Abstract
The objective of the present study was to assess the role of the 5-HT2A/2C receptor at two specific brain sites, i.e., the dorsal periaqueductal gray matter (DPAG) and the medial septal (MS) area, in maternal aggressive behavior after the microinjection of either a 5-HT2A/2C receptor agonist or antagonist. Female Wistar rats were microinjected on the 7th postpartum day with the selective agonist alpha-methyl-5-hydroxytryptamine maleate (5-HT2A/2C) or the antagonist 5-HT2A/2C, ketanserin. The agonist was injected into the DPAG at 0.2 (N = 9), 0.5 (N = 10), and 1.0 µg/0.2 µl (N = 9), and the antagonist was injected at 1.0 µg/0.2 µl (N = 9). The agonist was injected into the medial septal area (MS) at 0.2 (N = 9), 0.5 (N = 7), and 1.0 µg/0.2 µl (N = 6) and the antagonist was injected at 1.0 µg/0.2 µl (N = 5). For the control, saline was injected into the DPAG (N = 7) and the MS (N = 12). Both areas are related to aggressive behavior and contain a high density of 5-HT receptors. Non-aggressive behaviors such as horizontal locomotion (walking) and social investigation and aggressive behaviors such as lateral threat (aggressive posture), attacks (frontal and lateral), and biting the intruder were analyzed when a male intruder was placed into the female resident's cage. For each brain area studied, the frequency of the behaviors was compared among the various treatments by analysis of variance. The results showed a decrease in maternal aggressive behavior (number of bites directed at the intruder) after microinjection of the agonist at 0.2 and 1.0 µg/0.2 µl (1.6 ± 0.7 and 0.9 ± 0.3) into the DPAG compared to the saline group (5.5 ± 1.1). There was no dose-response relationship with the agonist. The present findings suggest that the 5-HT2A/2C receptor agonist has an inhibitory effect on maternal aggressive behavior when microinjected into the DPAG and no effect when microinjected into the MS. Ketanserin (1.0 µg/0.2 µl) decreased locomotion when microinjected into the DPAG and MS, but did not affect aggressive behavior. We interpret these findings as evidence for a specific role of 5-HT2A/2C receptors in the DPAG in the inhibition of female aggressive behavior, dissociated from those on motor activity.
5-HT receptors; Ketanserin; alpha-Methyl-5-hydroxytrypta- mine maleate; Microinjection; Serotonin
Braz J Med Biol Res, April 2005, Volume 38(04) 597-602 (Short Communication)
Maternal aggression in Wistar rats: effect of 5-HT2A/2C receptor agonist and antagonist microinjected into the dorsal periaqueductal gray matter and medial septum
R.M.M. de Almeida, M. Giovenardi, S.P. da Silva, V.P. de Oliveira and D.J. Stein
Laboratório de Neurociências, Centro 2, UNISINOS, São Leopoldo, RS, Brasil
 Text
Text
 References
References
 Correspondence and Footnotes
Correspondence and Footnotes
Correspondence and Footnotes
Correspondence and Footnotes
Correspondence and Footnotes
Correspondence and Footnotes
Abstract
The objective of the present study was to assess the role of the 5-HT2A/2C receptor at two specific brain sites, i.e., the dorsal periaqueductal gray matter (DPAG) and the medial septal (MS) area, in maternal aggressive behavior after the microinjection of either a 5-HT2A/2C receptor agonist or antagonist. Female Wistar rats were microinjected on the 7th postpartum day with the selective agonist a-methyl-5-hydroxytryptamine maleate (5-HT2A/2C) or the antagonist 5-HT2A/2C, ketanserin. The agonist was injected into the DPAG at 0.2 (N = 9), 0.5 (N = 10), and 1.0 µg/0.2 µl (N = 9), and the antagonist was injected at 1.0 µg/0.2 µl (N = 9). The agonist was injected into the medial septal area (MS) at 0.2 (N = 9), 0.5 (N = 7), and 1.0 µg/0.2 µl (N = 6) and the antagonist was injected at 1.0 µg/0.2 µl (N = 5). For the control, saline was injected into the DPAG (N = 7) and the MS (N = 12). Both areas are related to aggressive behavior and contain a high density of 5-HT receptors. Non-aggressive behaviors such as horizontal locomotion (walking) and social investigation and aggressive behaviors such as lateral threat (aggressive posture), attacks (frontal and lateral), and biting the intruder were analyzed when a male intruder was placed into the female resident's cage. For each brain area studied, the frequency of the behaviors was compared among the various treatments by analysis of variance. The results showed a decrease in maternal aggressive behavior (number of bites directed at the intruder) after microinjection of the agonist at 0.2 and 1.0 µg/0.2 µl (1.6 ± 0.7 and 0.9 ± 0.3) into the DPAG compared to the saline group (5.5 ± 1.1). There was no dose-response relationship with the agonist. The present findings suggest that the 5-HT2A/2C receptor agonist has an inhibitory effect on maternal aggressive behavior when microinjected into the DPAG and no effect when microinjected into the MS. Ketanserin (1.0 µg/0.2 µl) decreased locomotion when microinjected into the DPAG and MS, but did not affect aggressive behavior. We interpret these findings as evidence for a specific role of 5-HT2A/2C receptors in the DPAG in the inhibition of female aggressive behavior, dissociated from those on motor activity.
