Abstract
The visual system is a potential target for methylmercury (MeHg) intoxication. Nevertheless, there are few studies about the cellular mechanisms of toxicity induced by MeHg in retinal cells. Various reports have indicated a critical role for nitric oxide synthase (NOS) activation in modulating MeHg neurotoxicity in cerebellar and cortical regions. The aim of the present study is to describe the effects of MeHg on cell viability and NOS activation in chick retinal cell cultures. For this purpose, primary cultures were prepared from 7-day-old chick embryos: retinas were aseptically dissected and dissociated and cells were grown at 37ºC for 7-8 days. Cultures were exposed to MeHg (10 µM, 100 µM, and 1 mM) for 2, 4, and 6 h. Cell viability was measured by MTT method and NOS activity by monitoring the conversion of L-[H³]-arginine to L-[H³]-citrulline. The incubation of cultured retina cells with 10 and 100 µM MeHg promoted an increase of NOS activity compared to control (P < 0.05). Maximum values (P < 0.05) were reached after 4 h of MeHg incubation: increases of 81.6 ± 5.3 and 91.3 ± 3.7%, respectively (data are reported as mean ± SEM for 4 replicates). MeHg also promoted a concentration- and time-dependent decrease in cell viability, with the highest toxicity (a reduction of about 80% in cell viability) being observed at the concentration of 1 mM and after 4-6 h of incubation. The present study demonstrates for the first time the modulation of MeHg neurotoxicity in retinal cells by the nitrergic system.
Methylmercury; Chick retinal cell culture; Nitric oxide synthase; Cell viability; Neurotoxicity
Braz J Med Biol Res, March 2006, Volume 39(3) 415-418 (Short Communication)
Methylmercury intoxication activates nitric oxide synthase in chick retinal cell culture
A.M. Herculano1, M.E. Crespo-López2, S.M.A. Lima3, D.L.W. Picanço-Diniz4 and  Correspondence and Footnotes
Correspondence and Footnotes

 J.L.M. Do Nascimento1
J.L.M. Do Nascimento1
1Laboratório de Neuroquímica Molecular e Celular, Departamento de Fisiologia, 2Laboratório de Biologia Celular, Núcleo de Medicina Tropical, 3Laboratório de Neurobiologia, Departamento de Fisiologia, 4Laboratório de Neuroendocrinologia, Departamento de Fisiologia, Universidade Federal do Pará, Belém, PA, Brasil
 Text
Text
 References
References
 Correspondence and Footnotes
Correspondence and Footnotes
Correspondence and Footnotes
Correspondence and Footnotes
Correspondence and Footnotes
Correspondence and Footnotes
Abstract
The visual system is a potential target for methylmercury (MeHg) intoxication. Nevertheless, there are few studies about the cellular mechanisms of toxicity induced by MeHg in retinal cells. Various reports have indicated a critical role for nitric oxide synthase (NOS) activation in modulating MeHg neurotoxicity in cerebellar and cortical regions. The aim of the present study is to describe the effects of MeHg on cell viability and NOS activation in chick retinal cell cultures. For this purpose, primary cultures were prepared from 7-day-old chick embryos: retinas were aseptically dissected and dissociated and cells were grown at 37ºC for 7-8 days. Cultures were exposed to MeHg (10 µM, 100 µM, and 1 mM) for 2, 4, and 6 h. Cell viability was measured by MTT method and NOS activity by monitoring the conversion of L-[H3]-arginine to L-[H3]-citrulline. The incubation of cultured retina cells with 10 and 100 µM MeHg promoted an increase of NOS activity compared to control (P < 0.05). Maximum values (P < 0.05) were reached after 4 h of MeHg incubation: increases of 81.6 ± 5.3 and 91.3 ± 3.7%, respectively (data are reported as mean ± SEM for 4 replicates). MeHg also promoted a concentration- and time-dependent decrease in cell viability, with the highest toxicity (a reduction of about 80% in cell viability) being observed at the concentration of 1 mM and after 4-6 h of incubation. The present study demonstrates for the first time the modulation of MeHg neurotoxicity in retinal cells by the nitrergic system.
