Abstract
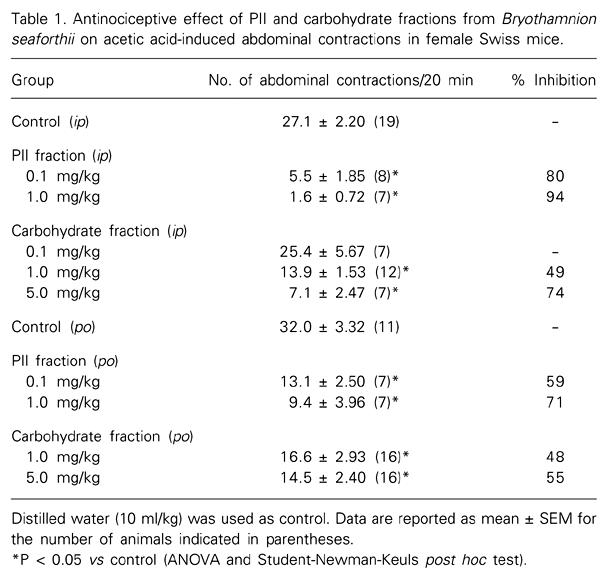
Bryothamnion seaforthii, a red alga common to the Northeastern coast of Brazil, was used to prepare the protein fraction F0/60 by ammonium sulfate precipitation. The chromatography of F0/60 on DEAE-Sephadel column resulted in two lectin fractions, PI and PII, which have antinociceptive properties in rodents. We determined the antinociceptive activity of the PII fraction and of a carbohydrate-containing fraction (CF) in mice. The CF was prepared from the dried algae, after digestion with 100 mM sodium acetate, pH 6.0, containing 5 mM cysteine, EDTA and 0.4% papain, at 60ºC. A 10% cetylpyridinium chloride was added to the filtrate, and the precipitate was dissolved with 2 M NaCl:ethanol (100:15, v/v) followed by the carbohydrate precipitation with ethanol. The final precipitate, in acetone, was dried at 25ºC. The PII fraction markedly inhibited acetic acid-induced abdominal writhing after ip administration (control: 27.1 ± 2.20; PII 0.1 mg/kg: 5.5 ± 1.85; 1 mg/kg: 1.6 ± 0.72 writhes/20 min) and after oral administration (control: 32.0 ± 3.32; PII 0.1 mg/kg: 13.1 ± 2.50; 1 mg/kg: 9.4 ± 3.96 writhes/20 min). PII was also effective against both phases of pain induced by 1% formalin (control, ip: 48.2 ± 2.40 and 27.7 ± 2.56 s; PII: 1 mg/kg, ip: 34.3 ± 5.13 and 5.6 ± 2.14 s; control, po: 44.5 ± 3.52 and 25.6 ± 2.39 s; PII 5 mg/kg, po: 26.5 ± 4.67 and 15.3 ± 3.54 s for the 1st and 2nd phases, respectively) and in the hot-plate test. The CF (ip) also displayed significant antinociceptive properties in all tests but at higher doses (1 and 5 mg/kg, ip and po). Thus, CF at the dose of 5 mg/kg significantly inhibited writhes (ip: 7.1 ± 2.47 and po: 14.5 ± 2.40 writhes/20 min) as well as the 1st (po: 19.6 ± 1.74 s) and 2nd (po: 7.1 ± 2.24 s) phases of the formalin test compared to controls ip and po. The antinociceptive effects of both the PII and CF in the formalin and hot-plate tests were prevented at least partially by pretreatment with the opioid receptor antagonist naloxone (2 mg/kg, sc). Moreover, both fractions retained antinociceptive activity in the acetic acid-induced writhing test following heating, a procedure which abolished the hemagglutinating activity of the fraction, presumably due to lectins also present. Finally, both fractions also prolonged the barbiturate-induced sleeping time. These results indicate that carbohydrate molecules present in the PII (26.8% carbohydrate) and CF (21% of the alga dried weight) obtained from B. seaforthii display pronounced antinociceptive activity which is resistant to heat denaturation and is mediated by an opioid mechanism, as indicated by naloxone inhibition.