Key words: 5-HT receptors, Ketanserin, a-Methyl-5-hydroxytrypta- mine maleate, Microinjection, Serotonin
Clinical and pre-clinical data indicate the involvement of the serotonergic system in the modulation of emotional behaviors (1) such as aggression (for a review, see Ref. 2) and violent and impulsive behavior (3,4). Most studies of neurochemical control of aggression across different species, including humans, have demonstrated that high levels of serotonergic activity are related to low levels of aggressive or violent behavior in males (5,6). In females, there are only a few studies relating aggression to the possible involvement of the serotonergic system (7-9).
Among several types of serotonin (5-hydroxytryptamine, 5-HT) receptors, the 5-HT1A, 5-HT1B and 5-HT2A/2C receptors have been shown to decrease aggressive behavior in different animal models (8,10-12). The 5-HT2A/2C receptor has been of particular interest to research on aggression ever since the finding that low doses of 1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane (DOI), a 5-HT2A/2C agonist, decrease aggression in a relatively specific manner in rats without impairing locomotor activity (8). Intracerebroventricular injections of this 5-HT2 receptor agonist (DOI) inhibited maternal aggression without affecting maternal care (7). Another 5-HT2 receptor agonist, 1-(4-bromo-2,5-dimethoxyphenyl)-2-aminopropane, at higher doses significantly reduced aggressive behavior in the resident-intruder rat model (13). 1-(3-Trifluoromethylphenyl)- piperazine (TFMPP), a 5-HT1B/2C receptor agonist, decreased aggression in mice (11). In male rats, TFMPP microinjected into the brain (lateral ventricle) reduced aggression quite specifically, did not decrease social interest or exploration, and sometimes even increased these behaviors when administered intraperitoneally (8,14). However, TFMPP (up to 1 mg/kg) was found to be ineffective in reducing offensive aggression in the resident rat model (13) or when it was administered locally into the dorsal raphe nucleus of rats. In maternal aggression this agonist, when injected icv at the 20 µg dose, did not change aggression (7). 2,5-Dimethoxy-4-methylamphetamine, another 5-HT2A/2C receptor agonist, dose-dependently decreased dominance in rats (14).
a-Methyl-5-hydroxytryptamine maleate is another agonist with high affinity and selectivity for 5-HT2A and 5-HT2C receptors (pKi = 6.1 and 7.3), respectively (15). The 5-HT2A/2C receptors are directly coupled to a phosphoinositol second messenger system. Brain regions such as the hippocampus, septum, preoptic area, amygdala, and dorsal periaqueductal gray matter (DPAG) are rich in 5-HT2A/2C receptors. Early studies have demonstrated that these areas are involved in aggressive and defensive behavior (9).
The aim of the present study was to assess the role of the 5-HT2A/2C receptor at two specific brain sites such as the DPAG and medial septal (MS) area in maternal aggressive behavior after the microinjection of either a 5-HT2A/2C receptor agonist or antagonist.
