Abstract
We investigated the systemic and regional hemodynamic effects of early crystalloid infusion in an experimental model of septic shock induced by intravenous inoculation with live Escherichia coli. Anesthetized dogs received an intravenous infusion of 1.2 x 10(10) cfu/kg live E. coli in 30 min. After 30 min of observation, they were randomized to controls (no fluids; N = 7), or fluid resuscitation with lactated Ringer's solution, 16 ml/kg (N = 7) or 32 ml/kg (N = 7) over 30 min and followed for 120 min. Cardiac index, portal blood flow, mean arterial pressure, systemic and regional oxygen-derived variables, blood lactate, and gastric PCO2 were assessed. Rapid and progressive cardiovascular deterioration with reduction in cardiac output, mean arterial pressure and portal blood flow (~50, ~25 and ~70%, respectively) was induced by the live bacteria challenge. Systemic and regional territories showed significant increases in oxygen extraction and in lactate levels. Significant increases in venous-arterial (~9.6 mmHg), portal-arterial (~12.1 mmHg) and gastric mucosal-arterial (~18.4 mmHg) PCO2 gradients were also observed. Early fluid replacement, especially with 32 ml/kg volumes of crystalloids, promoted only partial and transient benefits such as increases of ~76% in cardiac index, of ~50% in portal vein blood flow and decreases in venous-arterial, portal-arterial, gastric mucosal-arterial PCO2 gradients (7.2 ± 1.0, 7.2 ± 1.3 and 9.7 ± 2.5 mmHg, respectively). The fluid infusion promoted only modest and transient benefits, unable to restore the systemic and regional perfusional and metabolic changes in this hypodynamic septic shock model.
Cardiac output; Escherichia coli; Gas tonometry; Portal blood flow; Septic shock
Braz J Med Biol Res, June 2005, Volume 38(06) 873-884
Short-lasting systemic and regional benefits of early crystalloid infusion after intravenous inoculation of dogs with live Escherichia coli
A.G. Garrido,  L.F. Poli de Figueiredo, R.J. Cruz Jr., E. Silva and M. Rocha e Silva
L.F. Poli de Figueiredo, R.J. Cruz Jr., E. Silva and M. Rocha e Silva
Serviço de Fisiologia Aplicada, InCor, Hospital das Clínicas, Faculdade de Medicina, Universidade de São Paulo, São Paulo, SP, Brasil
 References
References
 Correspondence and Footnotes
Correspondence and Footnotes
Correspondence and Footnotes
Correspondence and Footnotes
Correspondence and Footnotes
Correspondence and Footnotes
Abstract
We investigated the systemic and regional hemodynamic effects of early crystalloid infusion in an experimental model of septic shock induced by intravenous inoculation with live Escherichia coli. Anesthetized dogs received an intravenous infusion of 1.2 x 1010 cfu/kg live E. coli in 30 min. After 30 min of observation, they were randomized to controls (no fluids; N = 7), or fluid resuscitation with lactated Ringer's solution, 16 ml/kg (N = 7) or 32 ml/kg
(N = 7) over 30 min and followed for 120 min. Cardiac index, portal blood flow, mean arterial pressure, systemic and regional oxygen-derived variables, blood lactate, and gastric PCO2 were assessed. Rapid and progressive cardiovascular deterioration with reduction in cardiac output, mean arterial pressure and portal blood flow (~50, ~25 and ~70%, respectively) was induced by the live bacteria challenge. Systemic and regional territories showed significant increases in oxygen extraction and in lactate levels. Significant increases in venous-arterial (~9.6 mmHg), portal-arterial (~12.1 mmHg) and gastric mucosal-arterial (~18.4 mmHg) PCO2 gradients were also observed. Early fluid replacement, especially with 32 ml/kg volumes of crystalloids, promoted only partial and transient benefits such as increases of ~76% in cardiac index, of ~50% in portal vein blood flow and decreases in venous-arterial, portal-arterial, gastric mucosal-arterial PCO2 gradients (7.2 ± 1.0, 7.2 ± 1.3 and 9.7 ± 2.5 mmHg, respectively). The fluid infusion promoted only modest and transient benefits, unable to restore the systemic and regional perfusional and metabolic changes in this hypodynamic septic shock model.
Key words: Cardiac output, Escherichia coli, Gas tonometry, Portal blood flow, Septic shock
Introduction
Severe sepsis and septic shock are associated with a complex systemic hemodynamic profile, widespread microcirculatory abnormalities and cellular alterations leading to an uncoupling of blood flow from metabolic tissue requirements. These alterations are implicated in multiple organ dysfunction development, responsible for the high mortality rates observed in septic patients (1-4).