Algae; Antinociceptive activity; Carbohydrate; Bryothamnion seaforthii
Braz J Med Biol Res, July 2004, Volume 37(7) 1071-1079
The alga Bryothamnion seaforthii contains carbohydrates with antinociceptive activity
L.A.P. Vieira1, A.L.P. Freitas1, J.P.A. Feitosa2, D.C. Silva1 and G.S.B. Viana3
Departamentos de 1Bioquímica e Biologia Molecular, 2Química Orgânica e Inorgânica, and 3Fisiologia e Farmacologia, Universidade Federal do Ceará, Fortaleza, CE, Brasil
 References
References
 Correspondence and Footnotes
Correspondence and Footnotes
Correspondence and Footnotes
Correspondence and Footnotes
Correspondence and Footnotes
Correspondence and Footnotes
Abstract
Bryothamnion seaforthii, a red alga common to the Northeastern coast of Brazil, was used to prepare the protein fraction F0/60 by ammonium sulfate precipitation. The chromatography of F0/60 on DEAE-Sephadel column resulted in two lectin fractions, PI and PII, which have antinociceptive properties in rodents. We determined the antinociceptive activity of the PII fraction and of a carbohydrate-containing fraction (CF) in mice. The CF was prepared from the dried algae, after digestion with 100 mM sodium acetate, pH 6.0, containing 5 mM cysteine, EDTA and 0.4% papain, at 60ºC. A 10% cetylpyridinium chloride was added to the filtrate, and the precipitate was dissolved with 2 M NaCl:ethanol (100:15, v/v) followed by the carbohydrate precipitation with ethanol. The final precipitate, in acetone, was dried at 25ºC. The PII fraction markedly inhibited acetic acid-induced abdominal writhing after ip administration (control: 27.1 ± 2.20; PII 0.1 mg/kg: 5.5 ± 1.85; 1 mg/kg: 1.6 ± 0.72 writhes/20 min) and after oral administration (control: 32.0 ± 3.32; PII 0.1 mg/kg: 13.1 ± 2.50; 1 mg/kg: 9.4 ± 3.96 writhes/20 min). PII was also effective against both phases of pain induced by 1% formalin (control, ip: 48.2 ± 2.40 and 27.7 ± 2.56 s; PII: 1 mg/kg, ip: 34.3 ± 5.13 and 5.6 ± 2.14 s; control, po: 44.5 ± 3.52 and 25.6 ± 2.39 s; PII 5 mg/kg, po: 26.5 ± 4.67 and 15.3 ± 3.54 s for the 1st and 2nd phases, respectively) and in the hot-plate test. The CF (ip) also displayed significant antinociceptive properties in all tests but at higher doses (1 and 5 mg/kg, ip and po). Thus, CF at the dose of 5 mg/kg significantly inhibited writhes (ip: 7.1 ± 2.47 and po: 14.5 ± 2.40 writhes/20 min) as well as the 1st (po: 19.6 ± 1.74 s) and 2nd (po: 7.1 ± 2.24 s) phases of the formalin test compared to controls ip and po. The antinociceptive effects of both the PII and CF in the formalin and hot-plate tests were prevented at least partially by pretreatment with the opioid receptor antagonist naloxone (2 mg/kg, sc). Moreover, both fractions retained antinociceptive activity in the acetic acid-induced writhing test following heating, a procedure which abolished the hemagglutinating activity of the fraction, presumably due to lectins also present. Finally, both fractions also prolonged the barbiturate-induced sleeping time. These results indicate that carbohydrate molecules present in the PII (26.8% carbohydrate) and CF (21% of the alga dried weight) obtained from B. seaforthii display pronounced antinociceptive activity which is resistant to heat denaturation and is mediated by an opioid mechanism, as indicated by naloxone inhibition.
Key words: Algae, Antinociceptive activity, Carbohydrate, Bryothamnion seaforthii
Introduction
Among the several algae species used in popular medicine are those belonging to the genera Codium, Sargassum, Gracilaria, and Hypnea (1,2). Several alga species occurring along the northeastern Brazilian coast are known to present the same type of biological activity such as the hemagglutinating activity (3-5). It has been speculated that some of the chemical constituents of algae, such as sulfated polysaccharides, may be responsible for their fibrinolytic, antithrombotic, anticoagulant, and antiviral activities (6-14). Furthermore, polysaccharides from the red microalga Porphyridium sp have been shown to be potent hypocholesterolemic agents (15). Sulfated polysaccharides are part of a complex group of macromolecules with a wide range of important biological properties and some of these compounds extracted from red algae might be used as low-cost, broad-spectrum antiviral agents (9). Recently (16), sulfated polysaccharides from red microalgae were shown to present potent anti-inflammatory properties.
