Abstract
The ability of the clinically used cephalosporins: cephalothin, cefotaxime and cefotiam to induce lipid peroxidation (LPO) and renal damage was compared to that of nephrotoxic cephaloridine under in vivo conditions. Glutathione was measured in rat liver or in renal cortex as non-protein sulfhydryls. LPO was measured in plasma, renal cortex and liver by the generation of malondialdehyde or as the increase in renal cortical concentration of conjugated dienes. Impairment of renal function was measured as the decrease in renal cortical accumulation of the organic anion p-aminohippurate (PAH). Administration of cephalosporins to rats as a single dose (2000 mg/kg, ip) induced a significant glutathione-depletion in the renal cortex with cephaloridine, and in the liver with cephaloridine, cephalothin and cefotiam. Treatment of rats with cephaloridine, cephalothin and cefotiam (200, 500, or 1000 mg kg-1 day-1, ip) for 5 days resulted in a dose-dependent increase of LPO in the renal cortex. While cephaloridine induced the highest concentration of conjugated diene, cefotaxime had no effect. Measurements of PAH accumulation in renal cortical slices from cephalosporin-treated rats showed a dose-dependent decrease in the renal cortical accumulation of PAH. Pretreatment with the antioxidants vitamin E or cyanidanol (400 mg kg-1 day-1, ip) 1 h before treatment with cephaloridine, cephalothin or cefotiam (1000 mg kg-1 day-1, ip) for 3 days inhibited cephalosporin-induced LPO and significantly reduced the impairment of renal cortical accumulation of PAH. The potential of different cephalosporins for inducing LPO and reducing PAH accumulation was ranked as follows: cephaloridine > cephalothin > cefotiam > cefotaxime.
Cephalosporins; Glutathione depletion; Lipid peroxidation; Nephrotoxicity; Antioxidants; Vitamin E
Braz J Med Biol Res, June 2007, Volume 40(6) 867-875
Protection against cephalosporin-induced lipid peroxidation and nephrotoxicity by (+)-cyanidanol-3 and vitamin E
 Correspondence and Footnotes
Correspondence and Footnotes
 C. Cojocel1, K.-L. Tolle2, H. El-Hajj1 and K. Baumann2
C. Cojocel1, K.-L. Tolle2, H. El-Hajj1 and K. Baumann2
1Department of Pharmacology and Toxicology, Faculty of Medicine, Kuwait University, Safat, Kuwait
2Department of Cell Physiology, Institute of Physiology, University of Hamburg, Hamburg, Germany
 References
References
 Correspondence and Footnotes
Correspondence and Footnotes
Correspondence and Footnotes
Correspondence and Footnotes
Correspondence and Footnotes
Correspondence and Footnotes
Abstract
The ability of the clinically used cephalosporins: cephalothin, cefotaxime and cefotiam to induce lipid peroxidation (LPO) and renal damage was compared to that of nephrotoxic cephaloridine under in vivo conditions. Glutathione was measured in rat liver or in renal cortex as non-protein sulfhydryls. LPO was measured in plasma, renal cortex and liver by the generation of malondialdehyde or as the increase in renal cortical concentration of conjugated dienes. Impairment of renal function was measured as the decrease in renal cortical accumulation of the organic anion p-aminohippurate (PAH). Administration of cephalosporins to rats as a single dose (2000 mg/kg, ip) induced a significant glutathione-depletion in the renal cortex with cephaloridine, and in the liver with cephaloridine, cephalothin and cefotiam. Treatment of rats with cephaloridine, cephalothin and cefotiam (200, 500, or 1000 mg kg-1 day-1, ip) for 5 days resulted in a dose-dependent increase of LPO in the renal cortex. While cephaloridine induced the highest concentration of conjugated diene, cefotaxime had no effect. Measurements of PAH accumulation in renal cortical slices from cephalosporin-treated rats showed a dose-dependent decrease in the renal cortical accumulation of PAH. Pretreatment with the antioxidants vitamin E or cyanidanol (400 mg kg-1 day-1, ip) 1 h before treatment with cephaloridine, cephalothin or cefotiam (1000 mg kg-1 day-1, ip) for 3 days inhibited cephalosporin-induced LPO and significantly reduced the impairment of renal cortical accumulation of PAH. The potential of different cephalosporins for inducing LPO and reducing PAH accumulation was ranked as follows: cephaloridine > cephalothin > cefotiam > cefotaxime.
