Abstract
The influence of melatonin on the developmental pattern of functional nicotinic acetylcholine receptors was investigated in embryonic 8-day-old chick retinal cells in culture. The functional response to acetylcholine was measured in cultured retina cells by microphysiometry. The maximal functional response to acetylcholine increased 2.7 times between the 4th and 5th day in vitro (DIV4, DIV5), while the Bmax value for [125I]-alpha-bungarotoxin was reduced. Despite the presence of alpha8-like immunoreactivity at DIV4, functional responses mediated by alpha-bungarotoxin-sensitive nicotinic acetylcholine receptors were observed only at DIV5. Mecamylamine (100 µM) was essentially without effect at DIV4 and DIV5, while dihydro-ß-erythroidine (10-100 µM) blocked the response to acetylcholine (3.0 nM-2.0 µM) only at DIV4, with no effect at DIV5. Inhibition of melatonin receptors with the antagonist luzindole, or melatonin synthesis by stimulation of D4 dopamine receptors blocked the appearance of the alpha-bungarotoxin-sensitive response at DIV5. Therefore, alpha-bungarotoxin-sensitive receptors were expressed in retinal cells as early as at DIV4, but they reacted to acetylcholine only after DIV5. The development of an alpha-bungarotoxin-sensitive response is dependent on the production of melatonin by the retinal culture. Melatonin, which is produced in a tonic manner by this culture, and is a key hormone in the temporal organization of vertebrates, also potentiates responses mediated by alpha-bungarotoxin-sensitive receptors in rat vas deferens and cerebellum. This common pattern of action on different cell models that express alpha-bungarotoxin-sensitive receptors probably reflects a more general mechanism of regulation of these receptors.
Retina; Development; Nicotinic acetylcholine receptors; Alpha-bungarotoxin; Melatonin; Microphysiometry
Braz J Med Biol Res, April 2005, Volume 38(04) 603-613
Influence of melatonin on the development of functional nicotinic acetylcholine receptors in cultured chick retinal cells
L.F.S. Sampaio1,3, D.E. Hamassaki-Britto2 and R.P. Markus1
1Laboratório de Cronofarmacologia, Departamento de Fisiologia, Instituto de Biociências, 2Departamento de Histologia e Embriologia, Instituto de Ciências Biomédicas, Universidade de São Paulo, São Paulo, SP, Brasil
3Laboratório de Bioquímica do Desenvolvimento do Sistema Nervoso, Departamento de Fisiologia, Centro de Ciências Biológicas, Universidade Federal do Pará, Belém, PA, Brasil
 References
References
 Acknowledgments
Correspondence and Footnotes
Acknowledgments
Acknowledgments
Correspondence and Footnotes
Acknowledgments
Abstract
The influence of melatonin on the developmental pattern of functional nicotinic acetylcholine receptors was investigated in embryonic 8-day-old chick retinal cells in culture. The functional response to acetylcholine was measured in cultured retina cells by microphysiometry. The maximal functional response to acetylcholine increased 2.7 times between the 4th and 5th day in vitro (DIV4, DIV5), while the Bmax value for [125I]-a-bungarotoxin was reduced. Despite the presence of a8-like immunoreactivity at DIV4, functional responses mediated by a-bungarotoxin-sensitive nicotinic acetylcholine receptors were observed only at DIV5. Mecamylamine (100 µM) was essentially without effect at DIV4 and DIV5, while dihydro-ß-erythroidine (10-100 µM) blocked the response to acetylcholine (3.0 nM-2.0 µM) only at DIV4, with no effect at DIV5. Inhibition of melatonin receptors with the antagonist luzindole, or melatonin synthesis by stimulation of D4 dopamine receptors blocked the appearance of the a-bungarotoxin-sensitive response at DIV5. Therefore, a-bungarotoxin-sensitive receptors were expressed in retinal cells as early as at DIV4, but they reacted to acetylcholine only after DIV5. The development of an a-bungarotoxin-sensitive response is dependent on the production of melatonin by the retinal culture. Melatonin, which is produced in a tonic manner by this culture, and is a key hormone in the temporal organization of vertebrates, also potentiates responses mediated by a-bungarotoxin-sensitive receptors in rat vas deferens and cerebellum. This common pattern of action on different cell models that express a-bungarotoxin-sensitive receptors probably reflects a more general mechanism of regulation of these receptors.
Key words: Retina, Development, Nicotinic acetylcholine receptors, Alpha-bungarotoxin, Melatonin, Microphysiometry
Introduction
Neuronal nicotinic acetylcholine receptors (nAChRs) are widely distributed pentameric transmembrane proteins acting as key molecules in cholinergic transmission at synapses, including the retina (1), where they play an important role in synaptogenesis (2). They are composed of combinations of subunits encoded by at least 12 different genes, and two main classes have been identified: the a-bungarotoxin-sensitive receptors made of a7, a8, a9, or a10 subunits, which can form homomeric or heteromeric receptors, and the a-bungarotoxin-insensitive receptors made of a2-a6 and ß2-ß4 subunits, which only form heteromeric receptors (3).
The a-bungarotoxin-sensitive nAChRs present in chick retina are composed of a7 and/or a8 subunits (1), whereas the a-bungarotoxin-insensitive channels in the chick retina include a variety of subtypes composed of a2, a3, a4, or a6 and ß2, ß3 or ß4 subunits, in the presence or absence of a5 or ß3 subunits (4). Chick amacrine and ganglion cells contain a3, a7, a8, ß2, and ß3 subunits (5,6), whereas bipolar cells appear to express only the a8 subunit (6). Some subunits (a3 and a8) can be detected as early as on embryonic day 5 (7), well before the appearance of choline acetyltransferase, the acetylcholine-synthesizing enzyme, which appears around day 6.5 (8).