Female Wistar rats (N = 83; 250-350 g) were used. Each pregnant female was housed individually in large observation cages (50 x 50 x 35 cm), with unrestricted access to water and food and maintained on a 12:12-h light:dark cycle (lights on at 4 am). The temperature and the acoustic isolation were controlled. On the 3rd day postpartum the females were tested for aggressive behaviors and only those who showed more than 3 bites during a 10-min test (about 75%) were used as subjects. To test aggression, male Wistar rats (N = 83) were used as stimulus intruder animals.
On the 4th postpartum day, the females were anesthetized with sodium thiopental (50 mg/kg, ip) placed in a stereotaxic frame (David Kopf, Tujunga, CA, USA), and implanted with a 23-gauge guide-cannula fixed with dental cement to the skull. A unilateral guide-cannula was aimed at the DPAG 1.9 mm lateral to the sagittal line, 4.0 mm below the dura mater and at a lateral angle of 22º, or at the MS 0.2 mm anterior to bregma, 1.0 mm lateral to the sagittal line, 4.0 mm below the dura mater and at a lateral angle of 10º. All parameters used were based on the stereotaxic coordinates of a rat brain atlas. Females were separated from the litters for 4 ± 1 h and divided into 2 groups according to the site of cannula placement: DPAG (N = 44) and MS (N = 39), and the behavioral tests were carried out on the 7th day postpartum.
a-Methyl-5-hydroxytryptamine maleate, a 5-HT2A/2C receptor agonist (RBI, Natick, MA, USA) and ketanserin, a 5-HT2A/2C receptor antagonist (RBI), were diluted in 0.9% saline. Each animal received only one injection per brain area of a-methyl-5-hydroxytryptamine maleate (0.2, 0.5 or 1.0 µg) or ketanserin (1.0 µg). The injection needle (27 gauge) was 2.0 mm longer than the guide cannula, which remained 2.0 mm above the area. For each brain area, animals were randomly assigned to microinjection with various doses of a-methyl-5-hydroxytryptamine maleate, ketanserin or saline. The solution was slowly infused using a Hamilton syringe connected by tubing to the injecting needle that was left in the DPAG and MS for a further minute after the injection. The injection procedure was performed outside the cage with the dams being gently restrained.
In order to reduce the effects of individual variations and/or any other non-specific influence, the experiments were grouped into blocks of animals for each brain area studied. Each block was randomly distributed among the various injections.
On the 7th day postpartum the microinjections were followed by resident-intruder tests. The behavioral recordings were started 10 min after the injection of either a-methyl-5-hydroxytryptamine maleate or saline and 20 min after the injection of ketanserin. The naive male intruder was placed into the female's cage and the behaviors were immediately videotaped for 10 min. The behaviors (frequency) analyzed during the tests of maternal aggression were as follows (9): non-aggressive, such as locomotion and social investigation, and aggressive, such as aggressive posture (lateral threat), attacks (frontal and lateral) and bites directed at the intruder.
After completion of all behavioral tests, the dams were deeply anesthetized with an overdose of sodium thiopental. Brains were perfused with 0.9% saline solution followed by 10% formol. They were removed and fixed in 10% formol and later cut into 50-µm coronal slices on a vibratome. The slices were placed on gelatinized microcover slides and stained with cresyl violet. The positions of the cannula tips were determined by microscopic analysis and only the animals with an exact localization were used for analysis (75%).
The frequency of each behavioral parameter for each animal group is reported as mean ± SEM. For each brain area studied, the frequency of the behavioral measures was compared among the various treatments by an analysis of variance (ANOVA), followed by the Newman-Keuls test when appropriate. In all cases, the alpha level was set at 0.05. Frequency was the parameter chosen because most of all behaviors showed a very short duration. Duration was recorded and analyzed, but the results led to the same conclusions as those reached for frequency.
Figure 1 shows that a-methyl-5-hydroxytryptamine maleate, at doses of 0.2 and 1.0 µg/0.2 µl microinjected into DPAG, significantly decreased the frequency of bites directed at the intruder (F(4,39) = 10.43, P = 0.001) when compared to the saline group. Also, the 5-HT2A/2C receptor agonist at the doses of 0.2, 0.5, and 1.0 µg/0.2 µl significantly decreased the frequency of bites (F(4,39) = 10.43, P = 0.001) compared to the ketanserin group.