Key words: Cephalosporins, Glutathione depletion, Lipid peroxidation, Nephrotoxicity, Antioxidants, Vitamin E
Introduction
First-generation cephalosporins such as cephaloridine and cephalothin and newer cephalosporins have been associated with nephrotoxicity in humans and in experimental animals (1,2). In contrast, third-generation cephalosporins such as cefotaxime and cefoperazone show practically no nephrotoxic effects (3). Several biochemical mechanisms have been proposed to explain cephaloridine nephrotoxicity. It has been suggested that biotransformation of cephaloridine to some toxic metabolite might be necessary for nephrotoxicity (4,5).
The most accepted hypothesis suggests that peroxidative injury is a possible mechanism of cephaloridine-induced nephrotoxicity (4,5).
Little is known about the in vivo occurrence of cephalosporin-induced lipid peroxidation (LPO) and its role in the development of cephalosporin nephrotoxicity. Cellular transport mechanisms involved in proximal tubular secretion of organic cations and anions have been investigated in detail.
The transport systems involved in the uptake of organic ions across the basolateral membrane exhibit broad substrate specificity to accommodate a large variety of chemically unrelated compounds. The hydrophobic moiety, the ability to form hydrogen bonds and the presence of ionic or partial electrical charges are common structural requirements for the renal transporters of organic ions (6). Cephalosporin antibiotics are excreted into the urine via organic anion transporters. Organic anion transporters can mediate cephalosporin nephrotoxicity, particularly that induced by cephaloridine (7,8).
An important function of vitamin E (a-tocopherol) in vivo is scavenging free radicals and inhibiting LPO. Vitamin E, a lipid-soluble antioxidant, is also capable of scavenging reactive oxygen species such as superoxide anion, singlet oxygen and peroxyl radicals (9). a-Tocopherol is the most biologically potent antioxidant of the four tocopherols that together constitute vitamin E (10). The flavonoid (+)-cyanidanol-3 (CND), also named (+)-catechin, is a naturally occurring flavonoid that has been shown to exert a number of liver-protecting actions: improvement of hepatic parenchimal regeneration, stabilization of the biomembrane structure by scavenging free radicals, and stimulation of certain types of immune function (11). CND has antioxidant properties and has been investigated in a number of LPO studies with liver, kidney and brain as target organs (12,13).
The present study was designed to investigate the protective role of vitamin E and CND against cephalosporin-caused LPO and nephrotoxicity under in vivo conditions.
Material and Methods
Male Wistar rats (200-250 g) were purchased from Winkelmann (Kirchborchen, Germany) and housed for use in the present in vivo studies.
Glutathione determination
The first series of experiments was designed to quantify reduced glutathione (GSH) depletion in kidney cortex and liver tissue 2 h after administration of the cephalosporin. Animals (5 rats per treatment group) were given a single ip injection of cephalothin, cefotiam, cefotaxime, or cephaloridine (2000 mg/kg). Control rats were treated with saline. In order to avoid diurnal changes in tissue GSH content, rats were treated between 7 and 9 am and sacrificed before 1 pm. In the present study, cephaloridine was used as a positive control for its peroxidative and nephrotoxic potential.
Portions of liver and kidney cortex (100 mg) were used for GSH determination. Reduced GSH was measured as the nonprotein sulfhydryl content (14). Kidney or liver samples were homogenized in 20 volumes of ice-cold 6 g/100 mL TCA and centrifuged at 10,000 g for 20 min. The resulting supernatant was then diluted with ice-cold 6 g/100 mL TCA and 0.5 mL of the diluted supernatant was added to 2 mL 0.3 M Na2HPO4 solution. Five milliliters of a solution of 40 mg/100 mL 5,5 dithio-bis-(2-nitrobenzoic acid) in 10 g/100 mL sodium citrate was then added, and the absorbance at 412 nm was measured immediately after mixing using a Beckman DU-70 spectrophotometer (Beckman, Fullerton, CA, USA).
Determination of malondialdehyde in plasma and renal cortex
In the second series of experiments, male Wistar rats (200 g) were treated ip with cephalothin, cefotiam, cefotaxime, and cephaloridine (200, 500, and 1000 mg kg-1 day-1) for 1, 2, 3, 4, and 5 days. Control rats were treated with 0.9 g/100 mL NaCl. Each treatment group consisted of at least 5 rats. At the end of each treatment period, rats were sacrificed and malondialdehyde (MDA) was measured in plasma, liver and kidney tissue.