The light-dark cycle modulates the function of many tissues, including central and peripheral cholinergic synapses. The number and the response of nAChRs located on sympathetic nerve terminals of rat vas deferens (9) present a diurnal rhythm (10). Nicotine administration affects rat locomotor activity during the day but not during the night, and the density of [125I]-a-bungarotoxin binding sites in rat hypothalamus is lower at the end of a 12-h dark period than during the light period (9). Acetylcholine-induced release of neurotransmitter from sympathetic nerve terminals of the vas deferens and cerebellum slices of the rat is higher in the dark phase than in the light phase of the day. In both cases the effect of acetylcholine is mediated by nAChRs sensitive to a-bungarotoxin (11,12). Melatonin, the hormone released by the pineal gland during the dark phase of the day, is responsible for the diurnal variation of nAChR-mediated responses. This rhythm is abolished in animals maintained under conditions of constant lighting (13), and in animals treated with propranolol (11), conditions which impair the nocturnal surge of melatonin. Melatonin replacement restores the response to cholinergic agonists in both cases.
Cultured cells from embryonic chick retina have been used to study neurodevelopment (14) and melatonin production by photoreceptors (15). Melatonin has a paracrine function in the retina and is produced by photoreceptors since embryonic day 7. In the early stages melatonin is synthesized tonically; the rhythmic synthesis of melatonin only starts at day 20 (16). Light-induced inhibition of melatonin biosynthesis is mediated by light-induced release of dopamine, which acts on D4 dopamine receptors expressed on the membrane of cells that synthesize melatonin (14). Accordingly, melatonin synthesis by photoreceptors is inhibited by quinpirole, a D4 dopamine receptor agonist. On the other hand, melatonin, by acting on MT2 melatonin receptors, blocks dopamine synthesis. The mutual antagonism between melatonin and dopamine is observed in vivo and in cultured retina.
In the present study, the influence of melatonin on the developmental pattern of functional nAChRs was investigated in a culture of embryonic chick retinal cells in which the synthesis of melatonin is not rhythmic (15,17). The functional response of nAChRs was determined by microphysiometry, (18). Firstly, the activity of a-bungarotoxin-sensitive and non-sensitive nAChRs was pharmacologically characterized and then the effect of melatonin on the nAChR-mediated response was tested.
Material and Methods
Culture of chick retinal cells
Fertilized chicken eggs were obtained from a local hatchery and incubated at 38ºC in a humidified atmosphere. Primary cultures of retinal cells were prepared at embryonic day 8 as described (19). Briefly, retinas were dissected and treated with trypsin and the cells dissociated in Dulbecco's modified Eagle's medium (DMEM). The cells were plated (1.76 x 104 cells/mm2) and incubated in DMEM containing 5% fetal calf serum, 80 U/ml penicillin and 80 µg/ml streptomycin at 37ºC for 4 to 8 days in an atmosphere of 5% CO2 plus air. The medium was replaced every other day. The cultures were tested after 4 (DIV4), 5 (DIV5), 6 (DIV6) or 8 (DIV8) days in vitro.
Immunocytochemistry
At DIV4 and DIV6, retinal cells, plated onto chamber slide systems (Lab Tek, Christchurch, Caterbury, New Zealand), were fixed for 10 min with 2% paraformaldehyde in 0.1 M sodium phosphate-buffered saline (PBS), pH 7.4. Cells were incubated overnight with 10 nM of a monoclonal antibody against the a3 or a8 subunit (mAb 305 and mAb 315, respectively, a kind gift by Dr. Jon Lindstrom (University of Pennsylvania, Philadelphia, PA, USA). The secondary antibody was an anti-rat IG antibody tagged with fluorescein isothiocyanate or tetramethylrhodamine isothiocyanate that was diluted 200 times. All antibodies were diluted in 0.3% Triton-X 100 in PBS. No immunoreactivity was observed after elimination of the primary antibodies or after incubation with normal rat serum.
Microphysiometry
Retinal cells, seeded onto 12-mm transwell inserts (Costar, Corning, NY, USA), and incubated for 4, 5, 6, or 8 days at 37°C in 5% CO2, were loaded into the sensor chambers of the Cytosensor microphysiometer (Molecular Devices, Palo Alto, CA, USA), a silicon-based biosensor system which continuously monitors the extracellular pH surrounding cells in culture, and reports receptor activation by measuring increases in extracellular acidification rate occurring in response to agonist stimulation (18). The chambers were perfused at a rate of 100 µl/min with bicarbonate-free DMEM (sodium bicarbonate replaced by 44 mM sodium chloride, 37°C, pH 7.4). Each measurement cycle consisted of a 2-min period, 110 s of pump on and 10 s of pump off (Figure 1). The rate of chamber acidification was determined as the slope of a linear least-square fit to the pH-time data and normalized to its baseline using the Cytosoft program (Molecular Devices).
Cells were exposed to acetylcholine for 14 s. The pump was on for 4 s, allowing the entry of the drug into the chamber, and off for the last 10 s, and the slope of the acidification rate was calculated. Responses to increasing concentrations of acetylcholine were measured after an equilibration period of 50 min. It is interesting to note that the acidification rate of retina cells was constant in the absence of the agonist. A 40-min washout period between exposures to acetylcholine was allowed to elapse in order to avoid desensitization. Atropine (1 µM) was perfused during the entire experiment in order to block muscarinic receptors.