The splanchnic territory, particularly the gut mucosa, is highly vulnerable to reductions in oxygen supply and prone to early injury in the course of shock (4-9). Incomplete splanchnic resuscitation with gut hypoxia or ischemia is one possible factor contributing to gastrointestinal tract barrier dysfunction and translocation of bacteria and bacterial products that have been implicated in the genesis, amplification and perpetuation of the systemic inflammatory response and sepsis (1,2,4,9-12).
Since hypovolemia is frequently present in the early stages of sepsis and is a key factor contributing to low oxygen delivery to the tissues, fluid replacement has been considered essential to restore and to avoid subsequent cardiovascular deterioration in severe sepsis to a septic shock state (1-3,10). Recently, early aggressive fluid resuscitation, guided by central venous oxygen saturation, markedly decreased mortality rate and organ dysfunction in patients with severe sepsis and septic shock (3). However, the disparity between systemic and regional variables has been well demonstrated (6,8,13-17), and the regional impact of early fluid resuscitation remains to be better defined. We studied the systemic and regional hemodynamic impact of early crystalloid infusion on experimental hypodynamic septic shock induced by an intravenous injection of live Escherichia coli.
Material and Methods
The study was approved by the Animal Care and Use Committee of the University of São Paulo Medical School and was conducted in compliance with the guidelines of the Brazilian Regulations for the Care and Use of Laboratory Animals.
Animal preparation
Twenty-one healthy male mongrel dogs weighing 14-20 kg were fasted for 12 h before the study, with free access to water. Anesthesia was induced with an intravenous injection of 0.1 mg/kg morphine sulfate followed by 25 mg/kg sodium pentobarbital. Additional doses of pentobarbital, 2 mg/kg, were used when required. A cuffed endotracheal tube was placed in the trachea to allow mechanical ventilation with a 1.0 inspired fraction of oxygen, at a tidal volume of 15 ml/kg (670 Takaoka ventilator, São Paulo, SP, Brazil). Respiratory rate was adjusted to maintain PaCO2 at 40 ± 5 mmHg. A urinary bladder catheter was placed for urinary drainage and measurement of urinary output. During surgical preparation, a heating pad was used to maintain normothermia, and the animals received lactated Ringer's solution, 20 ml kg-1 h-1, to compensate for fluid losses, including imperceptible perspiration. Lactated Ringer's is a 275-mOsm/l balanced electrolyte solution with 130 mEq/l sodium, 109 mEq/l chloride, 2.7 mEq/l calcium, 4 mEq/l potassium, and 28 mEq/l lactate. Each animal received an intravenous injection of ranitidine, 1.5 mg/kg.
The right common femoral artery was dissected and cannulated with a polyethylene catheter (PE240) to measure mean arterial pressure in the abdominal aorta and to collect arterial blood samples for blood gas and lactate analysis. A catheter (PE240) was introduced through the right common femoral vein for fluid infusion.
A balloon-tipped catheter (5-Fr Arrow® Balloon Thermodilution Catheter, Inc., Reading, PA, USA) was inserted into the pulmonary artery through the right external jugular vein under guidance of pressure waves, as determined by a multichannel monitor system. This catheter was connected to a cardiac computer (Vigilance, Baxter Edwards Critical Care, Irvine, CA, USA) for the measurement of cardiac output using 3-ml bolus injection of isotonic saline at 20ºC. All catheters were connected to disposable pressure transducers (Transpac Disposable Transducer; Abbott, Chicago, IL, USA) and to a computerized multichannel system for biological data acquisition (Acknowledge® III MP 100 WSW; Biopac Systems, Inc., Goleta, CA, USA).
Splenectomy was performed through a midline laparotomy to prevent splenocontraction and autotransfusion of erythrocytes. An ultrasonic flow probe (Transonic Systems, Inc., Ithaca, NY, USA) was placed around the portal vein for the measurement of transit time flow in this vessel (T206 Transonic Volume Flowmeter, Transonic Systems). A P240 catheter was threaded into the portal system via the splenic vein for portal blood sampling. The abdominal cavity was then carefully closed. After the surgical preparation was completed, the animals were allowed to recover for 30 min before the measuring protocol was started and the infusion of lactated Ringer's solution was discontinued.
A large gastric polyethylene tube was orally introduced and placed in the stomach and gastric lavage was performed with warm isotonic saline solution until a clear fluid was obtained at drainage. A 16F TRIP® tonometry catheter (Datex-Ohmeda Division, Instrumentarium Corp., Helsinki, Finland) was introduced orally and positioned in the large curvature of the stomach. The tonometry catheter was then connected to a calibrated gas capnometer (Tonocap, model TC-200, Tonometrics, Datex-Ohmeda Division) for gastric mucosal pCO2 measurement every 10 min.
