for correspondence: J.S. Cruz, Departamento de Bioquímica e Imunologia, ICB, UFMG, 31270-901 Belo Horizonte, MG, Brasil. Fax: +55-31-3441-5963. E-mail: jcruz@icb.ufmg.br
Research supported by CNPq, CAPES and TWAS. J.S. Cruz, V.F. Prado and P.S.L. Beirão are recipients of CNPq research fellowships. Received June 2, 2003. Accepted February 26, 2004.
- 1. Perez Reyes E (2003). Molecular physiology of low-voltage-activated T-type calcium channels. Physiological Reviews, 83: 117-161.
- 2. Huguenard JR (1998). Low-voltage-activated (T-type) calcium-channel genes identified. Trends in Neurosciences, 21: 451-452.
- 3. Perez Reyes E (1999). Three for T: molecular analysis of the low voltage-activated calcium channel family. Cellular and Molecular Life Sciences, 56: 660-669.
- 4. Berthier C, Monteil A, Lory P & Strube C (2002). Alpha(1H) mRNA in single skeletal muscle fibres accounts for T-type calcium current transient expression during fetal development in mice. Journal of Physiology, 539: 681-691.
- 5. Chemin J, Monteil A, Dubel S, Nargeot J & Lory P (2001). The alpha1I T-type calcium channel exhibits faster gating properties when overexpressed in neuroblastoma/glioma NG 108-15 cells. European Journal of Neuroscience, 14: 1678-1686.
- 6. Chemin J, Monteil A, Bourinet E, Nargeot J & Lory P (2001). Alternatively spliced alpha(1G) (Ca(V)3.1) intracellular loops promote specific T-type Ca(2+) channel gating properties. Biophysical Journal, 80: 1238-1250.
- 7. Todorovic SM, Perez-Reyes E & Lingle CJ (2000). Anticonvulsants but not general anesthetics have differential blocking effects on different T-type current variants. Molecular Pharmacology, 58: 98-108.
- 8. Armstrong CM & Matteson DR (1985). Two distinct populations of calcium channels in a clonal line of pituitary cells. Science, 227: 65-66.
- 9. Glassmeier G, Hauber M, Wulfsen I, Weinsberg F, Bauer CK & Schwarz JR (2001). Ca2+ channels in clonal rat pituitary cells (GH3/B6). Pflügers Archiv, 442: 577-587.
- 10. McRory JE, Santi CM, Hamming KS et al. (2001). Molecular and functional characterization of a family of rat brain T-type calcium channels. Journal of Biological Chemistry, 276: 3999-4011.
- 11. Kushmerick C, Kalapothakis E, Beirao PS et al. (1999). Phoneutria nigriventer toxin Tx3-1 blocks A-type K+ currents controlling Ca2+ oscillation frequency in GH3 cells. Journal of Neurochemistry, 72: 1472-1481.
- 12. Leao RM, Cruz JS, Diniz CR, Cordeiro MN & Beirao PS (2000). Inhibition of neuronal high-voltage activated calcium channels by the omega-Phoneutria nigriventer Tx3-3 peptide toxin. Neuropharmacology, 39: 1756-1767.
- 13. Herrington J & Lingle CJ (1992). Kinetic and pharmacological properties of low voltage-activated Ca2+ current in rat clonal (GH3) pituitary cells. Journal of Neurophysiology, 68: 213-233.
- 14. Bezanilla F & Armstrong CM (1977). Inactivation of the sodium channel. I. Sodium current experiments. Journal of General Physiology, 70: 549-566.
- 15. Frazier CJ, Serrano JR, George EG et al. (2001). Gating kinetics of the alpha1I T-type calcium channel. Journal of General Physiology, 118: 457-470.
- 16. Gomora JC, Murbartian J, Arias JM, Lee JH & Perez Reyes E (2002). Cloning and expression of the human T-type channel Ca(v)3.3: insights into prepulse facilitation. Biophysical Journal, 83: 229-241.
- 17. Murbartian J, Arias JM, Lee JH, Gomora JC & Perez Reyes E (2002). Alternative splicing of the rat Ca(v)3.3 T-type calcium channel gene produces variants with distinct functional properties(1). FEBS Letters, 528: 272-278.
- 18. Matteson DR & Armstrong CM (1986). Properties of two types of calcium channels in clonal pituitary cells. Journal of General Physiology, 87: 161-182.
- 19. Monteil A, Chemin J, Leuranguer V, Altier C, Mennessier G, Bourinet E, Lory P & Nargeot J (2000). Specific properties of T-type calcium channels generated by the human alpha 1I subunit. Journal of Biological Chemistry, 275: 16530-16535.
- 20. Hille B (2001). Ion Channels of Excitable Membranes Sinauer Associates, Inc., Sunderland, MA, USA.
Correspondence and Footnotes
Publication Dates
-
Publication in this collection
27 May 2004 -
Date of issue
June 2004
History
-
Received
02 June 2003 -
Accepted
26 Feb 2004