The following nAChR antagonists were used: a-bungarotoxin, mecamylamine and dihydro-ß-erythroidine (DHßE). Alpha-bungarotoxin was incubated for 60 min before the addition of acetylcholine. Mecamylamine (100 µM) was applied during the entire experiment, and a control was run in parallel. In the case of DHßE a complete concentration-response curve for the antagonist was obtained. After measuring the response to 250 µM acetylcholine in the absence of the antagonist, a series of successive 40-min incubations with increasing concentration of DHßE (10-100 µM) was performed and the response to 250 µM acetylcholine was measured again.
When appropriate, the cultures were pretreated with the antagonist of melatonin receptors luzindole or the D4 dopamine receptor agonist quinpirole for 48 h, at the concentrations specified in Results.
Binding assays
[125I]-Tyr54-a-bungarotoxin ([125I]-a-bungarotoxin) binding was conducted in intact cells at DIV4 and DIV5.[125I]-a-bungarotoxin was incubated for 1 h in DMEM (37ºC, pH 7.4), and the cells were then washed with cold buffer, left overnight in 0.1 M NaOH and counted for radioactivity in a Packard 2300 TR liquid scintillation spectrophotometer (Springfield, IL, USA). In association assays [125I]-a-bungarotoxin (4-10 nM) was incubated for 2.5-120 min, and equilibrium was reached at 60 min both on DIV4 and DIV5. Therefore, 60 min was the time chosen for saturation studies. Specific binding was calculated as the difference in binding obtained in the absence and presence of 1 mM (-)-nicotine. Nonspecific binding accounts for 20-50% of total binding.
Protein assay
Protein content was estimated by the method of Bradford (20) using bovine serum albumin as the standard.
Drugs and chemicals
Acetylcholine bromide, a-bungarotoxin, 2-benzyl-N-acetyltryptamine (luzindole), (-)-nicotine hydrogen tartrate, trypsin, mecamylamine hydrochloride, quinpirole, and DHßE were purchased from Research Biochemicals International (Natick, MA, USA). Fluorescein isothiocyanate and tetramethylrhodamine isothiocyanate were supplied by Jackson Laboratories (West Grove, PA, USA). Atropine was purchased from Merck (Rio de Janeiro, RJ, Brazil), and [125I]-a-bungarotoxin (138-146 Ci/mmol) from NEN Life Science Products, Inc. (Boston, MA, USA). Gibco-BRL (Grand Island, NY, USA) supplied DMEM and penicillin-streptomycin.
Statistical analysis
Concentration-response curves and binding saturation curves were fitted by non-linear equations (Graph-Pad software package; GraphPad, San Diego, CA, USA). The pD2 values reported correspond to the negative logarithm of the EC50 (effective concentration that induced half-maximal increase in extracellular acidification rate). The pD2 values were calculated for each dose-response curve, and the values are reported as the mean ± SEM of the number of cultures.
For binding assays, the maximal number of binding site (Bmax) and the apparent equilibrium dissociation constant (Kd) values, reported as means ± SEM, were calculated from three independent assays by fitting non-linear saturation curves for one binding site model. Data were analyzed statistically by the Student t-test or ANOVA followed by the Newman-Keuls test, when appropriate. Values of P < 0.05 were considered statistically significant. All analyses were performed using the Graph-Pad software package (GraphPad).
Results
a3- and a8-like immunoreactivity
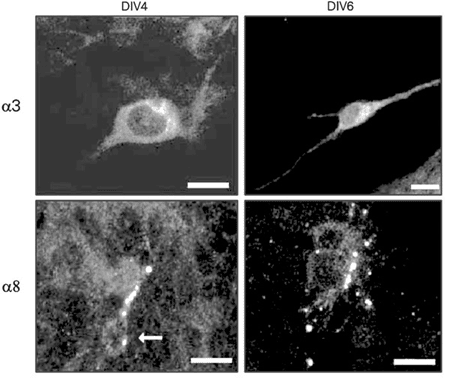
Confocal microscopy confirmed the presence of a3- and a8-like immunoreactivity in cultured retinal cells (Figure 1). These subunits seemed to be distributed throughout the cell, with a3 labeling especially pronounced in the perikarya and proximal dendrites, and a8 preferentially labeling processes and varicosities. Both subunits were detected in DIV4 and DIV6 cultures, indicating that no developmental stage-related change had occurred in the cellular distribution of a3- and a8-like immunoreactivity.
Chick retinal cell culture development and the response to acetylcholine
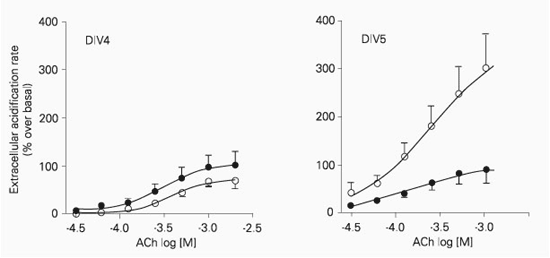
Acetylcholine (30 µM-2 mM) induced a concentration-dependent increase in extracellular acidification rate in retinal cells after DIV4, DIV5 and DIV6. The maximal increase in extracellular acidification rate was significantly higher (P < 0.001) at DIV5 and DIV6 when compared to DIV4 and DIV8 (Figure 2). On the other hand, the apparent affinity was not dependent on the number of days in vitro, since the pD2 values for DIV4 (3.40 ± 0.05, N = 7), DIV5 (3.57 ± 0.10, N = 11) and DIV6 (3.44 ± 0.05, N = 7) were not significantly different (P > 0.05).
In order to determine which receptor subtype mediates the acetylcholine-induced response, selective nAChR antagonists were used. Mecamylamine, a non-competitive antagonist of heteromeric channels, had no effect on DIV5 cultures (N = 3; data not shown), and only a very high concentration of mecamylamine (100 µM) caused a small reduction (15 ± 5%, P < 0.05, N = 4) in the response to 2 mM acetylcholine in DIV4 cultures (data not shown).