Bacterial preparation
A strain of E. coli O55, provided by Department of Bacteriology of Adolfo Lutz Institute, São Paulo, SP, Brazil, originating from the stool of a patient with gastrointestinal sepsis, was used in this study. The bacteria were stored in conservative milieu at room temperature, activated in Trypticase Soy Broth, plated in Trypticase Soy Agar (TSA) and incubated at 37ºC for 24 h. Aliquots were then suspended in sterile saline. The bacterial suspension was estimated turbidimetrically by comparing the newly grown bacterial suspension to known standards by spectrophotometry at 625 nm, in order to obtain a culture of desired bacterial density. The same suspension was subsequently quantified by plating successive 10-fold dilutions onto TSA plates and scoring visible colonies after 24 h of incubation at 37ºC. The target dose, as calculated by the methods outlined above, was 3.0 x 109 cells/ml or 0.6 x 1010 cfu/ml, and a dose of 1.2 x 1010 cfu/kg body weight was used to induce sepsis.
Data collection and analysis
Mean systemic and pulmonary arterial pressures, heart rate, and portal vein blood flow were continuously recorded. Cardiac output was determined by the thermodilution technique and is reported as cardiac index according to the estimated body surface area. Each determination was the arithmetic mean of three consecutive measurements when their differences did not exceed 10%. Central venous blood temperature was recorded with the thermistor in the pulmonary artery catheter. No measurement was made to keep body temperature constant after the induction of septic shock.
Blood gases, hemoglobin, hematocrit, and blood lactate levels were obtained from arterial, portal and mixed venous samples at baseline (T0) and then at 30, 60, 90, 120, 150, 180, and 210 min after the initiation of the bacterial infusion (Figure 1). All blood samples were analyzed with a Stat Profile Ultra Analyzer (Nova Biomedical, Waltham, MA, USA). The arterial oxygen content, mixed venous oxygen content, portal oxygen content, systemic oxygen delivery, mesenteric oxygen delivery, systemic oxygen extraction ratio, mesenteric oxygen extraction ratio, systemic oxygen consumption, mesenteric oxygen consumption, and arteriovenous oxygen content difference [C(a - v)O2] were calculated using standard formulae.
The following PCO2 gradients were calculated: PCO2(v - a), as the difference between mixed venous PCO2 and arterial PCO2; PCO2(p - a), as the difference between portal vein PCO2 and arterial PCO2; PCO2(g - a), as the difference between gastric mucosal PCO2, measured by gas tonometry, and arterial PCO2. The PCO2(v - a)/C(a - v)O2 ratio was also calculated.
Experimental protocol
After surgical preparation, the animals were allowed to stabilize for 30 min. At T0, a continuous infusion of E. coli at the dose of 1.2 x 1010 cfu/kg was started and maintained for 30 min in all groups (T30). After 30 min of observation (T60), the animals were randomized into three groups: no fluid treatment (CT; N = 7); fluid treatment with lactated Ringer's solution, 16 ml/kg (LR16; N = 7), or 32 ml/kg (LR32; N = 7) over 30 min (T90). The animals were followed without additional interventions for 120 min (T210). They were then euthanized with a pentobarbital overdose followed by 19.1% potassium chloride injection.
Statistical analysis
Data are reported as means ± SEM. Statistical analysis was performed using the Statistical Package for the Social Sciences for Windows software (version 6.0, SPSS Inc., Chicago, IL, USA). Differences between groups were analyzed using repeated measure analysis of variance and the post hoc Tukey test, with the level of significance set at P < 0.05.
Results
Systemic effects of live Escherichia coli infusion and fluid replacement
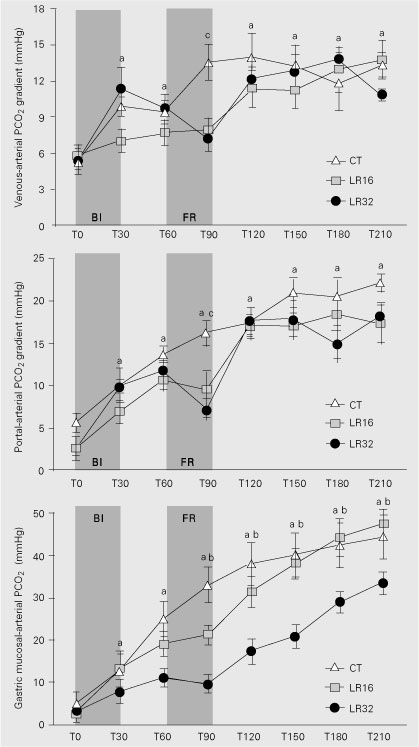
The infusion of a lethal dose of live E. coli promoted a hypodynamic septic shock state in all animals, characterized by hypotension and reduced cardiac index (Figure 2). A progressive significant increase in central temperature was detected in all groups (Figure 3). Increases in systemic oxygen extraction rate, venous-arterial PCO2 gradient and arterial lactate were observed (Table 1, Figure 4). No changes in systemic oxygen consumption or in PCO2(v - a)/C(a - v)O2 ratio were detected throughout the experiment (Table 1). Hemoglobin levels increased in all groups after bacterial infusion (Figure 3).