Key words: Methylmercury, Chick retinal cell culture, Nitric oxide synthase, Cell viability, Neurotoxicity
Methylmercury (MeHg) is an environmental pollutant with neurotoxic action (1). Many studies have demonstrated that the motor and visual systems are potentially affected in cases of MeHg intoxication (2).
Rodents intoxicated with MeHg exhibited cell damage in cerebellar and cortical regions; nevertheless, the molecular mechanisms activated by MeHg exposure in these regions are not understood. Various studies using in vivo and in vitro models have indicated an important role for the nitrergic system in the cytotoxicity induced by MeHg in cortex and cerebellum (3,4).
Activation of the nitrergic system leads to the generation of nitric oxide (NO) that has multiple roles in the brain. NO is considered to be an unusual neurotransmitter, a regulatory factor in the cerebral circulation and a neurotoxin when overproduced (5). NO is synthesized from L-arginine by nitric oxide synthase (NOS) activation. NOS enzyme can be expressed as three isoforms: neuronal (NOS-1), inducible (NOS-2) and endothelial (NOS-3). All of these isoforms reduce nitroblue tetrazolium to formazan in an NADPH-dependent process involving NADPH-diaphorase activity (6,7).
Although the cerebellum and cortex are the best-characterized targets of MeHg toxicity in both humans and rodents, some studies have demonstrated that retinal cells are also vulnerable to MeHg damage (8). However, the mechanisms of neurotoxicity induced by MeHg in these cells are poorly understood. The aim of the present study was to evaluate the effect of MeHg on NOS activity in retinal cells, as well as to correlate this activation with MeHg neurotoxicity.
For this purpose, primary chick retinal cell cultures were prepared from 7-day-old chick embryos as described previously (9). Briefly, retinas were aseptically dissected and tissue was treated with 0.05% trypsin saline solution. Dissociated cells were maintained in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum, 100 U/mL penicillin, 100 µg/mL streptomycin, and 500 µg/mL glutamine. About 5 x 106 retinal cells were grown in 35-mm culture dishes for 7-8 days in a 5% CO2 atmosphere. The cultures were exposed to MeHg (10 µM, 100 µM, and 1 mM) diluted in serum-free DMEM for 2, 4, and 6 h.
Cell viability in retinal cell cultures was measured by the method of Mosman (10). Active mitochondria of viable cells reduce the colorless tetrazolium salt MTT, forming dark blue insoluble formazan crystals. Control and MeHg-treated cells were washed twice with PBS and incubated for 3 h with 55 µL of an MTT stock solution (5 mg/mL). Formazan crystal formation was measured at 570 nm with a spectrophotometer and cell viability is reported as percent MTT reduction compared to control values. MeHg neurotoxicity was evaluated by the decrease in cell viability.
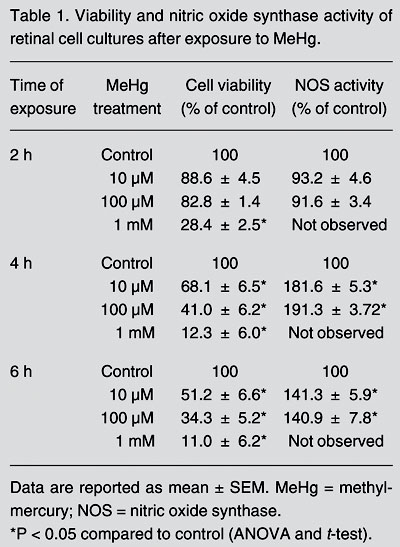
MeHg induced neurotoxic effects on retinal cells in a concentration- and time-dependent manner (Table 1). Retinal cell cultures exposed for 2 h to 10 and 100 µM MeHg did not show significant changes in cell viability. Longer incubation times (4 and 6 h) reduced cell viability by 32 and 49% for 10 µM MeHg, respectively, and by 59 and 66% for 100 µM MeHg. Incubation with 1 mM MeHg caused intense toxicity at all exposure periods, provoking the highest rates of cell death (Table 1), with 72, 88, and 89% of cells dying after 2, 4, and 6 h of incubation, respectively.