DHßE, an antagonist that blocks heteromeric receptors in chick retina (21), inhibited in a concentration-dependent manner (10-100 µM) the acetylcholine-induced response at DIV4 (Figure 3; IC50 = 16.9 µM). The response at DIV5 was not sensitive to DHßE (Figure 3).
Alpha-bungarotoxin, an antagonist that blocks receptors containing a7 or a8 subunits, presented a different profile. In DIV4 cultures, 10 nM a-bungarotoxin did not modify either the maximal response to or the apparent affinity for acetylcholine (control: pD2 = 3.40 ± 0.05; a-bungarotoxin: pD2 = 3.40 ± 0.20), but in DIV5 cultures a-bungarotoxin caused a sharp reduction in the response to acetylcholine (Figure 4), and no change in pD2 values (control: pD2 = 3.4 ± 0.05; a-bungarotoxin: pD2 = 3.4 ± 0.20). The concentration-response curve at DIV5 in the presence of a-bungarotoxin was similar to the response at DIV4 in the presence or absence of a-bungarotoxin (Figure 4).
[125I]-a-bungarotoxin binding sites
[125I]-a-bungarotoxin binding to DIV4 and DIV5 intact cells was saturable and fitted a curve modeling one binding site. Bmax and Kd were higher at DIV4 (Bmax = 233.10 ± 31.29 fmol/mg protein; Kd = 17.74 ± 4.46 nM, N = 4) than at DIV5 (Bmax = 86.94 ± 8.86 fmol/mg protein; Kd = 5.90 ± 1.62 nM, N = 4; Figure 5).
The effect of melatonin on the acetylcholine-induced response depends on the developmental stage of cultured retinal cells
The effect of melatonin on the development of the acetylcholine-induced response in cultured retinal cells was evaluated by blocking melatonin receptors with 100 µM luzindole since DIV2 or by inhibiting melatonin production by stimulating dopamine D4 receptors with 10 µM quinpirole since DIV3.
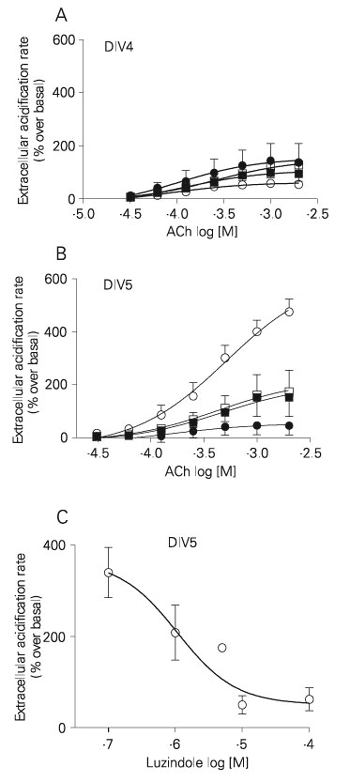
Pretreatment with luzindole (48 h) reduced the acetylcholine-induced increase in acidification rate at DIV5 from 516 ± 84 to 121 ± 85% (Figure 6B), while no significant difference was observed at DIV4 (63 ± 4.9 to 150 ± 41%; Figure 6A). The number of cells in culture estimated from the protein content was not modified (0.57 ± 0.04 mg/well in the absence and 0.51 ± 0.05 mg/well in the presence of luzindole). The pD2 values for acetylcholine in retinal cultures pretreated or not with luzindole were not changed either at DIV4 (pD2 value without luzindole = 4.09 ± 0.19; with luzindole = 3.98 ± 0.53) or DIV5 (pD2 value without luzindole = 3.32 ± 0.21; with luzindole = 3.28 ± 0.59). Incubation of the DIV4 retinal cultures with a-bungarotoxin did not modify the maximum response to acetylcholine in the presence or absence of luzindole, while it reduced the magnitude of the response to acetylcholine by DIV5 cells in the absence, but not in the presence, of luzindole pretreatment. The reduction by luzindole in the acetylcholine-induced maximal acidification rate at DIV5 was dependent on the concentration (Figure 6C). In these experiments each well was incubated with one concentration of luzindole for 48 h, and only the effect of 1 mM acetylcholine was tested. The maximal reduction of the acetylcholine-induced response was 86%, and the IC50 value for luzindole was 1.05 µM.
The dopamine D4 agonist, quinpirole (10 µM), was added to DIV3 cultures, and the cells were set up for microphysiometric recording at DIV5. The increase in extracellular acidification rate induced by 1 mM acetylcholine was 81.7 ± 0.5% (N = 3) smaller in cultures incubated with quinpirole when compared to cultures that were not incubated with the D4 dopamine receptor agonist. Therefore, quinpirole impairs the increase in the acetylcholine-induced effect that was observed at DIV5.
Confocal laser scanning images of retinal cells from 4th and 6th day in vitro (DIV4 and DIV6) cultures labeled with antibodies against a3 and a8 subunits, and visualized with tetramethylrhodamine isothiocyanate. a3-like immunoreactivity can be seen in the perykarion and proximal processes, and a8 in a varicose process. The arrow points to the somata of cells labeled with the a8 monoclonal antibody. Scale bar: 10 µm.
Acetylcholine-induced increase in extracellular acidification rate of retinal cells isolated from 8-day-old embryos and cultured for 4 (DIV4, triangles), 5 (DIV5, circles) and 6 (DIV6, squares) days in vitro. Cells (4 x 106) were exposed to increasing concentrations of acetylcholine for 10 s. The interval between subsequent concentrations was 40 min in order to avoid desensitization, and only one concentration-response curve was obtained for each well. Data are reported as means ± SEM for 5-13 independent cultures. The curves were fitted according to a sigmoid concentration-response curve.