Blood samples were collected into heparinized glass tubes and immediately centrifuged at 3000 rpm for 10 min. Plasma concentration of thiobarbituric acid-reactive substances was measured using a Beckman DU-70 Spectrophotometer and expressed as nmol/mL MDA by using an extinction coefficient of 1.56 x 105 M-1 cm-1 (15). LPO in the renal cortex was monitored by measuring the production of MDA using the thiobarbituric acid assay (16).
Determination of conjugated diene
The third series of experiments was designed to quantify concentrations of conjugated diene in microsomal lipids from the renal cortex of control and cephalosporin- treated rats (17). Rats were given a single ip injection of cephalosporin (2000 mg/kg) and were killed 18 h after administration. Experimental and control rats from each group were treated identically. Renal cortex microsomes were prepared as previously described (18,19). At the end of the preparation the microsomal pellet was weighed and an adequate volume of 0.3 M sucrose and 3 mM EDTA solution was added to dilute the microsomes to a final concentration of 300 mg/mL.
A 0.5-mL aliquot of the microsomal suspension was extracted with 9.5 mL 2:1 chloroform:methanol. The mixture was shaken for 15 min and filtered. The filtrate was separated into two phases by the addition of 2 mL water and the upper phase was discarded. Methanol (0.2 mL) was added to the lower phase, and absorbance was determined between 210 and 300 nm with a freshly prepared blank. Peak absorption for conjugated diene was measured at 239 nm. Cephalosporin-induced LPO was estimated as the mean difference spectrum from 210 to 300 nm between lipids from cephalosporin-treated rats and lipids from control rats using a Beckman DU-70 spectrophotometer.
Determination of p-aminohippurate in renal cortical slices
Renal cortical slices were prepared from kidneys of naive 200-g male Wistar rats after 4 days of saline or cephalosporin treatment (5 rats per treatment group). Rats were killed and kidneys were removed immediately, decapsulated and placed in ice-cold 0.9 g/100 mL NaCl. Kidney slices were prepared from the cortex of both kidneys and pooled together and a sample (80-120 mg) was used to determine p-aminohippurate (PAH) accumulation.
At the end of the first incubation, slices were removed from the incubation medium, blotted, transferred to a 4-mL cephalosporin-free incubation medium containing PAH and incubated for 90 min at 25ºC under 100% O2 in a GFL metabolic shaker (Gesellschaft für Labortechnik mbH, Burgwedel, Germany). The corresponding control medium consisted of 96.7 mM NaCl, 7.4 mM sodium phosphate buffer, 40 mM KCl and 0.74 mM CaCl2. Additionally the medium contained 10 mM lactic acid (20) and 74 mM PAH. After 90-min incubation, slices were removed, blotted, weighed, and homogenized in 10 mL 30 g/100 mL trichloroacetic acid and centrifuged. Two milliliters of incubation medium was treated similarly. After centrifugation, the supernatant fluid from slices and incubation medium was used to measure PAH concentration as previously described (21). The accumulation of PAH in renal cortical slices is reported as slice-to-medium (S/M) ratio, where S represents mg PAH/g tissue and M represents mg PAH/mL medium.
The fourth series of experiments was designed to quantify the effects of the antioxidants vitamin E and CND on cephalosporin-induced LPO and nephrotoxicity in vivo.
Male Wistar rats were given ip vitamin E (400 mg kg-1 day-1 in corn oil) or CND (400 mg kg-1 day-1 in 0.9 g/100 mL NaCl) 1 h prior to ip administration of cephalothin, cefotiam or cephaloridine (1000 mg kg-1 day-1) for 3 days. Control rats were treated with corn oil or with 0.9 g/100 mL NaCl.
Animals were killed 24 h after the last injection and MDA in plasma, kidney cortex and liver tissue as well as PAH accumulation in renal cortical slices were determined as described previously (22).
Statistical analysis
Data are reported as means ± SD and were analyzed statistically by one-way analysis of variance and the Student-Neumann-Keuls test, with the level of significance set at P < 0.05.