Effects of a-methyl-5-hydroxytryptamine maleate (5-HT2A/2C), ketanserine and/or saline microinjected into the dorsal periaqueductal gray matter (DPAG) in a dose range of 0.2-1.0 µg/0.2 µl. Data are reported as the mean frequency ± SEM of lateral threats, attacks and bites during a 10-min test against an intruder male by lactating female rats. N = number of animals. ªP < 0.05 compared to the saline group; bP < 0.05 compared to the ketanserin group (ANOVA followed by the Newman-Keuls test).
Similarly, the frequency of attacks was also reduced by 1.0 µg/0.2 µl of the 5-HT2A/2C receptor agonist (F(4,39) = 3.57, P = 0.01) compared to both the saline and ketanserin groups.
Locomotion (walking) and social investigation (sniffing the intruder) were used to evaluate the general conditions of the females, in order to detect possible symptoms of the 5-HT syndrome. The motor behaviors were not altered significantly by any dose of a-methyl-5-hydroxytryptamine maleate microinjected into the DPAG when compared to the data from the saline group (results not shown). Ketanserin at the dose of 1.0 µg/0.2 µl significantly decreased the frequency of walking (F(3,31) = 5.11, P = 0.005) only when compared to the data for the saline group (26.9 ± 3.3; 64.3 ± 4.9, respectively).
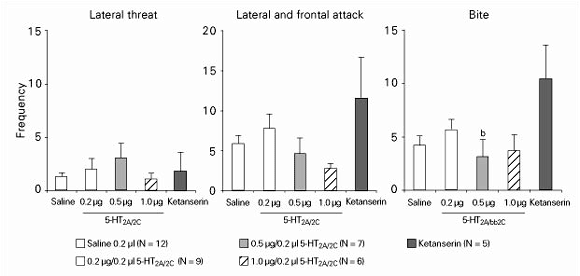
Figure 2 shows that a-methyl-5-hydroxytryptamine maleate microinjected into the MS at the dose of 0.5 µg/0.2 µl significantly decreased the frequency of bites directed at the intruder (F(4,34) = 2.78, P = 0.04) when compared to the values for the ketanserin group. The other doses did not alter significantly the maternal aggressive behavior toward the intruder (results not shown). The locomotor activity was not altered by the agonist. Ketanserin at the dose of 1.0 µg/0.2 µl significantly decreased the frequency of locomotion (F(4,34) = 2.97, P = 0.03) compared to the saline group (56.2 ± 5.2, 72.8 ± 8.7, respectively).
Effects of a-methyl-5-hydroxytryptamine maleate (5-HT2A/2C), ketanserine and/or saline microinjected into the medial septal area (MS) in a dose range of 0.2-1.0 µg/0.2 µl. Data are reported as the mean frequency ± SEM of lateral threats, attacks and bites during a 10-min test against a male intruder by lactating female rats. N = number of animals. ªP < 0.05 compared to the saline group (ANOVA followed by the Newman-Keuls test).
The present findings show that the 5-HT2A/2C receptor agonist a-methyl-5-hydroxytryptamine maleate has an inhibitory effect on maternal aggressive behavior when microinjected into the DPAG but not when microinjected into the MS. Ketanserin had no effect on aggressive behavior when microinjected into the DPAG or MS at the 1.0 µg dose, but this dose affected behavior since it reduced locomotor activity. It can be anticipated that higher ketanserin doses inhibit maternal aggression, but in a behaviorally non-specific manner.
The locomotion and the exploratory activity (sniffing the intruder) of the females showed no significant alteration after microinjection of a-methyl-5-hydroxytryptamine, indicating a high degree of behavioral specificity of this compound regarding both brain structures.
Most of experimental studies have used 5-HT agonists from the 5-HT1 family such as 8-OH-DPAT, a 5-HT1A receptor agonist, or TFMPP, a 5-HT1B/2C receptor agonist. Both agonists reduce aggression in different experimental procedures but cause impairment of motor activity or stimulation (7,9,12). However, several recently developed 5-HT1A receptor agonists like alnespirone and S-15535 have more specific anti-aggressive effects in male rats (16). The 5-HT1B receptor agonists, such as CP-94,253, anpirtoline and zolmitriptan, have shown anti-aggressive effects in male mice when administered systemically (17). Unfortunately, these compounds were not available for the present study and could not be examined in specific structures related to aggressive behavior in male or female subjects. In future experiments, it will be relevant to assess the role of the 5-HT1B receptor agonists in specific brain areas of female and male rats and mice.
