Abstract
The expression of P53, Bcl-2, Bax, Bag-1, and Mcl-1 proteins in CD5/CD20-positive B-chronic lymphocytic leukemia (B-CLL) cells from 30 typical CLL patients was evaluated before and after 48 h of incubation with 10-6 M fludarabine using multiparametric flow cytometric analysis. Protein expression was correlated with annexin V expression, Rai modified clinical staging, lymphocyte doubling time, and previous treatment. Our main goal was to determine the predictive value of these proteins in CLL cells in terms of disease evolution. Bcl-2 expression decreased from a median fluorescence index (MFI) of 331.71 ± 42.2 to 245.81 ± 52.2 (P < 0.001) after fludarabine treatment, but there was no difference between viable cells (331.57 ± 44.6 MFI) and apoptotic cells (331.71 ± 42.2 MFI) before incubation (P = 0.859). Bax expression was higher in viable cells (156.24 ± 32.2 MFI) than in apoptotic cells (133.56 ± 35.7 MFI) before incubation, probably reflecting defective apoptosis in CLL (P = 0.001). Mcl-1 expression was increased in fludarabine-resistant cells and seemed to be a remarkable protein for the inhibition of the apoptotic process in CLL (from 233.59 ± 29.8 to 252.04 ± 35.5; P = 0.033). After fludarabine treatment, Bag-1 expression was increased in fludarabine-resistant cells (from 425.55 ± 39.3 to 447.49 ± 34.5 MFI, P = 0.012), and interestingly, this higher expression occurred in patients who had a short lymphocyte doubling time (P = 0.022). Therefore, we could assume that Bag-1 expression in such situation might identify CLL patients who will need treatment earlier.
Chronic lymphocytic leukemia; Apoptosis; Fludarabine; P53; Bcl-2; Bax; Mcl-1; Bag-1
Abstract
Introduction
Material and Methods
Results
Braz J Med Biol Res, March 2006, Volume 39(3) 327-333
Fludarabine induces apoptosis in chronic lymphocytic leukemia - the role of P53, Bcl-2, Bax, Mcl-1, and Bag-1 proteins
 Correspondence and Footnotes
Correspondence and Footnotes
 J.R. Faria1, M. Yamamoto2, R.M.D. Faria1, J. Kerbauy2 and J.S.R. Oliveira2
J.R. Faria1, M. Yamamoto2, R.M.D. Faria1, J. Kerbauy2 and J.S.R. Oliveira2
1Serviço de Hematologia e Hemoterapia, Hospital das Clínicas, da Universidade Federal de Minas Gerais, Belo Horizonte, MG, Brasil
2Disciplina de Hematologia e Hemoterapia, Escola Paulista de Medicina, Universidade Federal de São Paulo, São Paulo, SP, Brasil
 References
References
 Correspondence and Footnotes
Correspondence and Footnotes
Correspondence and Footnotes
Correspondence and Footnotes
Correspondence and Footnotes
Correspondence and Footnotes
The expression of P53, Bcl-2, Bax, Bag-1, and Mcl-1 proteins in CD5/CD20-positive B-chronic lymphocytic leukemia (B-CLL) cells from 30 typical CLL patients was evaluated before and after 48 h of incubation with 10-6 M fludarabine using multiparametric flow cytometric analysis. Protein expression was correlated with annexin V expression, Rai modified clinical staging, lymphocyte doubling time, and previous treatment. Our main goal was to determine the predictive value of these proteins in CLL cells in terms of disease evolution. Bcl-2 expression decreased from a median fluorescence index (MFI) of 331.71 ± 42.2 to 245.81 ± 52.2 (P < 0.001) after fludarabine treatment, but there was no difference between viable cells (331.57 ± 44.6 MFI) and apoptotic cells (331.71 ± 42.2 MFI) before incubation (P = 0.859). Bax expression was higher in viable cells (156.24 ± 32.2 MFI) than in apoptotic cells (133.56 ± 35.7 MFI) before incubation, probably reflecting defective apoptosis in CLL (P = 0.001). Mcl-1 expression was increased in fludarabine-resistant cells and seemed to be a remarkable protein for the inhibition of the apoptotic process in CLL (from 233.59 ± 29.8 to 252.04 ± 35.5; P = 0.033). After fludarabine treatment, Bag-1 expression was increased in fludarabine-resistant cells (from 425.55 ± 39.3 to 447.49 ± 34.5 MFI, P = 0.012), and interestingly, this higher expression occurred in patients who had a short lymphocyte doubling time (P = 0.022). Therefore, we could assume that Bag-1 expression in such situation might identify CLL patients who will need treatment earlier.
