Abstract
The cytokinesis-block micronucleus (CBMN) assay is one of the standard cytogenetic tools employed to assess chromosomal damage subsequent to exposure to genotoxic/cytotoxic agents, and is widely applicable to plant, animal and human cells. In the present study, the CBMN assay was used to assess the baseline damage in binuclear human peripheral blood lymphocytes exposed to 25 µg/L p,p'-DDT for 1, 2, 24, and 48 h by measuring the frequency of micronuclei, nucleoplasmic bridges and nuclear buds. These new scoring criteria facilitated the detection of different types of clastogenic and aneugenic effects induced by this type of pollutant. With these criteria, CBMN can also be used to measure nucleoplasmic bridges which are considered to be consequences of chromosome rearrangements and nuclear buds which are biomarkers of altered gene amplification and gene dosage. The total number of micronuclei observed in binuclear human peripheral blood lymphocytes of the exposed samples (ranging from 32 to 47) was significantly greater (P < 0.05) than that detected in the unexposed (0 time) control sample, where the total number of micronuclei was 7. The number of nucleoplasmic bridges and nuclear buds obtained after 24 and 48 h was also significantly (P < 0.05) greater in the samples treated with p,p'-DDT than in the unexposed control samples. Thus, our results confirmed the usefulness of the new criteria applicable for the CBMN assay employed in measuring the DNA damage and its role of a sensitive cytogenetic biomarker.
p,p'-DDT; Peripheral blood human lymphocytes; Micronucleus assay; Nucleoplasmic bridges; Nuclear buds
Braz J Med Biol Res, June 2008, Volume 41(6) 473-476 (Short Communication)
Efficacy of HUMN criteria for scoring the micronucleus assay in human lymphocytes exposed to a low concentration of p,p'-DDT
 Correspondence and Footnotes
Correspondence and Footnotes
 V. Garaj-Vrhovac, G. Gajski and S. Ravlić
V. Garaj-Vrhovac, G. Gajski and S. Ravlić
Institute for Medical Research and Occupational Health, Mutagenesis Unit, Zagreb, Croatia
 Text
Text
 Correspondence and Footnotes
Correspondence and Footnotes
Correspondence and Footnotes
Correspondence and Footnotes
Correspondence and Footnotes
Correspondence and Footnotes
Abstract
The cytokinesis-block micronucleus (CBMN) assay is one of the standard cytogenetic tools employed to assess chromosomal damage subsequent to exposure to genotoxic/cytotoxic agents, and is widely applicable to plant, animal and human cells. In the present study, the CBMN assay was used to assess the baseline damage in binuclear human peripheral blood lymphocytes exposed to 25 µg/L p,p'-DDT for 1, 2, 24, and 48 h by measuring the frequency of micronuclei, nucleoplasmic bridges and nuclear buds. These new scoring criteria facilitated the detection of different types of clastogenic and aneugenic effects induced by this type of pollutant. With these criteria, CBMN can also be used to measure nucleoplasmic bridges which are considered to be consequences of chromosome rearrangements and nuclear buds which are biomarkers of altered gene amplification and gene dosage. The total number of micronuclei observed in binuclear human peripheral blood lymphocytes of the exposed samples (ranging from 32 to 47) was significantly greater (P < 0.05) than that detected in the unexposed (0 time) control sample, where the total number of micronuclei was 7. The number of nucleoplasmic bridges and nuclear buds obtained after 24 and 48 h was also significantly (P < 0.05) greater in the samples treated with p,p'-DDT than in the unexposed control samples. Thus, our results confirmed the usefulness of the new criteria applicable for the CBMN assay employed in measuring the DNA damage and its role of a sensitive cytogenetic biomarker.
Key words: p,p'-DDT; Peripheral blood human lymphocytes; Micronucleus assay; Nucleoplasmic bridges; Nuclear buds
Text
Over the last decade, the wide applicability of the in vitro micronucleus test in peripheral blood lymphocytes and the simplicity of scoring have made it an attractive cytogenetic tool to assess occupational and environmental exposures to genotoxic agents. The formation of micronuclei is considered to be an effective biomarker of diseases and processes associated with DNA damage. The cytokinesis-block micronucleus (CBMN) assay solved the problem of variations in micronucleus frequency caused by the alterations in the proportion of dividing cells that may occur when cells are exposed to genotoxic agents, by restricting the scoring of micronuclei to once-divided cells (1). Except for cytogenetic damage measured by the number and distribution of micronuclei, according to the new criteria for micronuclei scoring, the CBMN assay also detects the nucleoplasmic bridges, as well as nuclear buds. Current evidence suggests that nucleoplasmic bridges derive from dicentric chromosomes in which the centromeres have been pulled to the opposite poles of the cell during the anaphase stage, and are therefore indicative of the DNA mis-repair, chromosome rearrangement or telomere end-fusion. According to the new criteria applicable to the CBMN assay, nuclear buds arise from the elimination of the amplified DNA and possibly from the elimination of the DNA-repair complexes, which therefore, may be considered a marker of gene amplification and altered gene dosage. In this respect, the micronucleus assay has evolved into a comprehensive method employed in measuring chromosomal instability of the phenotype and altered cell viability and represents an effective tool to be used in research of cellular and nuclear dysfunctions caused by in vitro or in vivo exposure to toxic substances (2,3). The present study reports the results obtained with the CBMN assay, measuring the frequency of micronuclei, nucleoplasmic bridges and nuclear buds in binuclear human lymphocytes exposed to a very low concentration of p,p'-DDT.