The 5-HT2A/2C receptor agonist acts preferentially on excitatory postsynaptic receptor sites. The 5-HT2A receptor is coupled to the phosphoinositol signaling system. Its activation causes the hydrolysis of phosphatidyl-inositol 4,5-biphosphate located in the cell membrane through phospholipase C (a G protein-activated enzyme), giving rise to the formation of both inositol 1,4,5-triphosphate (IP3) and diacylglycerol, which act like intracellular second messengers. IP3 plays an important role in various physiological processes by releasing calcium from intracellular stores after binding to its specific receptors. The 5-HT2A/2C receptors are mainly located in the claustrum, tuberculum olfactorium, anterior olfactory nucleus, striatum, hypothalamus, septum, amygdala, and periaqueductal gray matter. Although binding studies found a higher density of these receptors in limbic areas, it is interesting to note that a-methyl-5-hydroxytryptamine maleate, the 5-HT2A/2C receptor agonist, is more effective in reducing the aggressive behavior in mesencephalic (DPAG) than in diencephalic or telencephalic areas (Giovenardi M, da Silva SP, de Oliveira VP and Stein DJ, unpublished data from our laboratory). It is possible that these receptors might have more sensitivity at these doses in mesencephalic areas of female rats. In the DPAG, the behavioral effects of the 5-HT2A/2C receptor agonist were more evident than in the septal structure. It is important to emphasize that the DPAG has been proposed as the final efferent structure that organizes aggressive behavior (18).
Electric stimulation of the DPAG leads to a chain of events collectively named the defense reaction. In the rat, such stimulation evokes behaviors which include brisk running, jumping and autonomic changes (19). Microinjection of the 5-HT1A agonist receptor (8-OH-DPAT) into the DPAG decreased the aggressive behavior of lactating females towards the intruder (9).
There are reciprocal pathways that connect the DPAG to the amygdala. The amygdala-periaqueductal gray system is considered to be an important anatomical substrate for autonomic responses of emotional behaviors (20). Both structures constitute the so-called aversion system with stimulatory functions on aggressive behaviors. Parts of the amygdala seem to synthesize environmental stimuli, and this information is conveyed to the DPAG where the degree of threat of those stimuli is processed (18). It may be proposed that the aversive nature is integrated and aggressive behavior is organized in the DPAG. The role of the DPAG is to integrate and emit the final response, in the present case the generation of aggressive behavior (9).
The present results suggest that activation of 5-HT2A/2C receptors in the DPAG, but not in the MS, is significant for the behaviorally specific inhibition of aggression, and this inhibitory role extends to aggression in lactating female rats.
Acknowledgments
The authors are grateful to Dr. Klaus A. Miczek for suggestions and comments about this paper.
Address for correspondence: R.M.M. de Almeida. Laboratório de Neurociências. Centro 2, UNISINOS, Av. Unisinos, 950, 93022-000, São Leopoldo, RS, Brasil. E-mail: rmalmeida@bios.unisinos.br
Presented at the XIX Annual Meeting of the Federação de Sociedades de Biologia Experimental, Águas de Lindóia, SP, Brazil, August 25-29, 2004, Research supported by UNISINOS and FAPERGS. Received April 5, 2004. Accepted January 27, 2005.
- 1. Iversen SD (1984). 5-HT and anxiety. Neuropharmacology, 23: 1553-1560.
- 2. Miczek KA, Fish EW, De Bold J & de Almeida RMM (2002). Social and neural determinants of aggressive behavior: pharmacotherapeutic targets at serotonin, dopamine and gamma-aminobutyric acid systems. Psychopharmacology, 163: 434-458.
- 3. Coccaro EF & Kavoussi RJ (1997). Fluoxetine and impulsive aggressive behavior in personality-disordered subjects. Archives of General Psychiatry, 54: 1081-1088.
- 4. Lindenmayer JP & Kotsaftis A (2000). Use of sodium valproate in violent and aggressive behaviors: a critical review. Journal of Clinical Psychiatry, 61: 123-128.