Key words: Chronic lymphocytic leukemia, Apoptosis, Fludarabine, P53, Bcl-2, Bax, Mcl-1, Bag-1
B-cell chronic lymphocytic leukemia (B-CLL) is a neoplasm that results in the accumulation of clonal CD5-positive B-cells with a low proliferation rate (1). In most patients, the circulating mature B-lymphocytes are largely quiescent G0 phase cells, which accumulate due to longer survival than normal B cells rather than to increased proliferation (2). Thus, B-CLL represents an example of a malignancy caused by failure of programmed cell death (3).
Bcl-2 was first described in follicular lymphomas with t(14;18) translocation (4). At least 20 different Bcl-2 family members have been reported, acting as death antagonist or agonist proteins. Group I Bcl-2 proteins can form heterodimers with group II and prevent the occurrence of programmed cell death (5). The mcl-1 gene encodes a 37-kDa protein with significant homology to Bcl-2, particularly in the carboxyl portion. At least in vitro, Mcl-1 has functions similar to those of Bcl-2, having the ability to heterodimerize with Bax and to neutralize Bax-mediated cytotoxicity (6). Bag-1 was first cloned from mouse cells as a novel protein which interacts with Bcl-2 and Bcl-XL, enhancing the ability of Bcl-2 to prevent apoptosis. Four protein isoforms with 50 (p50), 46 (p46), 33 (p33), and 29 kDa (p29) molecular masses have been described. Bag-1 interacts with the serine/threonine-specific protein kinase Raf-1 and stimulates its activity through a Ras-independent mechanism; m-RNA of Bag-1 binds to platelet-derived growth factor, increasing its ability to inhibit apoptosis (7). Mutations of the p53 tumor suppressor gene may facilitate genetic damage transmission and the emergence of neoplastic clones with survival advantage. Mutations of the p53 gene occur in 15% of B-CLL patients (8).
Fludarabine, a potent apoptosis-inducing drug, is useful for the treatment of indolent lymphoproliferative disorders. F-ara-A, in its fludarabine nucleoside form, is transported into the cells and converted to F-ara-ATP, an active metabolite for cell enzymes. The exact mechanism of apoptosis induction by F-ara-A in proliferative and quiescent cells has not been completely established; however, some data suggest that purine nucleoside analogs may also function as activators for d-ATP-dependent caspase pathways (9,10).
We evaluated in vitro, the expression of P53, Bcl-2, Bax, Bag-1, and Mcl-1 proteins in CD5-positive B-CLL cells in 30 typical CLL patients on two different occasions, i.e., before and after 48 h of fludarabine incubation. The protein expressions were correlated with annexin V expression, Rai modified clinical staging, lymphocyte doubling time (LDT), and the presence of previous treatment. Our main goal was to determine the value of these apoptotic proteins in CLL cells in order to predict the evolution of the disease.
Patients
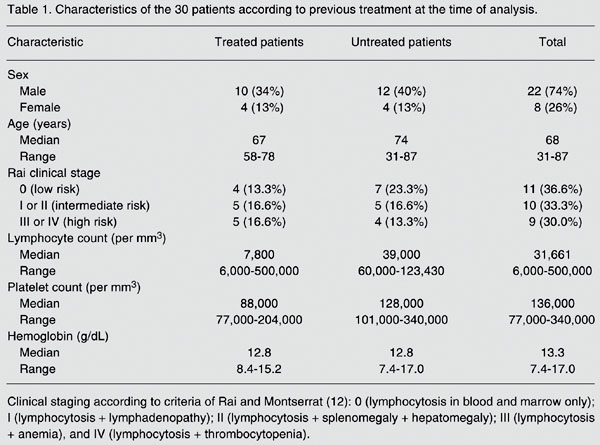
Thirty B-CLL patients, 8 women and 22 men with a median age of 68 years (range: 31 to 87 years) were studied. All patients fulfilled the National Cancer Institute criteria for the diagnosis of CLL. Fourteen patients had received previous therapies, but all had been off any treatment for at least one month before the study (11). Clinical staging was done according to the criteria of Rai and Montserrat (12). LDT was defined as the time needed to reach a double lymphocyte count in relation to the previous evaluation (Table 1) (13). The study was approved by the Institutional Ethics Review Board of the Medical School, Federal University of São Paulo, São Paulo, SP, Brazil, and subjects gave written informed consent to participate.