Results
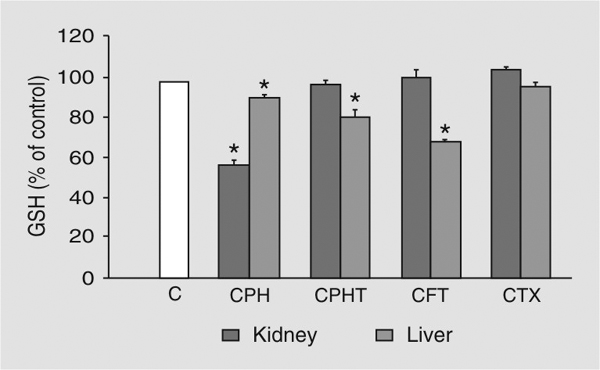
Cephaloridine induced a decrease of GSH content to 58% of control in the renal cortex of the rat kidney, while cephalothin, cefotiam and cefotaxime had no effect. At the same time cephaloridine, cephalothin and cefotiam induced a decrease of GSH content in the rat liver to 91, 81, or 69% of control, respectively, while cefotaxime had no effect (Figure 1).
After treatment of rats with cephaloridine (200, 500, 1000 mg kg-1 day-1), plasma MDA concentration reached the highest value of 1.05 nmol/mL at doses of 500 and 1000 mg kg-1 day-1 after 2 days of treatment and decreased to lower values after 3, 4 and 5 days of treatment at all doses tested (Figure 2). After cephalothin treatment at the same doses, the increase of plasma MDA concentration showed a pattern similar to that of cephaloridine with a peak of 0.85 nmol/mL after 3 days of treatment with 1000 mg kg-1 day-1. Similar treatment of rats with cefotiam caused a milder increase in plasma concentration of MDA with a peak of 0.7 nmol/mL one day after administration of cefotiam (1000 mg kg-1 day-1). Cefotaxime induced a slight increase in plasma MDA concentration during the treatment period.
Treatment of rats with cephaloridine, cephalothin or cefotiam for 4 days induced a dose-dependent increase of MDA content in the renal cortex of rats. After 4 days of cephalosporin treatment, the increase in MDA content of the renal cortex was highest after cephaloridine treatment while cefotaxime showed no dose-dependent increase in the MDA content (Figure 3). A drop in the MDA content of the renal cortex occurred, usually on the 5th day of treatment. The four cephalosporins investigated in this study caused no significant increase in MDA content in the rat liver tissue during the entire treatment period with 200 mg kg-1 day-1.
A small increase in MDA content in the liver tissue occurred after treatment of rats with the four cephalosporins (500 and 1000 mg kg-1 day-1) for 4 days. Eighteen hours after a single ip injection of rats with 2000 mg/kg cephalosporin, the concentration of conjugated diene in renal cortical microsomes was greater than control after cephaloridine, cephalothin and cefotiam treatment. While cephaloridine induced the highest concentration of conjugated diene, cefotaxime had no effect on the concentration of conjugated diene in renal cortical microsomes (Figure 4).
Treatment of rats for 4 days with cephaloridine, cephalothin and cefotiam induced a dose-dependent decrease in PAH accumulation by renal cortical slices (Figure 5). At the dose of 200 mg kg-1 day-1, the cephalosporins tested showed a significant effect on the renal cortical accumulation of PAH. At higher doses such as 1000 mg kg-1 day-1, cephaloridine, cephalothin and cefotiam significantly decreased PAH accumulation by renal cortical slices. After cefotaxime treatment, the PAH (S/M) ratio did not change significantly when compared to the control values at any of the doses used (Figure 5).
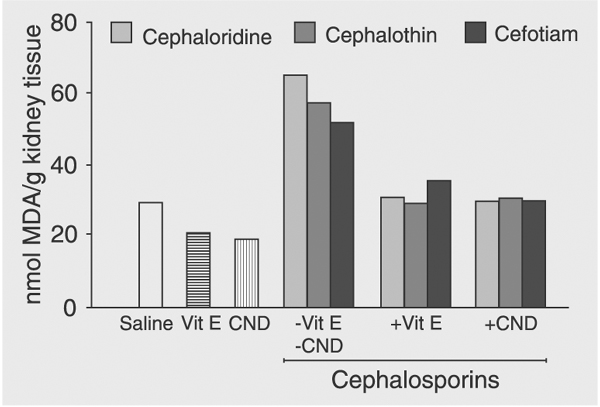
Pretreatment of rats with 400 mg kg-1 day-1 vitamin E or CND 1 h prior to cephalosporin administration (1000 mg kg-1 day-1) caused a complete inhibition of cephalosporin-induced MDA formation in the renal cortex after a 3-day treatment period (Figure 6). The antioxidants vitamin E and CND were equally effective in inhibiting MDA production in the liver 3 days after cephalosporin treatment of rats. PAH S/M values decreased significantly 3 days after cephaloridine, cephalothin or cefotiam treatment (1000 mg kg-1 day-1) and returned towards control values when rats were pretreated with 400 mg kg-1 day-1 CND or vitamin E 1 h prior to cephalosporin administration.