- 5. Coccaro EF, Kavoussi RJ, Trestman RL, Gabriel SM, Cooper TB & Siever LJ (1997). Serotonin function in human subjects: intercorrelations among central 5-HT indices and aggressiveness. Psychiatry Research, 73: 1-14.
- 6. Higley JD & Bennett AJ (1999). Central nervous system serotonin and personality as variables contributing to excessive alcohol consumption in non-human primates. Alcohol and Alcoholism, 34: 402-418.
- 7. de Almeida RMM & Lucion AB (1994). Effects of intracerebroventricular administration of 5-HT receptor agonists on the maternal aggression of rats. European Journal of Neuroscience, 264: 445-448.
- 8. Olivier B, Mos J, Van Oorschot R & Hen R (1995). Serotonin receptors and animal models of aggressive behavior. Pharmacopsychiatry, 28: 80-90.
- 9. de Almeida RMM & Lucion AB (1997). 8-OH-DPAT in the median raphe, dorsal periaqueductal gray and corticomedial amygdala nucleus decreases, but the medial septal area can increase maternal aggressive behavior in rats. Psychopharmacology, 134: 392-400.
- 10. Sanchez C & Hyttel J (1994). Isolation-induced aggression in mice: Effects of 5-hydroxytryptamine uptake inhibitors and involvement of postsynaptic 5-HT(1A) receptors. European Journal of Pharmacology, 264: 241-247.
- 11. Sanchez C, Arnt J & Moltzen EK (1996). The antiaggressive potency of (-)-penbutolol involves both 5-HT1A and 5-HT1B receptors and beta-adrenoceptors. European Journal of Pharmacology, 297: 1-8.
- 12. Miczek KA, Hussain S & Faccidomo S (1998). Alcohol-heightened aggression in mice: attenuation by 5-HT1A receptor agonists. Psychopharmacology, 139: 160-168.
- 13. Muehlenkamp F, Lucion AB & Vogel WH (1995). Effects of selective serotonergic agonists on aggressive behavior in rats. Pharmacology, Biochemistry and Behavior, 50: 671-674.
- 14. Bonson KR, Johnson RG, Fiorella D, Rabin RA & Winter JC (1994). Serotonergic control of androgen-induced dominance. Pharmacology, Biochemistry and Behavior, 49: 313-322.
- 15. Baxter G, Kennett G, Blaney F & Blackbum T (1995). 5-HT2 receptor subtypes: a family re-united? Trends in Pharmacological Sciences, 16: 105-110.
- 16. de Boer SF, Lesourd M, Mocaer E & Koolhaas JM (2000). Somatodendritic 5-HT1A autoreceptors mediate the anti-aggressive actions of 5-HT(1A) receptor agonists in rats: An ethopharmacological study with S-15535, alnespirone, and WAY-100635. Neuropsychopharmacology, 23: 20-33.
- 17. de Almeida RMM, Nikulina EW, Faccidomo S, Fish EW & Miczek KA (2001). Zolmitriptan, a 5-HT1B/D receptor agonist, alcohol, and aggression in male mice. Psychopharmacology, 157: 131-141.
- 18. Fanselow MS (1991). The midbrain periaqueductal gray as a coordinator of action in response to fear and anxiety. In: Depaulis A & Bandler R (Editors), The Midbrain Periaqueductal Gray Matter: Functional, Anatomical, and Neurochemical Organization. Plenum Press, New York, 151-173.
- 19. Bandler R & Shipley MT (1994). Columnar organization in the midbrain brain periaqueductal gray: modulates for emotional expression? Trends in Neurosciences, 17: 379-389.
- 20. Shipley MT, Ennis M, Rizvi TA & Behbehani MM (1991). Topographical specificity of forebrain inputs to the midbrain periaqueductal gray: evidence for discrete longitudinally organized input columns. In: Depaulis A & Bandler R (Editors), The Midbrain Periaqueductal Gray Matter: Functional, Anatomical, and Neurochemical Organization Plenum Press, New York, 417-448.
Correspondence and Footnotes
Publication Dates
-
Publication in this collection
13 Apr 2005 -
Date of issue
Apr 2005
History
-
Accepted
27 Jan 2005 -
Received
05 Apr 2004