Lymphocyte isolation and cell culture
Peripheral blood mononuclear cells were isolated by density gradient centrifugation using Lymphoprep (Nycomed, Oslo, Norway) (14). Freshly isolated mononuclear cells, 1 x 106/mL, were incubated in RPMI 1640/2 mM-glutamine medium (Gibco-BRL, Gaithersburg, MD, USA) and supplemented with 50 IU/mL penicillin, 100 µg/mL streptomycin, 20% fetal calf serum, and 10-6 M fludarabine for 48 h at 37ºC in a humidified 5% CO2 atmosphere.
Bcl-2, Bax, Bag-1, Mcl-1, and P53 analysis
The following monoclonal antibodies were used: Bcl-2 and P53 (Dako A/S, Glostrup, Denmark), Bax, Mcl-1 and Bag-1 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA), and CD5 and CD20 (Becton Dickinson, San Jose, CA, USA). CD5 was conjugated with phycoerythrin, CD20 with peridin chlorophyl, Bcl-2 and P53 with fluorescein isothiocyanate (FITC) and the others were pure lyophilized monoclonal antibodies (MoAb). As a negative control, isotype-matched immunoglobulins were used for each MoAb studied. Briefly, 1 x 106 cells were first stained with CD5/phycoerythrin and CD20/peridin chlorophyl. After permeabilization and fixation using a Fix and Perm kit (CALTAG, San Francisco, CA, USA), the cells were stained with P53/FITC and Bcl-2/FITC or Bax, Bag-1 and Mcl-1. For the last three MoAb, a second FITC-conjugated anti-rabbit immunoglobulin antibody (Dako) was used. Data acquisition and analysis were performed by FACScalibur flow cytometer (Becton Dickinson). The median fluorescence index (MFI) was recorded
in a linear mode (0-1023), as previously described (15,16).
Evaluation of in vitro apoptosis
Double staining for cellular annexin and DNA was done using the annexin-V-fluorescein kit (Roche/Boehringer-Mannheim, Penzburg, Bayern, Germany). The cells in HEPES buffer (part of the kit) were incubated with FITC-annexin and propidium iodide and acquisition and analysis were performed by flow cytometry (17). Morphological changes in cells undergoing apoptosis affect their light-scattering properties causing a reduction in their size and an increase in their complexity. These changes were used for gating apoptotic and viable cells. The expression of proteins and annexin was determined in the apoptotic and viable cells (18).
Statistical analysis
Data for mean protein expression by viable and apoptotic cells were compared by ANOVA before and after fludarabine incubation. ANOVA was also used to determine significant differences between Rai stages, LDT (high and low) and the patients' previous treatment.
Comparison of protein expression by viable cells before and after fludarabine incubation (Table 2)
After incubation with fludarabine P53 expression increased from 14.54 ± 2.0 to 26.40 ± 8.9 MFI (P = 0.003), Bag-1 expression increased from 425.55 ± 39.3 to 447.49 ± 34.5 MFI (P = 0.012), Mcl-1 expression increased from 233.59 ± 29.8 to 252.04 ± 35.5 MFI (P = 0.033), and annexin expression increased from 4.43 ± 3.17 to 19.73 ± 9.0% (P < 0.001). Bax expression did not differ significantly before (156.24 ± 32.2 MFI) and after (167.25 ± 26.6 MFI) incubation with fludarabine (P = 0.169).
Bag-1 expression increased from 430.69 ± 41.2 to 437.03 ± 37.22 within the long LDT group, while Bag-1 expression increased from 420.78 ± 42.6 to 465.93 ± 27.87 within the short LDT group. Therefore, Bag-1 presented a higher increase within the short LDT group (P = 0.022). Other proteins did not show significant variance when analyzed within the LDT group, the Rai modified stage group or the previous treatment group.
Comparison of protein expression by apoptotic cells before and after incubation with fludarabine (Table 2)
P53 expression increased from 6.46 ± 1.4 to 15.32 ± 9.2 MFI (P = 0.018), Bcl-2 expression decreased from 331.71 ± 42.2 to 245.81 ± 52.2 MFI (P < 0.001), Bax expression increased from 133.56 ± 35.7 to 154.33 ± 37.4 MFI (P = 0.050), and Mcl-1 expression decreased from 241.15 ± 44.0 to 204.28 ± 43.0 MFI (P = 0.009). Expression of the Bcl-2/Bax index decreased from 2.64 ± 0.7 to 1.67 ± 0.5 (P < 0.001) and annexin expression increased from 74.81 ± 20.8 to 86.91 ± 11.2% (P < 0.001).