In the untreated control group, circulatory and metabolic deterioration were progressive. In the treated groups, both fluid replacement regimens were associated with significant systemic hemodynamic changes including partial and transient increases in cardiac index and oxygen delivery, while there was a reduction in systemic oxygen extraction, and systemic oxygen consumption remained unchanged (Figure 2, Table 1). Treated groups also presented a transient hemodilution (Figure 3). By the end of the experimental protocol, these values no longer differed significantly from controls. Although arterial lactate remained higher than baseline values, a sustained and significant reduction was observed in group LR32 when compared to both LR16 and controls. The lactate levels in group LR16 showed a progressive increase throughout the study, being higher than both controls and LR32 (Table 1). Urinary output differed among the three groups according to the amount of fluid given: CT (1.87 ± 0.30 ml/kg), LR16 (3.39 ± 0.37 ml/kg, P < 0.001) and LR32 (8.83 ± 1.16 ml/kg, P < 0.001).
Regional effects of live Escherichia coli infusion and fluid replacement
Live E. coli infusion resulted in marked reductions in portal blood flow (Figure 2) and portal venous oxygen saturation (Table 2), while significant increases in portal oxygen extraction rate, portal lactate and portal-arterial PCO2 gradients were observed (Figure 4; Table 2). Those changes were progressive in controls.
Treated animals showed a partial increase in portal blood flow, which was greater in the LR32 group (Figure 2). Portal-arterial PCO2 gradients were transiently lower in both LR32 and LR16 than in controls (Figure 4). Additionally, portal oxygen extraction was transiently reduced only in LR32. At the end of experiment, no significant differences between groups could be detected for any regional variable (Table 2).
The gastric mucosal-arterial PCO2 gradient began to increase progressively and markedly after bacterial infusion in all groups. LR32 was the only group in which this increase was transiently ameliorated (Figure 4).
Schematic presentation of the experimental protocol. At each time point (T0-T210) blood samples were collected and systemic and regional hemodynamics was measured. Control = no fluids; LR16 and LR32 = fluid resuscitation with 16 or 32 ml/kg lactated Ringer's solution, respectively.
Mean arterial pressure, cardiac index and portal vein blood flow index during the experimental protocol. Data are reported as means ± SEM for N = 7 dogs in each group. BI = bacterial infusion; FR = fluid resuscitation. CT = control group; LR16 = group resuscitated with 16 ml/kg lactated Ringer's solution; LR32 = group resuscitated with 32 ml/kg lactated Ringer's solution. ªP < 0.05 vs T0, bP < 0.05 CT and LR16 vs LR32, cP < 0.05 LR32 and LR16 vs CT (repeated measure analysis of variance and post hoc Tukey's test).
Central temperature and hemoglobin levels during the experimental protocol. Data are reported as means ± SEM for N = 7 dogs in each group. BI = bacterial infusion; FR = fluid resuscitation. CT = control group; LR16 = group resuscitated with 16 ml/kg lactated Ringer's solution; LR32 = group resuscitated with 32 ml/kg lactated Ringer's solution. ªP < 0.05 vs T0, bP < 0.05 CT and LR16 vs LR32 (repeated measure analysis of variance and post hoc Tukey's test).
Venous-arterial pCO2 gradient, portal-arterial pCO2 gradient and gastric mucosal-arterial pCO2 gradient during the experimental protocol. Data are reported as means ± SEM for N = 7 dogs in each group. BI = bacterial infusion; FR = fluid resuscitation. CT = control group; LR16 = group resuscitated with 16 ml/kg lactated Ringer's solution; LR32 = group resuscitated with 32 ml/kg lactated Ringer's solution. ªP < 0.05 vs T0, bP < 0.05 CT and LR16 vs LR32, cP < 0.05 LR32 and LR16 vs CT (repeated measure analysis of variance and post hoc Tukey's test).