Dihydro-ß-erythroidine (DHßE) blocks the acetylcholine-induced increase in extracellular acidification rate of retinal cells in culture. Retinal cells cultured for 4 (DIV4, squares) or 5 (DIV5, circles) days in vitro were incubated in the absence or in the presence of different concentrations of the antagonist. DHßE inhibited the effect of 250 µM acetylcholine at DIV4 (IC50 = 16.9 µM) in a concentration-dependent manner but not at DIV5. C = control. Data are reported as means ± SEM for 3-5 independent experiments.
Blockade of acetylcholine-induced increase in extracellular acidification rate of retinal cells in culture by a-bungarotoxin. Retinal cells cultured for 4 (DIV4, left panel) or 5 (DIV5, right panel) days in vitro were stimulated with acetylcholine (ACh) in the presence (filled symbols) or absence (open symbols) of 10 nM a-bungarotoxin and incubated for 1 h. Data are reported as means ± SEM. a-Bungarotoxin inhibited the response in DIV5 cultures (N = 4, P < 0.01) but not in DIV4 cultures (N = 5) (two-way ANOVA).
[125I]-a-bungarotoxin (BTX) binding saturation curves for retinal cultures. Nonspecific binding was determined with 1 mM nicotine. Four-day (DIV4, squares) and 5-day (DIV5, circles) in vitro cultures were incubated for 60 min at 37ºC. Data are reported as means ± SEM for 3 independent experiments.
Pretreatment with luzindole (panels A and B - 100 µM; panel C - 0.1 to 100 µM) for 48 h inhibited the a-bungarotoxin-sensitive component of acetylcholine (ACh)-induced increases in extracellular acidification rate by retinal cultured cells. The effects on 4-day (DIV4) and 5-day (DIV5) in vitro cultures are shown in panels A and B, respectively. Open and filled symbols represent the effect in the absence and presence of 10 nM a-bungarotoxin, respectively. Squares and circles indicate the effect in cultures pretreated with 100 µM luzindole and untreated controls, respectively. Panel C shows a concentration-response curve for DIV5 cultures pretreated with luzindole (48 h) stimulated with 1 mM acetylcholine (IC50 = 1.05 µM). Each culture was pretreated with only one luzindole concentration. Data are reported as means ± SEM (N = 3-5).
Discussion
The present results demonstrate that, despite the immunostaining of a3- and a8-like subunits observed since DIV4, acetylcholine activated a-bungarotoxin-sensitive nAChRs of retinal cells only after the 5th day of culture. The appearance of the response of a-bungarotoxin-sensitive nAChRs was accompanied by a reduction in the number of [125I]-a-bungarotoxin binding sites. Melatonin produced by cultured retinal cells is essential for the appearance of the a-bungarotoxin-sensitive response. Inhibition of both retinal melatonin receptors and melatonin production impaired the development of the a-bungarotoxin-sensitive response.
Pharmacological characterization of functional nAChRs in retinal culture
The functional response of nAChRs to stimulation was measured by microphysiometry, a method that has been validated for several receptor-transduction pathways involving muscarinic (22), noradrenaline, adenosine-5'-triphosphate (23), and serotonin (24) receptors. In some cases the record of the variation of extracellular acidification rate was more sensitive than the measurement of second messengers (24). In the present study, we characterized the microphysiometric response of nAChRs to acetylcholine in the presence or absence of different selective antagonists. In all experiments the muscarinic receptors were blocked with atropine.
The acetylcholine-induced increase in extracellular acidification rate was enhanced 2.7 times between DIV4 and DIV5, and no further change in acetylcholine-induced response was observed between DIV5 and DIV6. Selective antagonists for different nAChR subtypes were used to investigate which nAChR subtypes mediate the acetylcholine- induced effect. Alpha-bungarotoxin blocked the acetylcholine-induced response in DIV5 and DIV6, but not in DIV4 cultures. Neither mecamylamine nor DHßE reduced the acetylcholine-induced increase in extracellular acidification rate in DIV5 retinal cells, indicating that at that stage the response to acetylcholine was mainly mediated by a-bungarotoxin-sensitive receptors. These antagonists were effective in DIV4 cultures.
Mecamylamine, known as a ganglionic antagonist, is a broad spectrum nAChR antagonist which preferentially blocks the a3ß4 nAChR subtype (25), but may also inhibit noncompetitively transfected human a4ß2 nAChRs (21). This antagonist blocks CA1 rat hippocampal interneuron a3ß4-containing nAChRs at 10 µM (25) and blocks rat cerebellum glutamate receptors at a ten times higher concentration (26). In several systems, including rat cerebellum (26) and 3-6-day-old chick retina (27), a-bungarotoxin-insensitive nAChRs evoke a calcium-dependent release of glutamate. In retinal cells in culture for 4 days a very high dose of mecamylamine (100 µM) was necessary to block the acetylcholine-induced response, suggesting that this antagonist was probably blocking glutamatergic receptors. Therefore, in spite of the presence of a3-like immunoreactivity in DIV4 cultured retinal cells, we cannot suggest that the acetylcholine-induced increase in extracellular acidification rate is being mediated by a3ß4-containing acetylcholine receptors, which are highly sensitive to mecamylamine.