Bcl-2 expression decreased from 332.74 ± 45.4 to 266.10 ± 49.6 within the untreated patient group and from 330.80 ± 40.7 to 228.05 ± 49.2 within the treated patient group. Therefore, Bcl-2 expression decreased more within the treated than the untreated group (P = 0.015). Other proteins did not show significant variance when analyzed within the previous treatment group, the Rai modified stage group or the LDT group.
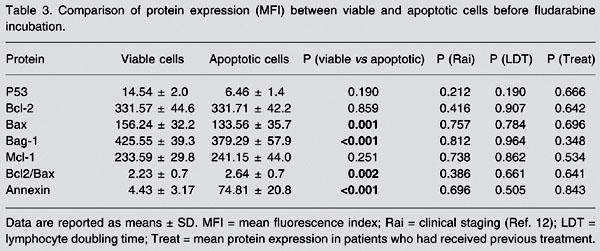
Comparison of protein expression between viable and apoptotic cells before incubation with fludarabine (Table 3)
Bax expression was higher in viable cells (156.24 ± 32.2 MFI) than in apoptotic cells (133.56 ± 35.7 MFI, P = 0.001). Bag-1 expression was higher in viable cells (425.55 ± 39.3 MFI) than in apoptotic cells (379.29 ± 57.9 MFI, P < 0.001) and the Bcl-2/Bax index was lower in viable cells (2.23 ± 0.7) than in apoptotic cells (2.64 ± 0.7, P = 0.002). Annexin expression was lower (4.43 ± 3.17%) in viable cells than in apoptotic cells (74.81 ± 20.8%, P < 0.001) and Bcl-2 expression was not significantly different between viable (331.57 ± 42.2 MFI) and apoptotic cells (331.71 ± 42.2 MFI, P = 0.859). None of the other proteins were significantly modified when analyzed within the LDT group, the Rai modified stage group or the previous treatment group.
Comparison of protein expression between viable and apoptotic cells after fludarabine incubation did not present relevant information and will not be discussed.
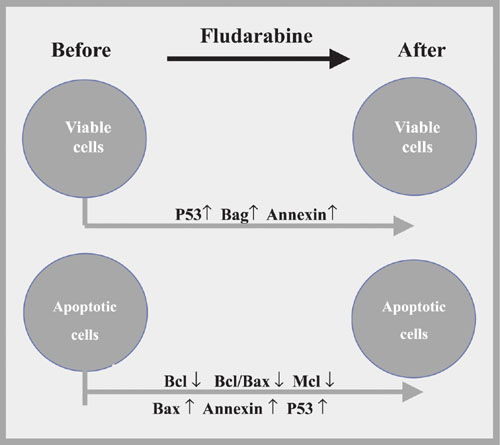
Significant modifications in the protein expressions before and after fludarabine incubation.
[View larger version of this image (? K JPG file)]
Comparison of patients with short and long lymphocyte doubling time (LDT) regarding Bag-1 expression before and after fludarabine incubation.
[View larger version of this image (? K JPG file)]
Characteristics of the 30 patients according to previous treatment at the time of analysis.
[View larger version of this image (? K JPG file)]
Comparison of protein expression (MFI) before and after fludarabine incubation in viable and apoptotic cells.
[View larger version of this image (? K JPG file)]
Comparison of protein expression (MFI) between viable and apoptotic cells before fludarabine incubation.
Discussion
[View larger version of this image (? K JPG file)]
We demonstrated an increase of annexin expression in viable (P < 0.001; Table 2) and apoptotic (P < 0.001; Table 2) cells by comparing CLL cells before and after fludarabine treatment (Figure 1).
There was a reduction of Bcl-2 expression after fludarabine treatment of apoptotic cells (P < 0.001; Table 2). On the other hand, untreated patients presented higher expression in these cells than treated patients (P = 0.015; Table 2). Therefore, we postulate that previous treatment might have reduced Bcl-2 expression by inducing CLL cells to apoptosis.
Comparison of viable and apoptotic cells showed a higher Bcl-2/Bax index in apoptotic cells before fludarabine treatment (spontaneous apoptosis) as a consequence of a lower Bax expression (P = 0.002; Table 3). These findings suggest a greater involvement of Bax in spontaneous apoptosis. Therefore, we can assume that the Bcl/Bax index better reflects the apoptosis process than isolated Bcl-2 level.