Discussion
Early fluid replacement has been considered essential to avoid sepsis-induced multiple organ dysfunction and death (1-3,11). Using an intravenous injection of a lethal dose of live E. coli, we reproduced the major hemodynamic and metabolic derangements of typical non-resuscitated hypodynamic septic shock. These striking alterations were detected at both the systemic and regional levels, with a remarkable involvement of splanchnic perfusion. Early fluid replacement promoted only partial and very transient benefits, only during the fluid infusion period, which were unable to restore sepsis-induced perfusional deficits.
The reductions of about 50% in cardiac output and 25% in mean arterial pressure were abrupt and began immediately after the initiation of bacterial infusion, as also reported by others (14,17,18). These severe alterations may have resulted from a combination of arterial and venous vasodilatation, hypovolemia and myocardial depression. Bacteria-induced alterations in peripheral vascular tone leading to venous pooling of blood and reduced venous return probably accounted for the immediate decrease in cardiac output and regional blood flows. The more modest decrease in mean arterial pressure in comparison to the greater decrease in cardiac output and flows probably reflected a complex combination of vasoconstriction and vasodilatation.
The increase in hemoglobin levels in our splenectomized dogs after the bacterial challenge suggested hypovolemia due to ongoing fluid losses, probably through increased microvascular permeability. Moreover, treated animals showed a very brief period of hemodilution followed by hemoconcentration, suggesting rapid extravascular redistribution of
the infused fluid volume.
Benefits, particularly with the larger volume of crystalloid, were only transient, possibly because of the ongoing capillary leakage as well as the sustained alterations in vascular tonus and cardiodepression. Experimental studies have clearly demonstrated the cardiodepressant effects of endotoxin and live bacteria (18-21). A reversible reduction in ejection fraction and biventricular dilatation is the typical pattern of cardiac performance during septic shock (18,19). A relevant clinical study by Rivers et al. (3) highlighted the important role of early fluid infusion and inotropic support in sepsis, in agreement with our experimental findings.
In response to declines in oxygen supply after bacteremia, an increase in systemic oxygen extraction rate was accompanied by changes in other global markers of perfusion, such as reduced mixed venous oxygen saturation and increased arterial lactate and venous-arterial PCO2 gradient. Baseline systemic oxygen consumption was low in our animals, possibly due to anesthesia and mechanical ventilation, and remained without significant alterations throughout the experimental protocol. This could be explained by the complex mixture between factors leading to decreases in VO2, such as low oxygen delivery and blood flow, and to increments in VO2 due to inflammatory reaction and cellular metabolic changes. In fact, it cannot be assumed that a critical oxygen delivery was reached. In this scenario, the observed hyperlactatemia could not have resulted exclusively from tissue hypoxia (22,23). Other mechanisms could have played a role, including inhibition of one or of several enzymatic reactions, such as pyruvate dehydrogenase, the ADP/ATP translocases, creatine kinase, and high aerobic metabolism increasing production of pyruvate or reduction in liver clearance (22-24).
Responses to fluid challenge suggested that perfusional deficits may have contributed to lactate increases. In the LR32 group we could observe a peak in lactate immediately after infusion, suggesting that a washout occurred, followed by a partial reduction in lactate levels. On the other hand, the LR16 group showed a progressive increase in lactate to levels even higher than control. We may speculate that the lack of fluid resuscitation in controls could be associated with limited lactate washout from tissues, thereby increasing less than expected.
The systemic venous-arterial PCO2 gradient inversely mirrored the alterations in cardiac output, as also observed in both clinical and experimental studies (17,25-27). A 50% decrease in cardiac output promoted an increase of about 66% in this gradient. Decreased blood flow with increase in tissue transit time and decreases in pulmonary blood flow are the main factor accounting for this phenomenon (27,28). Moreover, an increase in aerobic production of CO2, without the required proportional increase of blood flow, may also contribute to this finding (9,26,28).
Recently, the PCO2(v - a)/C(a - v)O2 ratio has been suggested as a global marker of anaerobic CO2 generation and thus of the occurrence of anaerobic metabolism whatever the blood flow conditions in critically ill patients (29). However, this ratio did not undergo marked changes throughout our study, in spite of oxygen delivery compromise and clear evidence of metabolic change. Two reasons may be suggested to account for this phenomenon. First, a critical oxygen delivery was not reached. Second, this ratio is a global marker of tissue hypoxia, unable to detect microregional tissue oxygen debt.
In our previous study, using a sublethal dose of live E. coli, a similar fluid regimen was able to restore most systemic and regional hemodynamic and metabolic variables (17). Gastric mucosal-arterial PCO2 gradient was the variable unable to improve after fluid infusion. In the present study, the lethal dose of live bacteria induced profound alterations in splanchnic perfusion. Portal blood flow decreased by 70% from baseline, paralleling the increases in portal oxygen extraction, portal-arterial PCO2 gradient and portal lactate. The magnitude of these regional changes was greater than the magnitude of systemic alterations. Moreover, the transient benefits after fluid replacement were much less evident within the splanchnic region. These factors explain why therapeutic interventions are sometimes ineffective in achieving adequate splanchnic oxygenation in sepsis, even when global hemodynamic and oxygen-derived variables appear to be adequate (2,4,6,8,9,17,30-35).