In addition, several types of lectins isolated from marine algae and hemagglutinins from red algae have been extensively studied and lectins from the marine red alga species Bryothamnion seaforthii and B. triquetrum (5) occurring along the northeastern Brazilian coast have also been investigated. We have reported that a lectin, which we named fraction PI, isolated from these species presents antinociceptive activity in several experimental models in mice (17). Significant anti-inflammatory, analgesic and free radical scavenging activities have also been detected in other species such as Chlorella stigmatophora and Phaeodactylum tricornutum (18). Recently (19) we demonstrated that B. seaforthii and B. triquetrum present not only one but two different fractions containing lectins (PI and PII). The lectins of both species have a high carbohydrate content which could be responsible, at least in part, for the antinociceptive activity.
The objective of the present investigation was to determine the mechanism of the antinociception observed in the PII lectin fraction and the role of the sulfated polysaccharides in this pharmacological effect.
Material and Methods
Drugs and reagents
Bovine serum albumin, naloxone, sodium pentobarbital, and cetylpyridinium chloride were purchased from Sigma (St. Louis, MO, USA). Morphine sulfate was from Cristália do Brasil S/A (Itapira, SP, Brazil) and cysteine was from Riedel-de-Haen (Seelze, Germany). Formaldehyde was purchased from Reagen (São Paulo, SP, Brazil) and acetic acid from Vetec (São Paulo, SP, Brazil). All other drugs and reagents used were of analytical grade.
Plant material
Bryothamnion seaforthii was collected on the northeastern Brazilian coast (Fleixeiras Beach, Trairi, Ceará State, Brazil), brought to the laboratory in water-ice bags and kept at -20ºC until use. The alga was identified by Dr. D.I.A. Teixeira (Universidade Federal do Ceará, Fortaleza, CE, Brazil) and a sample was deposited in the Prisco Bezerra Herbarium of the Federal University of Ceará (voucher No. 30.850).
Chemical fractionation
The F0/60 fraction and the lectin were prepared as described in Ref. 5. Briefly, algae were thawed, rinsed with distilled water, ground to a fine powder under liquid nitrogen, stirred for 4 h with three volumes of 20 mM phosphate buffer, pH 7.0, containing 0.15 M NaCl, filtered through a nylon mesh, and centrifuged at 7,000 g for 30 min at 4ºC. The supernatant was acidified to pH 1.0 with 2 N HCl, kept for 16 h under refrigeration, centrifuged and adjusted to pH 7.0 with 2 N NaOH, followed by the addition of ammonium sulfate crystals to 60% saturation. After 16 h, the precipitate was recovered by centrifugation (F0/60), resuspended in distilled water and dialyzed.
Fractions containing lectins (PI and PII) were obtained from lyophilized F0/60 by chromatography on DEAE-cellulose. The column was equilibrated and eluted with 20 mM sodium phosphate buffer, pH 7.6, followed by elution with 1 M NaCl. Active fractions (PI and PII) were re-chromatographed on the same column, combined, dialyzed against water, and lyophilized. The procedure for isolation of the fraction containing lectins is a simple and reproducible one, and provides an average yield of 10-15 mg protein/kg fresh algae. In the present study, only the PII fraction was used, and this fraction containing lectins, identified by hemagglutinating reaction, was dissolved in distilled water before use.
For the preparation of the fraction containing carbohydrate (CF), freshly collected algae were washed with distilled water and dried at 35ºC. Twenty grams of dried algae was wet in 1% (w/v) sodium hypochlorite and washed with distilled water. The dried tissue was suspended in 500 ml of 100 mM sodium acetate buffer, pH 6.0, containing 5 mM cysteine, 5 mM EDTA and 0.4% papain, and incubated at 60ºC for 24 h. The mixture was filtered and the supernatant solution saved. The residue was washed with distilled water and filtered and the two supernatants combined.