Depletion of rat tissue glutathione (GSH) following cephalosporin administration. Rats were injected with a single dose (2000 mg/kg, ip) of cephaloridine (CPH), cephalothin (CPHT), cefotiam (CFT), or cefotaxime (CTX) and killed 2 h later. Data are reported as percent of control (mean ± SD) for at least 5 rats. *P < 0.05 compared to animals receiving no cephalosporin (Student-Neumann-Keuls test). Hepatic and renal cortex concentrations of control rats (C) were 7.4 ± 0.3 and 4.6 ± 0.4 µmol/g tissue, respectively.
Time course of plasma malondialdehyde (MDA) concentration after ip administration of cephaloridine. Open circles = control (no cephalosporin); filled circles = 200 mg kg-1 day-1; open squares = 500 mg kg-1 day-1; filled squares = 1000 mg kg-1 day-1. Data are reported as means ± SD for at least 5 rats. *P < 0.05 compared to animals receiving no cephalosporin (Student-Neumann-Keuls test).
Dose-dependent increase of malondialdehyde (MDA) content in the renal cortex after ip cephalosporin administration for 4 days. Squares = cephaloridine; filled circles = cephalothin; triangles = cefotiam; open circles = cefotaxime. Data are reported as means ± SD for at least 5 rats. *P < 0.05 compared to control (Student-Neumann-Keuls test).
Increase of conjugated diene absorption (concentration) in renal cortical microsomes following cephalosporin administration. Rats were given a single ip injection of cephalosporin (2000 mg/kg) and were killed after 18 h. Data are reported as difference in spectrum between values obtained from treated animals and values from control rats receiving saline. Squares = cephaloridine; filled circles = cephalothin; triangles = cefotiam; open circles = cefotaxime. Data are reported as means ± SD for at least 5 rats.
Dose-dependent decrease of p-aminohippurate slice to medium ratio (PAH S/M) accumulation in rat renal cortical slices following ip cephalosporin administration for 4 days. Squares = cephaloridine; filled circles = cephalothin; triangles = cefotiam; open circles = cefotaxime. Data are reported as means ± SD for at least 5 rats. *P < 0.05 compared to control (Student-Neumann-Keuls test).
Inhibition of cephalosporin-induced malondialdehyde (MDA) production by the antioxidants vitamin E (Vit E) and cyanidanol (CND). Rats were pretreated with 400 mg kg-1 day-1 ip of the antioxidants Vit E or CND 1 h prior to treatment of rats with cephaloridine, cephalothin or cefotiam for 3 days (1000 mg kg-1 day-1, ip). Control rats were given saline, Vit E or CND. Data are reported as mean ± SD for at least 5 rats. *P < 0.05 compared to rats given cephalosporin alone (Student-Neumann-Keuls test).
Discussion
The present in vivo study was carried out in rats to compare the peroxidative potential and nephrotoxicity of cephaloridine with clinically used cephalosporins at dosages close to the therapeutic dose range or higher. The differences in the extent of the peroxidative damage induced by cephaloridine, cephalothin and cefotiam may have been due to differences in the pharmacokinetics of these cephalosporins. The concentration of these parenteral cephalosporins in proximal tubule cells, the target of their nephrotoxicity, is determined by the interplay between the ability to be absorbed and metabolized by the hepatic and renal cells on the one hand and their glomerular and biliary elimination on the other hand.
Because of its zwitterionic structure and its apparent inability to be metabolized, cephaloridine has a unique pharmacokinetic behavior and nephrotoxicity. Cephaloridine has a higher biliary elimination than cephalothin or cefotiam in the isolated perfused rabbit liver (23) and shows little or no hepatotoxicity. Cephaloridine shows the highest intracellular accumulation in renal proximal tubule cells and appears to be one of the most nephrotoxic compounds among the known cephalosporins.