We observed that the portal-arterial PCO2 gradient accompanied the changes of portal blood flow while the gastric mucosal-arterial PCO2 gradient did not parallel either systemic or regional blood flow trends, suggesting that microcirculatory blood flow distribution within the gastrointestinal wall cannot be predicted from macrocirculatory regional or systemic blood flow.
In fact, several experimental and clinical studies have failed to demonstrate a good correlation between gastric mucosal-arterial PCO2 gradient and hepatosplanchnic blood flow (8,17,32-39).
Some special characteristics of the splanchnic microvascular bed may contribute to these findings. The counter-current blood flow exchange system between the arterial and venous circulation within the superficial mucosal layer renders the gastrointestinal tract particularly sensitive to neuronal and systemic vasoconstrictors. There is a high concentration of receptors for systemically released vasoconstrictors within the splanchnic microcirculation. Moreover, the intestinal tract possesses a lower capillary density and is unable to recruit capillaries to augment local blood flow to match increases in metabolic needs. This results in low perfusion-to-oxygen demand ratios and subsequent tissue dysoxia even when systemic variables are adequate (4,9). Also, derangements in cellular energy metabolism, such as uncoupling of oxidative phosphorylation, inhibition of mitochondrial respiration, and reduced availability of substrates induced by dysoxia, cytokines and/or bacteria, non-responsive to changes in the regional oxygen supply, should be considered in this context (4,9,12,13,33).
There are limitations in our experimental model and protocol, as is also true for most other models of sepsis and septic shock (40). Our model induced an immediate hemodynamic collapse. This behavior is seldom observed in clinical sepsis, although it may mimic the extreme clinical sepsis such as seen in meningococcemia, pneumococcal bacteremia in asplenic individuals, and Gram-negative bacteremia in the setting of profound granulocytopenia. Moreover, no septic patient is treated with a fixed volume of fluid challenge without additional interventions. However, our goal was to address the acute impact of live bacteria and a single, but early fluid challenge with crystalloids, on systemic and regional levels, thereby allowing us to detect the long-lasting effects of this intervention. Crystalloid solutions are the most readily available and the least expensive resuscitation fluid, and are widely used in the management of most forms of shock (1). Normal saline resuscitation may lead to a hyperchloremic metabolic acidosis, but lactated Ringer's solution does not result in acidosis (1). And, in spite of the short period of fluid challenge, the amount of fluid used is the same as administered in the initial management of septic patients, and its salt load has been efficient to restore systemic perfusion in experimental hemorrhagic shock and severe sepsis (8,17). Prolonged fluid infusion, distinct types of fluids, inotropes and other interventions could alter substantially our findings and could result in a typical resuscitated hyperdynamic septic shock (2). Those questions should be evaluated in specifically designed experimental studies.
Although caution must be exercised when drawing clinical implications from animal studies, we believe that we were able to simulate the complex cardiovascular and metabolic changes observed in hypodynamic septic shock, including the disparity between systemic and regional parameters. The differentiation between the direct microvascular and metabolic effects of live bacteria from those produced by decreases in oxygen supply remains to be clarified.
Within the limitations of our study protocol, we conclude that early crystalloid infusion resulted in partial and transient benefits, essentially during the fluid infusion, which were especially poor in the splanchnic territory. These findings suggest significant early capillary leakage with rapid extravascular redistribution of the crystalloid administered, and emphasize that further studies are needed to search for fluids more efficient in maintaining the intravascular volume in septic patients. Furthermore, the modest effects of fluid resuscitation at the regional level emphasize that monitoring the gastrointestinal territory is probably desirable to improve splanchnic resuscitation, which may play an important role in the development of multiple organ failure and death during severe sepsis and septic shock.
Address for correspondence: L.F. Poli de Figueiredo, Serviço de Fisiologia Aplicada, HC, FM, USP, Rua Dr. Enéas C. Aguiar, 44, 05403-900, São Paulo, SP, Brasil. Fax: +55-11-3085-7887. E-mail: expluiz@incor.usp.br
Research supported by FAPESP (No. 98/15658-0). Received July 30, 2004. Accepted January 24, 2005.
- 1. Hollenberg SM, Ahrens TS, Annane D et al. (2004). Practice parameters for hemodynamic support of sepsis in adult patients: 2004 update practice parameters for hemodynamic support of sepsis in adult patients. Critical Care Medicine, 32: 1928-1948.