DHßE, the broad spectrum non-a7 and non-a8 nAChR antagonist, is a pharmacological tool for the study of non-a-bungarotoxin-sensitive receptors (28). In cells stably transfected with a4ß2-nAChRs, the Ki value for DHßE varies from 20 nM (29) to 3.5 µM (21), whereas the functional responses were fully blocked by 100 nM in rat a4ß2 receptors expressed in HEK293 cells (29) or presented an IC50 value of 1.5 µM in human a4ß2 nAChRs expressed in human epithelial cells (21). Native a4ß2-nAChRs expressed in rat brain (30,31) and a4ß4-nAChRs expressed in 1-day-old chick retina (20) bind DHßE with nM affinity. In rat striatal neurons DHßE inhibits a4ß2 nAChR-induced dopamine release in the µM range (32). In addition, human and rat a2ß2 nAChRs expressed in Xenopus oocytes are less sensitive to DHßE than a4ß2 nAChRs (33,34). A quantitative comparison of dose-inhibition curves for acetylcholine-induced current in a2ß4- and a4ß2-containing receptors expressed in Xenopus oocytes (RNA ratio 1:1) showed a significantly lower sensitivity in a2ß4-containing receptors (IC50 = 30 µM) than in a4ß2-containing receptors (9.6 µM) (28). In our study, DHßE inhibited the acetylcholine-induced increase in extracellular acidification rate in DIV4 cultures with an IC50 value of 38 µM. This value is much higher than that observed for the classical a4ß2 nAChRs, but is in the range observed for a2ß4-containing nAChRs. Both a2 and ß4 units are known to be expressed in embryonic chick retina (4,19). Therefore, in DIV4 cultures acetylcholine stimulated a-bungarotoxin-insensitive receptors with a pharmacological profile, considering the minimal effect of mecamylamine and the IC50 of DHßE similar to that of a2ß4 nAChRs.
In DIV5 cultured retinal cells acetylcholine promoted a more efficient response, which was blocked by a-bungarotoxin, known to antagonize a7- and a8-containing receptors (6). Alpha8-nAChR is the predominant subtype in embryonic and post-hatched chick retina (35) and, in addition, isolated a7- and a7a8-containing receptors have also been detected (6,35). The Kd values of a-bungarotoxin at a8-binding sites in embryonic (6) and post-hatched chick retina (36) are 17.4 and 5.5 nM, respectively, while for DIV4 and DIV5 cultured retinal cells the Kd values determined in the present study were 17.9 and 5.9 nM, respectively. Therefore, similar changes in Kd values were observed between equivalent developmental phases in vivo and in vitro. The coincidence between the appearance of an a-bungarotoxin-sensitive response and the decrease in Kd may be considered evidence that the receptor with a higher affinity is the functional one. Another important consideration is that a reduction in the number of a-bungarotoxin binding sites was observed between DIV4 and DIV5 cultures, confirming previous reports (37). Chronic exposure of a-bungarotoxin-sensitive nAChRs to agonists such as nicotine is classically associated with up-regulation of the number of receptors determined in binding assays (for a review, see Ref. 38). This was named the paradox of nicotinic acetylcholine up-regulation by nicotine. The mechanism underlying this desensitization is not well understood, but it is known that it does not require protein synthesis and that it is supposed to be a result of recruitment or stabilization of the receptors at the membrane surface (for a review, see Ref. 39). Therefore, it was not a surprise that the reduction in the number of a-bungarotoxin binding sites was accompanied by the appearance of the functional response between DIV4 and DIV5 retinal cultures.
Effect of melatonin on the development of the a-bungarotoxin-sensitive acetylcholine-induced response
Melatonin has been shown to modulate the expression and the activity of a7 nAChRs in sympathetic nerve terminals of rat vas deferens (10,12,13) and cerebellum (11). The release of noradrenaline in the vas deferens or of glutamate in the cerebellum is increased by melatonin. The cultured chick retina cells were found to be an interesting model to evaluate whether the effect of melatonin on a-bungarotoxin-sensitive nAChRs is a more general mechanism since the cells develop a robust response mediated by these receptors between the 4th and the 5th day in culture.
Melatonin is continuously synthesized by cultured retinal cells (15), and this synthesis can be blocked by stimulating D4 dopamine receptors with quinpirole (14). On the other hand, the effect of melatonin on chick retina is blocked by luzindole, a classical inhibitor of melatonin G-protein-coupled receptors. In order to test if the development of the response mediated by acetylcholine-induced a-bungarotoxin-sensitive nAChRs depends on melatonin produced by the cells in culture, we blocked the melatonin receptors with luzindole, or inhibited melatonin synthesis with quinpirole. Both strategies resulted in the inhibition of the development of the greater a-bungarotoxin-sensitive response to acetylcholine in DIV5 cultures, while they had no effect on the smaller a-bungarotoxin-insensitive response observed at DIV4. This result agrees with the functional responses observed in the rat vas deferens (13) and in the cerebellum (11), where it was shown that melatonin increases the functional response to stimulation of a-bungarotoxin-sensitive acetylcholine receptors.
We have shown that in cultured chick retina the a-bungarotoxin-insensitive nAChRs are active before the sensitive ones, and that the development of the functional nAChRs sensitive to a-bungarotoxin is dependent on the production of melatonin. The data obtained with the embryo retinal cells in culture are somewhat similar to those observed in rat sympathetic nerve terminals and in the cerebellum (11,12). In all cases melatonin only interferes with nAChRs sensitive to a-bungarotoxin. Since melatonin is a key hormone in the chronophysiology of vertebrates, this common pattern probably reflects a more general mechanism of regulation of a-bungarotoxin-sensitive nAChRs.
The authors would like to thank Prof. Alberto A.G.F.C. Ribeiro, Institute of Bioscience, USP, for the use of the confocal microscope, and the technician Waldir Caldeira for excellent assistance with the confocal microscope.