Analysis of the Bag-1 protein disclosed important features, and probably it is the most remarkable finding in our study. Lower Bag-1 expression was observed in viable cells than in apoptotic cells in pre-fludarabine incubations (P < 0.001; Table 3). The comparison of viable cells before and after fludarabine indicated an increase of Bag-1 after incubation (P = 0.012; Table 2). These results suggested that Bag-1 is a remarkable mechanism for cell survival after fludarabine treatment. Also, Bag-1 increased in patients with shorter LDT (P = 0.022; Table 2, Figure 2), as confirmed by ANOVA, particularly observed in viable cells resistant to fludarabine. Kitada et al. (6) linked high Bag-1 protein levels to a poor treatment response, suggesting that Bag-1 and Bcl-2 interactions enhance resistance to apoptosis. Plate et al. (19) unexpectedly observed that some anti-apoptotic genes such as bag and akt were positively regulated in CLL cells after treatment with fludarabine. They concluded that Bag-1 is a protein associated with cell survival which interacts with Bcl-2, increasing its effectiveness. This seems to be important because Bcl-2 alone cannot inhibit all apoptotic pathways triggered to remove cells from the organism. However, it should be taken into account that the increase of Bag-1 in cases of reduced LDT was seen mainly in viable cells after fludarabine incubation. It was notable that in this situation Bag-1 can be used to identify patients who will present short LDT and might have some influence in determining those patients who will need early therapeutic measures.
Mcl-1 protein expression was increased in fludarabine-resistant cells (P < 0.033; Table 2) and decreased in apoptotic cells (P = 0.009; Table 3). Most Mcl-1 studies have been conducted on non-lymphoid cells, showing that Mcl-1 expression is induced by IL-4 and inhibited by TGF-ß. Bcl-2 and Mcl-1 are able to form heterodimers with Bax and then neutralize their activities. High Mcl-1 levels were associated with poor remission achievement (6). However, we could not associate Mcl-1 protein expression with LDT, prior treatment, or Rai modified stage.
P53 is a highly unstable protein with a very short half-life in cell culture. However, P53 accumulates after cell exposures to DNA-damaging agents such as doxorubicin, 5-fluoruracil, etoposide, and ion radiation (20). P53 protein expression has been correlated with progressive disease when high protein levels are caused by p53 gene mutation (21). Our results revealed P53 protein up-regulation after fludarabine incubation in the viable (P = 0.003; Table 2) and apoptotic (P = 0.018; Table 2) cells. Since we did not find a relation between P53 expression and LDT, staging or treatment, our results agree with data reported in other studies which did not evaluate p53 gene mutation (22,23).
In the present study we have shown that: 1) Bax protein was important to induce programmed cell death seen in the apoptotic cells after incubation with fludarabine, 2) the Bcl-2/Bax index indicated apoptotic characteristics better than Bcl-2 itself, 3) the Mcl-1 protein found in resistant cells may be one of the proteins responsible for apoptosis inhibition in CLL patients, and 4) Bag-1 protein presented high expression in viable cells after fludarabine incubation, indicating that Bag-1 is the most important protein in apoptosis resistance. Its determination can predict CLL patients who demonstrate a short LDT and indicate who will need early therapy.
 Address for correspondence: J.R. Faria, Av. Bandeirantes, 1732/302, 30315-000 Belo Horizonte, MG, Brasil. E-mail: jrfaria@oi.com.br
Address for correspondence: J.R. Faria, Av. Bandeirantes, 1732/302, 30315-000 Belo Horizonte, MG, Brasil. E-mail: jrfaria@oi.com.br
Publication supported by FAPESP. Received November 9, 2004. Accepted September 6, 2005.
- 1. Sakai A, Marti GE, Caporaso N et al. (2000). Analysis of expressed immunoglobulin heavy chain genes in familial B-CLL. Blood, 95: 1413-1419.
- 2. Bentley DP & Pepper CJ (2000). The apoptotic pathway: a target for therapy in chronic lymphocytic leukemia. Hematological Oncology, 18: 87-98.
- 3. Kaufmann SH & Gores GJ (2000). Apoptosis in cancer: cause and cure. BioEssays, 22: 1007-1017.
- 4. Yunis JJ, Oken MM, Kaplan ME et al. (1982). Distinctive chromosomal abnormalities in histologic subtypes of non-Hodgkin's lymphoma. New England Journal of Medicine, 307: 1231-1236.