Cephalothin and cefotaxime are metabolized in the liver (24) and probably also in the kidney by deacetylation of their acetoxy-methyl side chain and the metabolites formed are eliminated in the bile (23) or by glomerular filtration (24). Previous research (4) provided evidence that cephaloridine undergoes cyclic reduction-oxidation reactions with transfer of one electron from reduced cephaloridine to oxygen and subsequent formation of superoxide anion, hydrogen peroxide and possibly hydroxy radical and singlet oxygen.
Reduced GSH, a sulfhydryl-containing tripeptide, belongs to the endogenous antioxidant defense system where GSH acts as a radical scavenger or as an antioxidant. Consequently, reactive oxygen species and/or hydroperoxides are inactivated via the enzymes catalase and/or GSH peroxidase, respectively (4). As a result of its tissue defensive action, GSH content in the renal or hepatic tissue was depleted in this study to different degrees. GSH depletion could be related to intracellular cephalosporin concentration and to the intrinsic potency of these compounds to react with GSH. These findings are in good agreement with previous results (25) showing a decrease in GSH concentration in the renal cortex after cephaloridine administration to rats and rabbits. The decrease in GSH, parallel to the increase in oxidized glutathione (GSSG) content and the consequent depletion of NADPH may explain in part the nephrotoxic effects of cephaloridine (5). Because GSH has a strong reductive potential, it can also act as a nonspecific, nonenzymatic scavenger of the free radicals produced by the interaction between cephaloridine and oxygen.
The results of the present study provided a description of the time course of cephalosporin-induced peroxidative injury under in vivo conditions. A relevant increase in the concentration of conjugated diene occurred as early as 18 h after the administration of a single injection of cephaloridine, cephalothin or cefotiam. The highest plasma concentration of MDA occurred 24, 48, and 72 h after daily administration of cefotiam, cephaloridine or cephalothin, respectively.
Accumulation and/or production of MDA in the renal cortex reached the highest value after 4 days of cephalosporin treatment.
Cefotaxime had no relevant effect either on the plasma concentration of MDA or on the MDA content in the renal cortical slices. The delayed occurrence of the peroxidative injury observed in the present study is in good agreement with early data showing that cephaloridine induced renal injuries at therapeutic doses after intracellular accumulation of cephalosporin at high concentrations (26).
In the present study, the decrease in plasma MDA concentration on the 4th and 5th days of treatment and the drop in MDA content of the renal cortex on the 5th day of treatment suggest an adaptive response which could be explained as the induction of the MDA-metabolizing enzyme aldehyde dehydrogenase (27,28). It is of interest to note that in the present study there was no relevant increase in the liver content of MDA during the 5 days of treatment (data not shown). This suggests that either cephaloridine does not lead to formation of reactive oxygen species in the liver cells or, more likely, the antioxidative defense mechanisms of the liver completely detoxify the reactive species which might be formed. Alternatively, MDA formed is readily metabolized by the liver aldehyde dehydrogenase (27,28).
In vivo hepatic and possibly renal metabolism of cephalosporins such as cephalothin, cefotaxime and cefotiam (23,24), on the one hand, and lack of the metabolism of cephaloridine (25) on the other hand, could explain the milder peroxidative damage observed in vivo with cephalothin, cefotaxime and cefotiam. In addition, differences in the extent of cephalosporin-induced LPO and the renal damage induced under in vivo conditions could be explained in part by the fast distribution of the formed MDA to different tissues and its oxidative metabolism through aldehyde dehydrogenase in cytoplasm, microsomes or mitochondria (28).
The results of the present in vivo study are in good agreement with in vitro and in vivo studies showing that cephaloridine induced a dose-dependent decrease in PAH accumulation by renal cortical slices (25, 29,30). These alterations were correlated with the dose-dependent increase in LPO measured as MDA content in renal cortical slices (29,30). Cefotaxime showed no relevant effects either on LPO or on renal cell function in the present in vivo study.
Results of another study (31) indicated that 6-hydroxydopamine decreased renal PAH accumulation and that resveratrol, a naturally occurring phytoalexin, inhibited the 6-hydroxydopamine-induced time-dependent decrease of PAH accumulation in a concentration-dependent manner. Ascorbic acid and Fe2+ caused significant alteration in PAH transport and this alteration in renal cortical cell function could be correlated with LPO (32).
In the present in vivo study, both antioxidants vitamin E and (+)-cyanidanol-3 were effective in decreasing the cephalosporin-induced LPO and significantly improving the renal cell function measured as accumulation of PAH by renal cortical slices, thus displaying protective effects. Cephaloridine cytotoxicity was also inhibited by probenecid (30,33), a specific inhibitor of the renal PAH transport. In a model of acute renal failure, treatment with vitamin E preserved proximal reabsorptive and secretory functions (34).