Correspondence and Footnotes

Address for correspondence: R.P. Markus, Departamento de Fisiologia, Instituto de Biociências, USP, Rua do Matão, Travessa 14, 05508-900, São Paulo, SP, Brasil. Fax: +55-11-3091-7612. E-mail: rpmarkus@usp.br
Research supported by FAPESP (No. 00/0659-2). L.F.S. Sampaio was the recipient of a graduate fellowship from CNPq; D.E. Hamassaki-Britto and R.P. Markus are recipients of research fellowships from CNPq. Received April 30, 2004. Accepted February 10, 2005.
- 1. Britto LR, Hamassaki-Britto DE, Ferro ES, Keyser KT, Karten HJ & Lindstrom JM (1992). Neurons of the chick brain and retina expressing a-bungarotoxin-sensitive and a-bungarotoxin-insensitive nicotinic acetylcholine receptors: an immunohistochemical analysis. Brain Research, 590: 193-200.
- 2. Wong WT & Wong RO (2001). Changing specificity of neurotransmitter regulation of rapid dendritic remodeling during synaptogenesis. Nature Neuroscience, 4: 351-352.
- 3. Le Novere N, Corringer PJ & Changeux JP (2002). The diversity of subunit composition in nAChRs: evolutionary origins, physiologic and pharmacologic consequences. Journal of Neurobiology, 53: 447-456.
- 4. Vailati S, Moretti M, Longhi R, Rovati GE, Clementi F & Gotti C (2003). Developmental expression of heteromeric nicotinic receptor subtypes in chick retina. Molecular Pharmacology, 63: 1329-1337.
- 5. Hamassaki-Britto DE, Brzozowska-Prechtl A, Karten HJ, Lindstrom JM & Keyser KT (1991). GABA-like immunoreactive cells containing nicotinic acetylcholine receptors in the chick retina. Journal of Comparative Neurology, 313: 394-408.
- 6. Keyser KT, Britto LR, Schoepfer R, Whiting P, Cooper J, Conroy W, Brzozowska-Prechtl A, Karten HJ & Lindstrom JM (1993). Three subtypes of a-bungarotoxin-sensitive nicotinic acetylcholine receptors are expressed in chick retina. Journal of Neuroscience, 13: 442- 454.
- 7. Hamassaki-Britto DE, Gardino PF, Hokoç JN, keyser KT, Karten HJ, lindstrom JM & britto LRG (1994). Differential development of a-bungarotoxin-sensitive and a-bungarotoxin-insensitive nicotinic acetylcholine receptors in the chick retina. Journal of Comparative Neurology, 347: 161-170.
- 8. Spira AW, Millar TJ, Ishimoto I, Epstein ML, Johnson CD, Dahl JL & Morgan IG (1987). Localization of choline acetyltransferase-like immunoreactivity in the embryonic chick retina. Journal of Comparative Neurology, 260: 526-538.
- 9. Morley BJ & Garner LL (1990). Light-dark variation in response to chronic nicotine treatment and the density of hypothalamic a-bungarotoxin receptors. Pharmacology, Biochemistry, and Behavior, 37: 239-245.
- 10. Carneiro RC, Cipolla-Neto J & Markus RP (1991). Diurnal variation of the rat vas deferens contraction induced by stimulation of presynaptic nicotinic receptors and pineal function. Journal of Pharmacology and Experimental Therapeutics, 259: 614-619.
- 11. Markus RP, Santos JM, Zago W & Reno LA (2003). Melatonin nocturnal surge modulates nicotinic receptors and nicotine-induced [3H]-glutamate release in rat cerebellum slices. Journal of Pharmacology and Experimental Therapeutics, 305: 525-530.
- 12. Zago WM & Markus RP (1999). Melatonin modulation of presynaptic nicotinic acetylcholine receptors located on short noradrenergic neurons of the rat vas deferens: a pharmacological characterization. Brazilian Journal of Medical and Biological Research, 32: 999-1006.
- 13. Carneiro RC, Pereira EP, Cipolla-Neto J & Markus RP (1993). Age-related changes in melatonin modulation of sympathetic neurotransmission. Journal of Pharmacology and Experimental Therapeutics, 266: 1536-1540.
- 14. Zawilska JB, Derbiszewska T & Nowak JZ (1997). Prolonged exposure of chicks to light or darkness differentially affects the quinpirole-evoked suppression of serotonin N-acetyltransferase activity in chick retina: an impact on dopamine D4-like receptor. Journal of Pineal Research, 22: 59-64.
- 15. Haque R, Alonso-Gomez AL, Chaurasia SS & Iuvone PM (2003). Diurnal regulation of arylalkylamine N-acetyltransferase activity in chicken retinal cells in vitro: analysis of culture conditions. Molecular Vision, 9: 52-59.
- 16. Iuvone PM (1990). Development of melatonin synthesis in chicken retina: regulation of serotonin N-acetyltransferase activity by light, circadian oscillations, and cyclic AMP. Journal of Neurochemistry, 54: 1562-1568.
- 17. Sampaio LFS & Paes-de-Carvalho R (1998). Developmental regulation of group III metabotropic glutamate receptors modulating adenylate cyclase activity in the avian retina. Neurochemistry International, 33: 367-374.
- 18. McConnell HM, Owicki JC, Parce JW, Miller DL, Baxter GT, Wada HG & Pitchford S (1992). The cytosensor microphysiometer: biological application of silicon technology. Science, 257: 1906-1912.