Viability and nitric oxide synthase activity of retinal cell cultures after exposure to MeHg.
NOS activity was measured by monitoring the conversion of L-[H3]-arginine to L-[H3]-citrulline (11). Control and treated retinal cell cultures were homogenized in HEPES buffer, pH 7.4, and centrifuged at 10,000 g for 10 min. The supernatant was incubated with 1 mM NADPH, 0.45 mM CaCl2 and 1 µCi L-[H3]-arginine at 37ºC for 10 min and the reaction was stopped with 5% TCA and ethyl ether. Samples were then run through columns packed with Dowex-50W ion exchange resin (Na+ form) to remove L-arginine. The L-[H3]-citrulline produced by NOS was quantified by liquid scintillation spectroscopy. Finally, protein content was determined and NOS activity is reported as percentage of control value (32.2 ± 2.3 fmol of L-[H3]-citrulline formed per mg protein/min).
Two hours of intoxication with 10 and 100 µM MeHg did not induce significant changes in NOS activity (Table 1). However, NOS activity increased by 82 and 91% after 4 h of exposure to 10 and 100 µM MeHg, respectively. Similarly, a longer exposure period (6 h) to these MeHg concentrations resulted in an increase of approximately 41% in NOS activity. Therefore, maximum induction occurred after 4 h of incubation with MeHg.
The increase in NOS activity in chick retinal cell cultures after MeHg intoxication was similar to that observed in cortical and cerebellar regions of rodents intoxicated with this metal (3,4). The exact pathway of NOS activation evoked by MeHg toxicity is not known, but may involve an increase of extracellular concentration (12) and subsequent stimulation of NMDA receptors (13), an increase of intracellular calcium levels with binding to calmodulin, and final activation of NOS (14,15).
To evaluate the role of the nitrergic system in the toxicity induced by MeHg, a co-treatment with 3 mM L-nitro- arginine (L-NARG), an inhibitor of NOS activity, was performed in retinal cell cultures (Table 2). In these cultures, L-NARG exerted protective effects against the neurotoxic effects of 10 and 100 µM MeHg at the 4-h time point, preventing the death of 22 and 30% of cells, respectively. However, the protective effect of L-NARG was not evident after 6 h of MeHg exposure.
Interestingly, in cultured retinal cells, 6 h of exposure to 10 µM MeHg reduced cell viability by approximately 50%, while in a previous study using cortical cell cultures this reduction in cell viability by the same MeHg concentration was observed only after 24 h of incubation (16). These results suggest that retinal cell cultures are more sensitive than cortical cultures to MeHg toxicity.
Since co-treatment with L-NARG protected against MeHg toxicity only at the 4-h time point, the NO produced by the retinal cells may be responsible for MeHg-mediated neurotoxicity only during the early stages of intoxication. For longer periods of intoxication, NOS activation by MeHg was lower but L-NARG was not able to protect against MeHg neurotoxicity. Therefore, other events such as increased intracellular calcium levels or decreased glutathione (GSH) levels that have been already shown to occur in other tissues (17) could be responsible for neurotoxicity to retinal cells after prolonged periods of intoxication.
The present study is the first, to our knowledge, to show the modulation of MeHg neurotoxicity by the nitrergic system in retinal cells.
Acknowledgments
We thank Makarú Ldta. for kindly providing chick embryos for our experiments.
 Address for correspondence: J.L.M. Do Nascimento, Laboratório de Neuroquímica, Departamento de Fisiologia, UFPA, Campus Universitário do Guamá, Rua Augusto Correia, 166075-110 Belém, PA, Brasil. Fax: +55-91-8123-1601. E-mail: jlmn@ufpa.br or aherculanos@yahoo.com.br
Address for correspondence: J.L.M. Do Nascimento, Laboratório de Neuroquímica, Departamento de Fisiologia, UFPA, Campus Universitário do Guamá, Rua Augusto Correia, 166075-110 Belém, PA, Brasil. Fax: +55-91-8123-1601. E-mail: jlmn@ufpa.br or aherculanos@yahoo.com.br
Presented at the Symposium on Sensory and Neuropsychological Losses Due to Mercury Intoxication and to Other Neurodegenerative Processes. Studies in Humans and in Animal Models. Águas de Lindóia, SP, Brazil, August 24, 2004. Research supported by CAPES-RENOR, CNPq-PNOPG, and FINEP-PNOPG. A.M. Herculano had a CAPES-PROF fellowship for graduate level programs. J.L.M. Do Nascimento and M.E. Crespo-López are recipients of CNPq and DCR-SECTAM/CNPq research fellowships, respectively. Received April 28, 2005. Accepted October 25, 2005.