- 5. Bertram JS (2000). The molecular biology of cancer. Molecular Aspects of Medicine, 21: 167-223.
- 6. Kitada S, Andersen J & Akar S et al. (1998). Expression of apoptosis-regulating proteins in chronic lymphocytic leukemia: correlations with in vitro and in vivo chemoresponses. Blood, 91: 3379-3389.
- 7. Yang X, Chernenko G, Hao Y et al. (1998). Human BAG-1/RAP46 protein is generated as four isoforms by alternative translation initiation and overexpressed in cancer cells. Oncogene, 17: 981-989.
- 8. Cordone I, Masi S, Mauro FR et al. (1998). p53 expression in B-cell chronic lymphocytic leukemia: a marker of disease progression and poor prognosis. Blood, 91: 4342-4349.
- 9. Consoli U, El-Tounsi I, Sandoval A et al. (1998). Differential induction of apoptosis by fludarabine monophosphate in leukemic B and normal T cells in chronic lymphocytic leukemia. Blood, 91: 1742-1748.
- 10. Huang P, Sandoval A, Van Den Neste E et al. (2000). Inhibition of RNA transcription: a biochemical mechanism of action against chronic lymphocytic leukemia cells by fludarabine. Leukemia, 14: 1405-1413.
- 11. Cheson BD, Benett JM, Grever M et al. (1996). National Cancer Institute-Sponsored Working Group Guidelines for chronic lymphocytic leukemia: revised guidelines for diagnosis and treatment. Blood, 87: 4990-4997.
- 12. Rai KR & Montserrat E (1987). Prognostic factors in chronic lymphocytic leukemia. Seminars in Hematology, 24 (Suppl 4): 252-256.
- 13. Montserrat E, Sanchez-Bisono J, Viñolas N et al. (1986). Lymphocyte doubling time in chronic lymphocytic leukaemia: analysis of its prognostic significance. British Journal of Haematology, 62: 567-575.
- 14. Boyum A (1974). Separation of blood leucocytes, granulocytes and lymphocytes. Tissue Antigens, 4: 269-274.
- 15. Deneys V, Mazzon AM, Marques JL et al. (2001). Reference values for peripheral blood B-lymphocyte subpopulations: a basis for multiparametric immunophenotyping of abnormal lymphocytes. Journal of Immunological Methods, 253: 23-36.
- 16. Kimura O, Sugamura K, Kijima T et al. (1996). Flow cytometric examination of p53 protein in primary tumors and metastases to the liver and lymph nodes of colorectal cancer. Diseases of the Colon and Rectum, 39: 1428-1433.
- 17. Pepper C, Bentley P & Roy T (1996). Regulation of clinical chemoresistance by bcl2 and bax oncoproteins in B-cell chronic lymphocytic leukaemia. British Journal of Haematology, 95: 513-517.
- 18. Pepper C, Thomas A, Hoy T et al. (1999). Chlorambucil resistance in B-cell chronic lymphocytic leukaemia is mediated through failed Bax induction and selection of high Bcl-2-expressing subclones. British Journal of Haematology, 104: 581-588.
- 19. Plate JMD, Petersen KS, Buckingham L et al. (2000). Gene expression in chronic lymphocytic leukemia B cells and changes during induction of apoptosis. Experimental Hematology, 28: 1214-1224.
- 20. Hupp TR, Lane DP & Ball KL (2000). Strategies for manipulating the p53 pathway in the treatment of human cancer. Biochemical Journal, 15: 1-17.
- 21. Morabito F, Filangeri M, Callea I et al. (1997). Bcl-2 protein expression and p53 gene mutation in chronic lymphocytic leukemia: correlation with in vitro sensitivity to chlorambucil and purine analogs. Haematologica, 82: 16-20.
- 22. Aguilar-Santelises M, Rottenberg ME, Lewin N et al. (1996). Bcl-2, bax and p53 expression in relation to in vitro survival and clinical progression. International Journal of Cancer, 69: 114-119.
- 23. El Rouby S, Thomas A, Costin D et al. (1993). p53 gene mutation in B-cell chronic lymphocytic leukemia is associated with drug resistance and is independent of MDR1/MDR3 gene expression. Blood, 82: 3452-3459.
Publication Dates
-
Publication in this collection
06 Mar 2006 -
Date of issue
Mar 2006
History
-
Accepted
06 Sept 2005 -
Received
09 Nov 2004