- 19. De-Mello MCF, Ventura ALM, Paes-de-Carvalho A & De-Mello FG (1982). Regulation of dopamine- and adenosine-dependent adenylate cyclase systems of chicken embryo retina cells in culture. Proceedings of the National Academy of Sciences, USA, 79: 5708-5712.
- 20. Bradford MM (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72: 248-254.
- 21. Alkondon M, Pereira EF, Eisenberg HM & Albuquerque EX (2000). Nicotinic receptor activation in human cerebral cortical interneurons: a mechanism for inhibition and disinhibition of neuronal networks. Journal of Neuroscience, 20: 66-75.
- 22. Barabino B, Vailati S, Moretti M, McIntosh JM, Longhi R, Clementi F & Gotti C (2001). An a4ß4 nicotinic receptor subtype is present in chick retina: identification, characterization and pharmacological comparison with the transfected a4ß4 and a6ß4 subtypes. Molecular Pharmacology, 59: 1410-1417.
- 23. Ikeda K, Kobayashi S, Suzuki M, Miyata K, Yamada T & Honda K (1999). Ca2+ mobilization and activation of extracellular acidification rate by carbachol inacutely dispersed cells from guinea pig detrusor: fura 2 fluorometry and microphysiometry using the cytosensor. Life Sciences, 65: 1569-1577.
- 24. Ferreira ZS, Garcia CR, Spray DC & Markus RP (2003). P2Y(1) receptor activation enhances the rate of rat pinealocyte-induced extracellular acidification via a calcium-dependent mechanism. Pharmacology, 69: 33-37.
- 25. Dunlop J, Zhang Y, Smith DL & Schechter LE (1998). Characterization of 5-HT1A receptor functional coupling in cells expressing the human 5-HT1A receptor as assessed with the cytosensor microphysiometer. Journal of Pharmacological and Toxicological Methods, 40: 47-55.
- 26. Eaton JB, Peng JH, Schroeder KM, George AA, Fryer JD, Krishnan C, Buhlman L, Kuo YP, Steinlein O & Lukas RJ (2003). Characterization of human alpha 4 beta 2-nicotinic acetylcholine receptors stably and heterologously expressed in native nicotinic receptor-null SH-EP1human epithelial cells. Molecular Pharmacology, 64: 1283-1294.
- 27. Reno LA, Zago W & Markus RP (2004). Release of [3H]-L-glutamate by stimulation of nicotinic acetylcholine receptors in rat cerebellar slices. Neuroscience, 124: 647-653.
- 28. Sheardown MJ (1997). The triggering of spreading depression in the chick retina by nicotinic receptor agonists. European Journal of Pharmacology, 337: 209-212.
- 29. Khiroug SS, Khiroug L & Yakel JL (2004). Rat nicotinic acetylcholine receptor alpha2beta2 channels: comparison of functional properties with alpha4beta2 channels in Xenopus oocytes. Neuroscience, 124: 817-822.
- 30. Sabey K, Paradiso K, Zhang J & Steinbach JH (1999). Ligand binding and activation of rat nicotinic alpha4beta2 receptors stably expressed in HEK293 cells. Molecular Pharmacology, 55: 58-66.
- 31. Williams M & Robinson JL (1984). Binding of the nicotinic cholinergic antagonist, dihydro-beta-erythroidine, to rat brain tissue. Journal of Neuroscience, 4: 2906-2911.
- 32. Matsubayashi H, Amano T, Seki T, Sasa M & Sakai N (2004). Postsynaptic alpha4beta2 and alpha7 type nicotinic acetylcholine receptors contribute to the local and endogenous acetylcholine-mediated synaptic transmissions in nigral dopaminergic neurons. Brain Research, 1005: 1-8.
- 33. Harvey SC, Maddox FN & Luetje CW (1996). Multiple determinants of dihydro-beta-erythroidine sensitivity on rat neuronal nicotinic receptor alpha subunits. Journal of Neurochemistry, 67: 1953-1959.
- 34. Chavez-Noriega LE, Crona JH, Washburn MS, Urrutia A, Elliott KJ & Johnson EC (1997). Pharmacological characterization of recombinant human neuronal nicotinic acetylcholine receptors h alpha 2 beta 2, h alpha 2 beta 4, h alpha 3 beta 2, h alpha 3 beta 4, h alpha 4 beta 2, h alpha 4 beta 4 and h alpha 7 expressed in Xenopus oocytes. Journal of Pharmacology and Experimental Therapeutics, 280: 346-356.
- 35. Gotti CM, Moretti R, Longhi L, Bricini B, Balestra F & Clementi F (1994). Expression of a-bungarotoxin receptor subtypes in chick central nervous system during development. Journal of Receptor Research, 14: 335-346.
- 36. Gotti C, Moretti M, Maggi R, Longhi R, Hanke W, Klinke N & Clementi F (1997). Alpha7 and alpha8 nicotinic receptor subtypes immunopurified from chick retina have different immunological, pharmacological and functional properties. European Journal of Neuro science, 9: 1201-1211.
- 37. De Pomerai DI, Kotecha B, Flor-Henry M, Fullick C, Young A & Gali MAH (1983). Expression of differentiation markers by chick embryo neuroretinal cells in vivo and in culture. Journal of Embryology and Experimental Morphology, 77: 201-220.
- 38. Wonnacott S (1990). The paradox of nicotinic acetylcholine receptor upregulation by nicotine. Trends in Pharmacological Sciences, 11: 216-218.
- 39. Dani JA & Heinemann S (1996). Molecular and cellular aspects of nicotine abuse. Neuron, 16: 905-908.
Acknowledgments
Publication Dates
-
Publication in this collection
13 Apr 2005 -
Date of issue
Apr 2005
History
-
Received
30 Apr 2004 -
Accepted
10 Feb 2005