- 2. Reinhart K, Sakka SG & Meier-Hellmann A (2000). Haemodynamic management of a patient with septic shock. European Journal of Anaesthesiology, 17: 6-17.
- 3. Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, Peterson E & Tomlanovich M (2001). Early goal-directed therapy in the treatment of severe sepsis and septic shock. New England Journal of Medicine, 345: 1368-1377.
- 4. Pastores SM, Katz DP & Kvetan V (1996). Splanchnic ischemia and gut injury in sepsis and the multiple organ dysfunction syndrome. American Journal of Gastroenterology, 91: 1697-1710.
- 5. Fink MP (1991). Gastrointestinal mucosal injury in experimental models of shock, trauma, and sepsis. Critical Care Medicine, 19: 627-641.
- 6. Hamilton-Davies C, Mythen MG, Salmon JB, Jacobson D, Shukla A & Webb AR (1997). Comparison of commonly used clinical indicators of hypovolaemia with gastrointestinal tonometry. Intensive Care Medicine, 23: 276-281.
- 7. Knichwitz G, Rötker J, Möllhoff T, Richter KD & Brussel T (1998). Continuous intramucosal pCO2 measurement allows the early detection of intestinal malperfusion. Critical Care Medicine, 26: 1550-1557.
- 8. Cruz Junior RJ, Yada-Langui MM, Poli de Figueiredo LF & Rocha e Silva M (2002). Effects of hemorrhage and rapid fluid resuscitation on splanchnic blood flow and gastrointestinal mucosal perfusion evaluated by gas tonometry. ABCD Arquivos Brasileiros de Cirurgia Digestiva, 15: 74-78.
- 9. Silva E & Poli de Figueiredo LF (2002). Gas tonometry. In: Vincent JL (Editor), The Sepsis Text. Kluwer Academic Publishers, New York.
- 10. Doig CJ, Sutherland LR, Sandham JD, Fick GH, Verhoef M & Meddings JB (1998). Increased intestinal permeability is associated with the development of multiple organ dysfunction syndrome in critically ill ICU patients. American Journal of Respiratory and Critical Care Medicine, 158: 444-451.
- 11. Oi Y, Aneman A, Svensson M, Ewert S, Dahlqvist M & Haljamae H (2000). Hypertonic saline-dextran improves intestinal perfusion and survival in porcine endotoxin shock. Critical Care Medicine, 28: 2843-2850.
- 12. Ding J, Magnotti LJ, Huang Q, Xu DZ, Condon MR & Deitch EA (2001). Hypoxia combined with Escherichia coli produces irreversible gut mucosal injury characterized by increased intestinal cytokine production and DNA degradation. Shock, 16: 189-195.
- 13. Whitworth PW, Cryer HM, Garrison RN, Baumgarten TE & Harris PD (1989). Hypoperfusion of the intestinal microcirculation without decreased cardiac output during live Escherichia coli sepsis in rats. Circulatory Shock, 27: 111-122.
- 14. Schneider AJ, Groeveneld AB, Teule GJ, Wesdorp RI & Thijs LG (1991). Total body blood volume redistribution in porcine E. coli septic shock: effect of volume loading, dobutamine, and norepinephrine. Circulatory Shock, 35: 215-222.
- 15. Vallet B, Lund N, Curtis SE, Kelly D & Cain SM (1994). Gut and muscle tissue PO2 in endotoxemic dogs during shock and resuscitation. Journal of Applied Physiology, 76: 793-800.
- 16. Hiltebrand LB, Krejci V, Banic A, Erni D, Wheatley AM & Sigurdsson GH (2000). Dynamic study of the distribution of microcirculatory blood flow in multiple splanchnic organs in septic shock. Critical Care Medicine, 28: 3233-3241.
- 17. Lagoa CE, Poli de Figueiredo LF, Cruz Junior RJ, Silva E & Rocha e Silva M (2004). Effects of fluid resuscitation on splanchnic perfusion in a canine severe sepsis model. Critical Care, 8: R221-R228.
- 18. Natanson C, Danner RL, Fink MP, MacVittie TJ, Walker RI, Conklin JJ & Parrillo JE (1988). Cardiovascular performance with E. coli challenges in a canine model of human sepsis. American Journal of Physiology, 254 (Part 3): H558-H559.
- 19. Natanson C, Fink MP, Ballantyne HK, MacVittie TJ, Conklin JJ & Parrillo JE (1986). Gram-negative bacteremia produces both severe systolic and diastolic cardiac dysfunction in a canine model that simulates human septic shock. Journal of Clinical Investigation, 78: 259-270.