Sulfated polysaccharides were precipitated with 17 ml of 10% (w/v) cetylpyridinium chloride. After standing at room temperature for 48 h, the mixture was centrifuged at 2,500 g for 20 min at 4ºC and pellets were washed with 600 ml of a 0.05% (w/v) cetylpyridinium chloride solution and centrifuged at 2,500 g for 20 min. The remaining pellet was then dissolved with 150 ml of 2 M NaCl:ethanol (100:15, v/v) and precipitated with 300 ml absolute ethanol. After 24 h at 4ºC, the precipitate was collected by centrifugation at 2,500 g for 20 min at 4ºC, washed with 300 ml of 80% ethanol, followed by the same volume of absolute ethanol, and finally by the same volume of acetone. The final precipitate was dried at room temperature. The yield was about 21% by weight. The carbohydrate-containing fraction was dissolved in distilled water before use. For the determination of the degree of sulfation, the carbohydrate fraction was submitted to infrared spectrophotometry in KBr from 4000 to 400 cm-1 using a model 100 Perkin Elmer spectrophotometer (Perkin Elmer, San Francisco, CA, USA). Both the PII and carbohydrate fractions were dissolved in distilled water before use and their pH was 7.0. This vehicle was used in all control groups.
Animals
Swiss mice of both sexes weighing 20 to 25 g, from the Animal House of the Federal University of Ceará, maintained on a 12-h light/dark cycle with free access to water and food were used. Since both the PII and carbohydrate fractions were dissolved in distilled water, this vehicle was used for all control groups. Experiments were carried out according to the Guide for the Care and Use of Laboratory Animals of the US Department of Health and Human Services.
Evaluation of antinociceptive activity
Writhing test. Male and female mice were used in each of the two experiments performed. Animals were treated with the PII or carbohydrate fractions from B. seaforthii for 30 min (intraperitoneal, ip, administration) or 60 min (oral, po, administration) before receiving a 0.6% acetic acid injection (10 ml/kg, ip), and the number of contractions was recorded for 20 min after a 10-min interval (20). Naloxone (2 mg/kg, ip) was injected 15 min before the fractions or morphine (used as standard).
Formalin test. Twenty microliters of a 1% formalin solution was injected into the right hind paw of male Swiss mice (25 g), and the licking time was recorded after the first 5 min (1st phase) and after 20 min (2nd phase), for 5 min each time. Animals were pretreated with lectin or carbohydrate fractions, 30 or 60 min before for ip or po administration, respectively. Naloxone, an opioid antagonist, was injected 15 min before the fractions, and morphine was used as standard (21,22).
Hot-plate test. Male Swiss mice (25 g) were pre-selected according to their reactions to a thermal stimulus (jumping or licking of hind limbs when placed on a hot plate at 55ºC). Latency times were recorded immediately before and 30, 60, 90, and 120 min after drug administration up to a maximum time of 40 s in order to avoid paw lesions (23). In order to detect a possible involvement of the opioid system, animals were pretreated with naloxone 15 min before treatment with the fractions or morphine.
Barbiturate-induced sleeping time
Sleep was induced in female mice by ip administration of 40 mg/kg sodium pentobarbital by the method of Ferrini et al. (24). Sodium pentobarbital was injected 30 min (ip) or 60 min (po) after the administration of PII or carbohydrate fractions and latency time to sleep (time to lose the righting reflex) and sleeping time (duration of time to recover the righting reflex) were determined.
Hemagglutination assay
Hemagglutination assays were conducted on rabbit erythrocytes according to the procedure described by Chiles and Bird (25). Approximately 4 ml of the erythrocyte suspension was washed four times with three volumes of cold PBS and the final pellet was used to prepare a 2% (v/v) suspension. Serial two-fold dilutions of the algal fractions were then prepared with PBS and added to 96-well round bottom microtiter plates. An equal volume of the erythrocyte suspension was added to each well and the plates were shaken and allowed to stand at room temperature for 24 h. The activity of the fractions was reported as the minimum amount of protein causing agglutination.
Statistical analysis
Data were analyzed statistically by ANOVA and by the Bonferroni t-test as the post hoc test, with the level of significance set at P < 0.05. This test is based on the Student t statistic and adjusts the level of significance observed to the fact that multiple comparisons are made. We also used the Student-Newman-Keuls test to detect homogeneity of the subset means.