The results of the present study are in good agreement with findings of in vitro studies indicating inhibition of cephaloridine-induced LPO after concomitant incubation of renal cortical slices with vitamin E, N,N'-diphenyl-p-phenylenediamine or promethazin (29,30). Similarly, administration of vitamin E and N,N'-diphenyl-p-phenylenediamine before cisplatin injection into rats attenuated the marked increase in blood urea nitrogen and creatinine concentrations induced by cisplatin (35,36). Taken together, the results of these antioxidant studies suggest that LPO mediates the effects of cephalosporins on renal organic anion and cation transport and vitamin E has a protective effect against cephalosporin-induced lipid peroxidation and renal damage.
The peroxidative potential and nephrotoxicity of different cephalosporins could be ranked as follows: cephaloridine > cephalothin > cefotiam > cefotaxime. The antioxidants (+)-cyanidanol-3 and vitamin E decreased cephalosporin-induced lipid peroxidation and protected against cephalosporin-induced nephrotoxicity.
Research supported by Kuwait University, Research Administration (No. MR 01/05). Received December 7, 2006. Accepted April 13, 2007.
- 1. Appel GB, Neu HC. The nephrotoxicity of antimicrobial agents (first of three parts). N Engl J Med 1977; 296: 663-670.
- 2. Wachsmuth ED. Adaptation to nephrotoxic effects of cephaloridine in subacute rat toxicity studies. Toxicol Appl Pharmacol 1982; 63: 446-460.
- 3. Tune BM, Wu KY, Fravert D, Holtzman D. Effect of cephaloridine on respiration by renal cortical mitochondria. J Pharmacol Exp Ther 1979; 210: 98-100.
- 4. Cojocel C, Hannemann J, Baumann K. Cephaloridine-induced lipid peroxidation initiated by reactive oxygen species as a possible mechanism of cephaloridine nephrotoxicity. Biochim Biophys Acta 1985; 834: 402-410.
- 5. Kuo CH, Maita K, Sleight SD, Hook JB. Lipid peroxidation: a possible mechanism of cephaloridine-induced nephrotoxicity. Toxicol Appl Pharmacol 1983; 67: 78-88.
- 6. Ullrich KJ. Renal transporters for organic anions and organic cations. Structural requirements for substrates. J Membr Biol 1997; 158: 95-107.
- 7. Khamdang S, Takeda M, Babu E, Noshiro R, Onozato ML, Tojo A, et al. Interaction of human and rat organic anion transporter 2 with various cephalosporin antibiotics. Eur J Pharmacol 2003; 465: 1-7.
- 8. Uwai Y, Saito H, Inui K. Rat renal organic anion transporter rOAT1 mediates transport of urinary-excreted cephalosporins, but not of biliary-excreted cefoperazone. Drug Metab Pharmacokinet 2002; 17: 125-129.
- 9. Fujimoto K, Ohmura H, Kaneda T. Biological antioxidant activities of bromophenols and certain other antioxidants. Agric Boil Chem 1986; 50: 101-108.
- 10. Burton GW, Joyce A, Ingold KU. Is vitamin E the only lipid-soluble, chain-breaking antioxidant in human blood plasma and erythrocyte membranes? Arch Biochem Biophys 1983; 221: 281-290.
- 11. Di Nola F. (+)-Cyanidanol-3 in acute viral hepatitis. Lancet 1980; 2: 1379-1380.
- 12. Feher J, Blazovics A, Cornides A, Vereckei A. Effect of (+)-cyanidanol-3 on rat brain lipid peroxidation. Br J Exp Pathol 1985; 66: 161-164.
- 13. Ritter J, Kahl R, Hildebrandt AG. Effect of the antioxidant (+)-cyanidanol-3 on H2O2 formation and lipid peroxidation in liver microsomes. Res Commun Chem Pathol Pharmacol 1985; 47: 48-58.
- 14. Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys 1959; 82: 70-77.
- 15. Yagi K. Assay for blood plasma or serum. Methods Enzymol 1984; 105: 328-331.
- 16. Buege JA, Aust SD. Microsomal lipid peroxidation. Methods Enzymol 1978; 52: 302-310.