- 20. Schneider AJ, Teule GJ, Kester AD, Heidendal GA & Thijs LG (1986). Biventricular function during volume loading in porcine E. coli with emphasis on right ventricular function. Circulatory Shock, 18: 53-63.
- 21. Mulder MF, van Lambalgen AA, van der Bos GC & Thijs LG (1996). The fall of cardiac output in endotoxemic rats cannot explain changes in organ blood flow: a comparison between endotoxin and low venous return shock. Shock, 5: 135-140.
- 22. Gutierrez G & Wulf ME (1996). Lactic acidosis in sepsis: a commentary. Intensive Care Medicine, 22: 6-16.
- 23. Vary T (1996). Sepsis-induced alterations in pyruvate dehydrogenase complex activity in rat skeletal muscle: effects on plasma lactate. Shock, 6: 89-94.
- 24. Bellomo R, Kellum JA & Pinsky M (1996). Transvisceral lactate fluxes during early endotoxemia. Chest, 110: 198-204.
- 25. Zhang H & Vincent JL (1993). Arteriovenous differences in PCO2 and pH are good indicators of critical hypoperfusion. American Review of Respiratory Diseases, 148: 867-871.
- 26. Creteur J, De Backer D & Vincent JL (1999). Does gastric tonometry monitor splanchnic perfusion? Critical Care Medicine, 27: 2480-2484.
- 27. Bakker J, Vincent JL, Gris P, Leon M, Coffernils M & Kahn RJ (1992). Veno-arterial carbon dioxide gradient in human septic shock. Chest, 101: 509-515.
- 28. Vallet B, Teboul JL, Cain S & Curtis S (2000). Venoarterial CO2 difference during regional ischemic or hypoxic hypoxia. Journal of Applied Physiology, 89: 1317-1321.
- 29. Mekontso-Dessap A, Castelain V, Anguel N, Bahloul M, Schauvliege F, Richard C & Teboul JL (2002). Combination of venoarterial PCO2 difference with arteriovenous O2 content difference to detect anaerobic metabolism in patients. Intensive Care Medicine, 28: 272-277.
- 30. Hinshaw LB (1996). Sepsis/septic shock: participation of the microcirculation: An abbreviated review. Critical Care Medicine, 24: 1072-1078.
- 31. Ince C & Sinaasappel M (1999). Microcirculatory oxygenation and shunting in sepsis and shock. Critical Care Medicine, 27: 1369-1377.
- 32. Creteur J, De Backer D & Vincent JL (1999). A dobutamine test can disclose hepatosplanchnic hypoperfusion in septic patients. American Journal of Respiratory and Critical Care Medicine, 160: 839-845.
- 33. Fink MP, Rothschild HR, Deniz YF, Wang HL, Lee PC & Cohn SM (1989). Systemic and mesenteric oxygen metabolism in endotoxic pigs. Effects of ibuprofen and meclofenamate. Journal of Applied Physiology, 67: 1950-1957.
- 34. Oud L & Kruse JA (1996). Progressive gastric intramucosal acidosis follows resuscitation from hemorrhagic shock. Shock, 6: 61-65.
- 35. Oud L & Haupt MT (1999). Persistent gastric intramucosal ischemia in patients with sepsis following resuscitation from shock. Chest, 115: 1390-1396.
- 36. Meier-Hellmann A, Specht M, Hannemann L, Hassel H, Bredle DL & Reinhart K (1996). Splanchnic blood flow is greater in septic shock treated with norepinephrine than in severe sepsis. Intensive Care Medicine, 22: 1354-1359.
- 37. Meier-Hellmann A, Bredle DL, Specht M, Hannemann L & Reinhart K (1999). Dopexamine increases splanchnic blood flow but decreases gastric mucosal pH in severe septic patients treated with dobutamine. Critical Care Medicine, 27: 2166-2171.
- 38. De Backer D, Zhang H, Cherkhaoui S, Borgers M & Vincent JL (2001). Effects of dobutamine on hepato-splanchnic hemodynamics in an experimental model of hyperdynamic endotoxic shock. Shock, 15: 208-214.
- 39. De Backer D, Creteur J, Silva E & Vincent JL (2003). Effects of dopamine, norepinephrine, and epinephrine on the splanchnic circulation in septic shock: which is the best? Critical Care Medicine, 31: 1659-1667.
- 40. Garrido AG, Poli de Figueiredo LF & Rocha e Silva M (2004). Experimental models of sepsis and septic shock: an overview. Acta Cirúrgica Brasileira, 19: 82-88.
Correspondence and Footnotes
Publication Dates
-
Publication in this collection
28 Sept 2005 -
Date of issue
June 2005
History
-
Accepted
24 Jan 2005 -
Received
30 July 2004