Results
The alga Bryothamnion seaforthii contains carbohydrates with antinociceptive activity. L.A.P. Vieira, A.L.P. Freitas, J.P.A. Feitosa, D.C. Silva and G.S.B. Viana. Brazilian Journal of Medical and Biological Research, 37 (7): 1071, 2004.
The alga Bryothamnion seaforthii contains carbohydrates with antinociceptive activity. L.A.P. Vieira, A.L.P. Freitas, J.P.A. Feitosa, D.C. Silva and G.S.B. Viana. Brazilian Journal of Medical and Biological Research, 37 (7): 1071, 2004.
In the hot-plate test (Figure 1), increases in the reaction time to thermal stimuli of 31, 58 and 37%, and 47, 45 and 48% were detected 30 and 60 s after PII administration at doses of 1, 5 and 10 mg/kg, ip, respectively. In this case, the effect was also totally reversed by naloxone pretreatment. Morphine, used as a positive standard, increased the reaction time to thermal stimuli by 75 and 71% after 30 and 60 min, respectively. As expected, in this case, the effect was totally blocked in the presence of naloxone. The carbohydrate fraction (5 mg/kg, ip) increased the reaction time to thermal stimuli by 25, 38 and 38% 30, 60 and 90 min after its administration, respectively. Similarly, the administration of 10 mg/kg, po, also increased the reaction time by 37 and 28% after 60 and 90 min, respectively. The effect of the carbohydrate fraction was reversed by pretreatment with naloxone (Figure 1).
The alga Bryothamnion seaforthii contains carbohydrates with antinociceptive activity. L.A.P. Vieira, A.L.P. Freitas, J.P.A. Feitosa, D.C. Silva and G.S.B. Viana. Brazilian Journal of Medical and Biological Research, 37 (7): 1071, 2004.
The alga Bryothamnion seaforthii contains carbohydrates with antinociceptive activity. L.A.P. Vieira, A.L.P. Freitas, J.P.A. Feitosa, D.C. Silva and G.S.B. Viana. Brazilian Journal of Medical and Biological Research, 37 (7): 1071, 2004.
Table 4 shows the treatment of the carbohydrate fraction with 1 M NaOH followed by heating at 80ºC for 1 h to destroy carbohydrate sulfate groups. This treatment (after pH adjustment to 7.0 with 1 N HCl) did not change significantly the antinociceptive effect of the fraction, as demonstrated in the writhing test with mice. Inhibition of about 90% was observed with PII at the dose of 2 mg/kg, ip, before and after heating. Similarly, percent inhibition ranged from 70 to 84% (before heating) and from 86 to 97% (after heating), with the administration of the carbohydrate fraction (1, 5 and 20 mg/kg, ip). This treatment decreased by 75% the degree of sulfation of the carbohydrate fraction, indicating that sulfate groups are not important for antinociception.
Effect of PII (A) and carbohydrate (B) fractions from Bryothamnion seaforthii on the hot-plate test in male mice. Data are reported as means ± SEM. P < 0.05 avs controls, bvs morphine, cvs 10 mg/kg PII or 5 mg/kg CF at the same period of time (ANOVA and Bonferroni as a post hoc test). CF = carbohydrate fraction; Mor = morphine, 4 mg/kg, ip; Nal = naloxone, 2 mg/kg, sc.
Discussion
Sulfated polysaccharides are present in several marine organisms, including sponges (26), marine invertebrates (10), and red algae (15,27). Sulfated polysaccharides have many biological functions which depend on the binding of specific carbohydrate structures to several types of protein molecules, including lectins (11,28-31). Recently reported data (14) have shown that polysaccharides isolated from brown algae possess significant anti-inflammatory and antithrombotic activities. In addition, polysaccharides with a fucosylated chondroitin sulfate structure were shown to present anticoagulant and antithrombotic activities (10).
The anticoagulant and antiproliferative effects of low molecular weight fucans with different sulfate content depend on their degree of sulfation (11). Polysaccharides, identified as sulfated D-galactans isolated from marine red algae, present anticoagulant activity whose potency is a function of the chemical structure of the molecule, and some investigators have suggested that conformational analysis may explain the differences in biological activity among sulfated polysaccharide molecules. In addition, a pharmacological study (18) of hydrosoluble and liposoluble extracts of the marine microalgae Chlorella stigmatophora and Phaeodactylum tricornutum showed significant anti-inflammatory, analgesic, and free scavenging activities in the hydrosoluble components from both species. No activity was detected in the liposoluble fractions and the activated constituents present in the hydrosoluble fractions probably are of a polysaccharide nature.