- 17. Recknagel RO, Glende EA Jr. Spectrophotometric detection of lipid conjugated dienes. Methods Enzymol 1984; 105: 331-337.
- 18. Netter KJ. A method for the direct measurement of O-demethylation in liver microsomes and its use in studying microsome inhibition by SKF-525-A. Naunyn Schmiedebergs Arch Exp Pathol Pharmakol 1960; 238: 292-300.
- 19. Dent JG, Graichen ME, Schnell S, Lasker J. Constitutive and induced hepatic microsomal cytochrome P-450 monooxygenase activities in male Fischer-344 and CD rats. A comparative study. Toxicol Appl Pharmacol 1980; 52: 45-53.
- 20. Cross RJ, Taggart JV. Renal tubular transport: accumulation of p-aminohippurate by rabbit kidney slices. Am J Physiol 1950; 161: 181-190.
- 21. Smith HW, Finkelstein N, Aliminosa L, Crawford B, Graber M. The renal clearances of substituted hippuric acid derivatives and other aromatic acids in dog and man. J Clin Invest 1945; 25: 388-404.
- 22. Cojocel C. Biochemical aspects of the renal tolerance for cefpirome and other cephalosporins. Arzneimittelforschung 1990; 40: 1140-1144.
- 23. Brogard JM, Arnaud JP, Blickle JF, Levy P, Dorner M, Lautier F. Biliary elimination of cefotiam, an experimental and clinical study. Chemotherapy 1986; 32: 222-235.
- 24. Chamberlain J, Coombes JD, Dell D, Fromson JM, Ings RJ, MacDonald CM, et al. Metabolism of cefotaxime in animals and man. J Antimicrob Chemother 1980; 6 (Suppl A): 69-78.
- 25. Kuo CH, Hook JB. Depletion of renal glutathione content and nephrotoxicity of cephaloridine in rabbits, rats, and mice. Toxicol Appl Pharmacol 1982; 63: 292-302.
- 26. Tune BM, Fravert D. Mechanisms of cephalosporin nephrotoxicity: a comparison of cephaloridine and cephaloglycin. Kidney Int 1980; 18: 591-600.
- 27. Draper HH, McGirr LG, Hadley M. The metabolism of malondialdehyde. Lipids 1986; 21: 305-307.
- 28. Marnett LJ, Buck J, Tuttle MA, Basu AK, Bull AW. Distribution and oxidation of malondialdehyde in mice. Prostaglandins 1985; 30: 241-254.
- 29. Goldstein RS, Pasino DA, Hewitt WR, Hook JB. Biochemical mechanisms of cephaloridine nephrotoxicity: time and concentration dependence of peroxidative injury. Toxicol Appl Pharmacol 1986; 83: 261-270.
- 30. Cojocel C, Laeschke KH, Inselmann G, Baumann K. Inhibition of cephaloridine-induced lipid peroxidation. Toxicology 1985; 35: 295-305.
- 31. Cojocel C, Thomson MS. Protective effect of resveratrol against 6-hydroxydopamine-induced impairment of renal p-aminohippurate transport. Arch Toxicol 2004; 78: 525-532.
- 32. Fujimoto Y, Fujita T. Effect of lipid peroxidation on p-aminohippurate transport by rat kidney cortical slices. Br J Pharmacol 1982; 76: 373-379.
- 33. Jung KY, Takeda M, Shimoda M, Narikawa S, Tojo A, Kim DK, et al. Involvement of rat organic anion transporter 3 (rOAT3) in cephaloridine-induced nephrotoxicity: in comparison with rOAT1. Life Sci 2002; 70: 1861-1874.
- 34. Arreola-Mendoza L, Reyes JL, Melendez E, Martin D, Namorado MC, Sanchez E, et al. Alpha-tocopherol protects against the renal damage caused by potassium dichromate. Toxicology 2006; 218: 237-246.
- 35. Sugihara K, Gemba M. Modification of cisplatin toxicity by antioxidants. Jpn J Pharmacol 1986; 40: 353-355.
- 36. Ajith TA, Usha S, Nivitha V. Ascorbic acid and alpha-tocopherol protect against anticancer drug cisplatin induced nephrotoxicity in mice: a comparative study. Clin Chem Acta 2007; 375: 82-86.
Correspondence and Footnotes
Publication Dates
-
Publication in this collection
18 June 2007 -
Date of issue
June 2007
History
-
Accepted
13 Apr 2007 -
Received
07 Dec 2006