- 1. Crespo-López ME, Herculano AM, Corvelo TC et al. (2005). Mercurio y neurotoxicidad. Revista de Neurologia, 40: 441-447.
-
2WHO (1990). Environmental Health Criteria 101. Methylmercury World Health Organization, International Program on Chemical Safety (IPCS), Geneva, Switzerland.
- 3. Himi T, Ikeda M, Sato I et al. (1996). Purkinje cells express neuronal nitric oxide synthase after methylmercury administration. Brain Research, 718: 189-192.
- 4. Yamashita T, Ando Y, Sakashita K et al. (1997). Role of nitric oxide in the cerebellar degeneration during methylmercury intoxication. Biochimica et Biophysica Acta, 1334: 303-311.
- 5. Dawson TM, Dawson VL & Snyder SH (1992). A novel neuronal messenger molecule in brain: the free radical, nitric oxide. Annals of Neurology, 32: 297-311.
- 6. De Faria MH, Do Nascimento JLM & Paes de Carvalho R (1995). Biochemical characterization of NADPH-diaphorase in the chick embryo retina: stimulation by calcium ions and inhibition by arginine analogs. Brazilian Journal of Medical and Biological Research, 28: 252-255.
- 7. Hope BTG, Michael J, Knigge KM et al. (1991). Neuronal NADPH diaphorase is a nitric oxide synthase. Proceedings of the National Academy of Sciences, USA, 88: 2811-2814.
- 8. Goto Y, Shigematsu J, Tobimatsu S et al. (2001). Different vulnerability of rat retinal cells to methylmercury exposure. Current Eye Research, 23: 171-178.
- 9. Do Nascimento JL, Kubrusly RC, Reis RA et al. (1998). Atypical effect of dopamine in modulating the functional inhibition of NMDA receptors of cultured retina cells. European Journal of Pharmacology, 343: 103-110.
- 10. Mosman T (1983). Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assay. Journal of Immunological Methods, 65: 55-63.
- 11. Bredt DS & Snyder SH (1989). Nitric oxide mediates glutamate-linked enhancement of cGMP levels in the cerebellum. Proceedings of the National Academy of Sciences, USA, 86: 9030-9033.
- 12. Aschner M, Du YL, Gannon M et al. (1993). Methylmercury-induced alterations in excitatory amino acid transport in rat primary astrocyte cultures. Brain Research, 602: 181-186.
- 13. Garthwaite J (1991). Glutamate, nitric oxide and cell-cell signalling in the nervous system. Trends in Neurosciences, 14: 60-67.
- 14. Sarafian T & Verity MA (1990). Methylmercury stimulates protein 32P phospholabeling in cerebellar granule cell culture. Journal of Neurochemistry, 55: 913-921.
- 15. Bredt DS, Glatt CE, Hwang PM et al. (1991). Nitric oxide synthase protein and mRNA are discretely localized in neuronal populations of the mammalian CNS together with NADPH diaphorase. Neuron, 7: 615-624.
- 16. Sanfeliu C, Sebastià J & Kim SU (2001). Methylmercury neurotoxicity in cultures of human neurons, astrocytes, neuroblastoma cells. Neurotoxicology, 22: 317-327.
- 17. Sanfeliu C, Sebastià J, Cristòfol R et al. (2003) Neurotoxicity of organomercurial compounds. Neurotoxicity Research, 5: 283-306.
Correspondence and Footnotes
Publication Dates
-
Publication in this collection
06 Mar 2006 -
Date of issue
Mar 2006
History
-
Received
28 Apr 2005 -
Accepted
25 Oct 2005