A recent study (32) demonstrated antiulcer activity of polysaccharides from marine algae and antipeptic activity has been observed in several types of sulfated polysaccharides such as dextran sulfate, carrageenan and fucoidan (29,33). The cited investigators showed that non-sulfated polysaccharides such as mannan and dextran had no antipeptic activity.
In the present study, we showed that the lectin fraction (PII) isolated from the B. seaforthii F0/60 fraction as well as the carbohydrate fraction isolated from the fresh alga possess a potent and dose-related antinociceptive activity against acetic acid-induced abdominal contractions and in the formalin and hot-plate tests in mice. The effects were manifested after both ip and oral administration, indicating that active compounds are well absorbed by the gastrointestinal tract. It seems that sulfate moieties in the polysaccharide molecules are not involved in antinociception, since treatment of the carbohydrate fraction with 1 M NaOH followed by heating at 80ºC for 1 h did not modify the effect. This treatment not only destroys the sulfate moieties but also denatures any protein/lectin present as a contaminant in the carbohydrate fraction.
In addition, in the formalin test, PII and the carbohydrate fraction seemed to act predominantly on the 2nd phase of the response, suggesting that their antinociceptive effect is mainly against pain of an inflammatory nature. Both fractions were also active in the hot-plate test, significantly increasing the animal's reaction time to the thermal stimulus. The presence of the opioid antagonist naloxone reversed at least in part the antinociceptive effect, indicating the involvement of the opioid system in the effects of both PII and the carbohydrate fraction. The antinociceptive effect was also manifested at a central level since both fractions significantly increased the barbiturate-induced sleeping time in mice in a dose-dependent manner after both ip and oral administration. A maximum effect was already observed at the dose of 1 mg/kg, ip. These data are consistent with the observation that both the PII and carbohydrate fractions decrease spontaneous locomotor activity in the open-field test, with no alteration in motor coordination as measured by the rota rod test (34).
The carbohydrate fraction was isolated from fresh algae and the yield was about 21%. On the other hand, the carbohydrate content present in the lectin fraction (PII from the DEAE cellulose column) was reasonably high (close to 40% of the alga dried weight) and thus carbohydrate type compounds are probably responsible for the biological activity of the lectin. Also, the antinociceptive effect of the lectin fraction was well maintained after heating at 120ºC for 15 min. Under these conditions, its hemagglutinating activity, used as an index of lectin activity, almost completely disappeared. In addition, we recently showed that the treatment of the F0/60 fraction with 0.1% sodium-dodecyl sulfate plus 0.1% mercaptoethanol, heating at 100ºC for 1 min, or the enzymatic treatment with a mixture of trypsin and papain, all of which cause protein denaturation, did not interfere with the observed antinociceptive effect (34). Besides, the removal of the sulfate groups from the carbohydrate molecules by treatment with 1 N NaOH, followed by heating at 80ºC for 1 h, actually increased the antinociception observed with the carbohydrate fraction.
In conclusion, our results indicate that carbohydrate molecules present in the PII and carbohydrate fractions of the lectin from B. seaforthii display pronounced antinociceptive activity which is resistant to heat denaturation and is mediated to a significant extent via opioid mechanisms. Furthermore, the fact that heating did not change the action of PII, but both heating and NaOH treatment enhanced the action of the carbohydrate fraction, may suggest that the antinociceptive actions of both fractions are mediated by multiple carbohydrate compounds. The present findings, taken together with some of our previous data (18), show that lectins and carbohydrates from some species of red algae of the Northeastern Brazilian coast present significant analgesic and anti-inflammatory properties and could therefore be of potential therapeutical use.
Acknowledgments
The authors are grateful to Ms. Vilani Rodrigues Bastos for technical assistance.
Address for correspondence: G.S.B. Viana, Departamento de Fisiologia e Farmacologia, UFCe, Rua Cel. Nunes de Melo, 1127, Rodolfo Teófilo, 60430-270 Fortaleza, CE, Brasil.
Research supported by CNPq. Received January 14, 2003. Accepted April 13, 2004.
- 1. Sampaio AH, Ainouz IL, Freitas ALP & Benevides NMB (1993). Hemaglutininas de algas marinhas. Revista Brasileira de Fisiologia Vegetal, 5: 171-177.
- 2. Lim SN, Cheung PC, Ooi VE & Ang PO (2002). Evaluation of antioxidative activity of extracts from a brown seaweed, Sargassum siliquastrum Journal of Agriculture and Food Chemistry, 50: 3862-3866.
- 3. Ainouz IL & Sampaio AH (1991). Screening of Brazilian marine algae for hemagglutinins. Botanica Marina, 34: 211-214.
- 4. Ainouz IL, Sampaio AH, Benevides NMB, Freitas ALP, Costa FHF, Carvalho MR & Pinheiro-Joventino F (1992). Agglutination of enzyme treated erythrocytes by Brazilian marine algae. Botanica Marina, 35: 475-479.
- 5. Ainouz IL, Sampaio AH, Freitas ALP, Benevides NMB & Mapurunga S (1995). Comparative study on hemagglutinins from the red algae Bryothamnion seaforthii and B triquetrum Revista Brasileira de Fisiologia Vegetal, 7: 15-19.
- 6. Colliec S, Fisher AM, Tapon-Bretaudiere J, Boisson C, Durant P & Josefonvicz J (1991). Anticoagulant properties of a fucoidan fraction. Thrombosis Research, 64: 143-154.
- 7. Nishino T, Aizu Y & Nagumo T (1991). The influence of sulfate content and molecular weight of a fucan sulfate from the brown seaweed Ecklonia kurome on its antithrombin activity. Thrombosis Research, 64: 723-731.
- 8. Mauray S, Sternberg C, Theveniaux J, Millet J, Sinquin C, Tapon-Bretaudiere J & Fischer AM (1995). Venous antithrombotic and anticoagulant activities of a fucoidan fraction. Thrombosis and Haemostasis, 74: 1280-1285.
- 9. Carlucci MJ, Pujol CA, Ciancia M, Noseda MD, Matulewicz MC, Damonte EB & Cerezo AS (1997). Antiherpetic and anticoagulant properties of carrageenans from the red seaweed Gigartina skottsbergii and their cyclized derivatives: correlation between structure and biological activity. International Journal of Biological Macromolecules, 20: 97-105.
- 10. Mourão PA & Pereira MS (1999). Searching for alternatives to heparin: sulfated fucans from marine invertebrates. Trends in Cardiovascular Medicine, 9: 225-232.
- 11. Haroun-Bouhedja F, Ellouali M, Sinquin C & Boisson-Vidal C (2000). Relationship between sulfate groups and biological activities of fucans. Thrombosis Research, 100: 453-459.
- 12. Duarte ME, Noseda DG, Noseda MD, Tulio S, Pujol CA & Damonte EB (2001). Inhibitory effect of sulfated galactans from the marine alga Bostrychia montagnei on herpes simplex virus replication in vitro Phytomedicine, 8: 53-58.
- 13. Choi HS & Sa YS (2001). Fibrinolytic and antithrombotic protease from Spirodela polyrhiza. Bioscience, Biotechnology, and Biochemistry, 65: 781-786.
- 14. Trento F, Cattaneo F, Pescador R, Porta R & Ferro L (2001). Antithrombin activity of an algal polysaccharide. Thrombosis Research, 102: 457-465.
- 15. Dvir I, Chayoth R, Sod-Moriah U, Shany S, Nyska A, Stark AH, Madar Z & Arad SM (2000). Soluble polysaccharides and biomass of red microalga Porphyridium sp. alter intestinal morphology and reduce serum cholesterol in rats. British Journal of Nutrition, 84: 469-476.
- 16. Matsui MS, Muizzuddin N, Arad S & Marenus K (2003). Sulfated polysaccharides from red microalgae have antiinflammatory properties in vitro and in vivo Applied Biochemistry and Biotechnology, 104: 13-22.
- 17. Vieira LAP, Andrade MCH, Bastos MVR, Freitas ALP & Viana GSB (1999). Efeito analgésico periférico e central da lectina de Bryothamnion seaforthii Kutzing. XIV Annual Meeting of the Federação de Sociedades de Biologia Experimental, Caxambu, MG, Brazil (Abstract 12.187, 392).
- 18. Guzman S, Gato A & Calleja JM (2001). Antiinflammatory, analgesic and free radical scavenging activities of the marine microalgae Chlorella stigmatophora and Phaeodactylum tricornutum Phytotherapy Research, 15: 224-230.
- 19. Viana GSB, Freitas ALP, Lima MML, Vieira LAP, Andrade MCH & Benevides NMB (2002). Antinociceptive activity of sulfated carbohydrates from the red algae Bryothamnion seaforthii (Turner) Kütz. and B. triquetrum (S.G. Gmel.) M. Howe. Brazilian Journal of Medical and Biological Research, 35: 713-722.
- 20. Koster R, Anderson M & De Beer EJ (1959). Acetic acid for analgesic screening. Federation Proceedings, 18: 412-417.
- 21. Fasmer OB, Berge OG & Hole K (1985). Changes in nociception after lesions of descending serotoninergic pathways induced with 5,6-dihydroxytryptamine: different effects in the formalin and tail flick test. Neuropharmacology, 24: 729-734.
- 22. Hunskaar S & Hole K (1987). The formalin test in mice: dissociation between inflammatory and non-inflammatory pain. Pain, 30: 103-114.
- 23. Woolfe G & MacDonald AD (1944). The evaluation of the analgesic action of pethidine hydrochloride (demerol). Journal of Pharmacology and Experimental Therapeutics, 80: 300-307.
- 24. Ferrini R, Miragoli G & Taccardi B (1974). Neuropharmacological studies on SB 5833, a new psychotherapeutic agent of the benzodiazepine class. Arzneimittel-Forschung, 24: 2029-2032.
- 25. Chiles TC & Bird KT (1989). A comparative study of animal erythrocyte agglutinins from marine algae. Comparative Biochemistry and Physiology, 94B: 107-111.
- 26. Zierer MS & Mourão PA (2000). A wide diversity of sulfated polysaccharides are synthesized by different species of marine sponges. Carbohydrate Research, 328: 209-216.
- 27. Farias WRL, Valente AP, Pereira MS & Mourão PAS (2000). Structure and anticoagulant activity of sulfated galactans. Journal of Biological Chemistry, 275: 29299-29307.
- 28. Mulloy B, Mourão PA & Gray E (2000). Structure/function studies of anticoagulant sulphated polysaccharides using NMR. Journal of Biotechnology, 77: 123-135.
- 29. Shibata H, Kimura-Takagi I, Nagaoka M, Hashimoto S, Aiyama R, Iha M, Ueyama S & Yokokura T (2000). Properties of fucoidan from Cladosiphon okamuranus Tokida in gastric mucosal protection. Biofactors, 11: 235-245.
- 30. Mourão PA, Boisson-Vidal C, Tapon-Bretaudiere J, Drouet B, Bros A & Fischer A (2001). Inactivation of thrombin by a fucosylated chondroitin sulfate from echinoderm. Thrombosis Research, 102: 167-176.
- 31. Matsubara K, Matsubara Y, Bacic A, Liao M, Horik K & Miyazawa K (2001). Anticoagulant properties of sulfated galactan preparation from a marine green alga, Codium cylindricum International Journal of Biological Macromolecules, 28: 395-399.
- 32. Nagaoka M, Shibata H, Kimura-Tagagi I, Hashimoto S, Aiyama R, Ueyama S & Yokokua T (2000). Anti-ulcer effects and biological activities of polysaccharides from marine algae. Biofactors, 12: 267-274.
- 33. Shibata H, Nagaoka M, Takagi IK, Hashimoto S, Aiyama R & Yokokura T (2001). Effect of oligofucose derivatives on acetic acid-induced gastric ulcer in rats. Bio-Medical Materials and Engineering, 11: 55-61.
- 34. Vieira LAP (2002). Estudos bioquímicos e farmacológicos das algas Bryothamnion seaforthii (Turner) Kütz, Bryothamnion triquetrum (S.G. Gmel) M. Howe e Botryocladia occidentalis (Borgesen) Kylin. Master's thesis, Universidade Federal do Ceará, Fortaleza, CE, Brazil.
Correspondence and Footnotes
Publication Dates
-
Publication in this collection
23 June 2004 -
Date of issue
July 2004
History
-
Received
14 Jan 2003 -
Accepted
13 Apr 2004