Dichlorodiphenyltrichloroethane (DDT) is a pesticide that was once extensively used to control insects affecting agricultural crops, and those carrying various diseases such as malaria and typhus; however, it is now used in only a small number of countries to control malaria. Technical-grade DDT (1,1,1-trichloro-2,2-bis(p-chlorophenyl)ethane) is a mixture of three forms, p,p'-DDT (85%), o,p'-DDT (15%) and o,o'-DDT (trace amounts), but may also contain DDE (1,1-dichloro-2,2-bis(p-chlorophenyl)ethylene) and DDD (1,1-dichloro-2,2-bis(p-chlorophenyl)ethane) as contaminants; both are breakdown products of DDT (4). In the most developed countries, DDT and DDT-based products were prohibited for use as pesticides in the 1970s and 1980s, because of their persistence, bioaccumulation and carcinogenicity (5). However, in other countries in which malaria is a long-standing public health problem, DDT is still the main pesticide used for mosquito control. Much research shows that DDT affects nervous, reproductive, and immunological systems and can provoke several types of cancer (6-9). DDT and its metabolites have also been tested in numerous genotoxicity studies conducted in animals and bacterial systems, but the number of studies on humans is insufficient (10-12). Mean blood concentration of DDT in humans ranges from 27.1 µg/L in children up to 67.8 µg/L in adults (12). Considering the lack of data on the effect of DDT on the cellular genome, and taking into account its usage in some countries of the Third World and its environmental persistence, the aim of this study was to assess the genotoxic potential of a low concentration of aqueous (25 µg/L) p,p'-DDT upon in vitro exposure of human peripheral blood lymphocytes of different duration, by using the new criteria for scoring micronucleus assay measuring the frequency of micronuclei, nucleoplasmic bridges and nuclear buds.
Peripheral blood samples were obtained from a healthy 25-year-old female donor. Blood was collected by venipuncture and stored in heparinized tubes (Becton Dickinson, NJ, USA). Blood was subsequently divided into aliquots and sored at 4°C. All experiments were conducted on peripheral blood lymphocytes cultivated in Euroclone medium (Sigma, USA) at 37°C in the presence of phytohemagglutinin.
The DDT metabolite was administered in the form of p,p'-DDT (Sulpeco, USA). Just before the beginning of the experiment, p,p'-DDT was dissolved in saline buffer at 25°C and added to the lymphocyte cultures at a final concentration of 25 µg/L for 0 (control), 1, 2, 24, and 48 h.
Micronucleus assay was performed in agreement with the guidelines described by Fenech et al. (13,14). According to the new scoring criteria, not only distribution of micronuclei, but also the frequency of nucleoplasmic bridges and nuclear buds were scored (1-3,15). Cytochalasine-B (Sigma) at a final concentration of 3 µg/mL was added 44 h after culture initiation. The cultures were harvested at 72 h. The lymphocytes were fixed in methanol-acetic acid solution (3:1), air-dried and stained with 5% Giemsa solution (Sigma). One thousand binuclear lymphocytes were analyzed on duplicate slides (difference below 2%) for the evaluation of micronuclei incidence and their distribution per micronuclear cells, as well as nucleoplasmic bridges and nuclear buds. The total number and distribution of micronuclei, nucleoplasmic bridges and nuclear buds scored in binucleated cells were evaluated using the chi-square test.
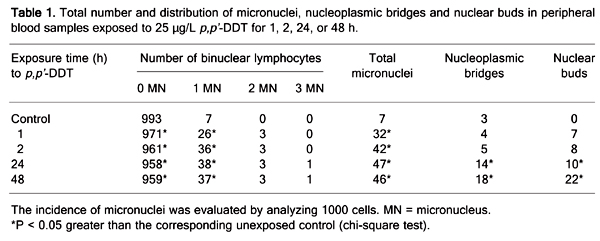
Table 1 summarizes the frequency and distribution of micronuclei, nucleoplasmic bridges and nuclear buds in the exposed and control groups. The total number of micronuclei observed in binuclear peripheral blood lymphocytes of the exposed samples (ranging from 32 to 47) was significantly (P < 0.05) greater than that detected in the unexposed (0 time) control sample, where the total number of micronuclei was 7. At 24 and 48 h, the number of nucleoplasmic bridges (ranging from 10 to 22) and nuclear buds (ranging from 14 to 18) were also significantly (P < 0.05) greater in samples treated with p,p'-DDT than in the unexposed control (ranging from 0 to 3). The p,p'-DDT treatment caused primary DNA damage as opposed to untreated control lymphocytes.
Due to its uncontrolled use during the last half of the 20th century, DDT, probably the best known and most used insecticide in the world, has induced changes in both working and living environments, and may cause adverse effects on human health. Even though its use has been prohibited in most countries on the basis of ecological considerations and its negative impact on wildlife, it is still used in some developing countries in an attempt to control malaria. Thus, it can still pose a health risk for humans, even in countries that banned its use 30 years ago (4,5).
Genotoxic studies of DDT are mainly based on investigations on animal models, and its genotoxicity has been evaluated using standard cytogenetic techniques. Research results obtained by the investigation of cytogenetic effects of DDT presented in the mouse spleen indicate that DDT induces a statistically significant increase in chromosomal aberrations at a dose of 5.5 mg/kg after a 24-h exposure (16). The results obtained in humans under in vitro exposure of peripheral blood indicated that the correlation between DDT dose and the number of cells containing chromosomal aberrations is non-existent. However, when the Poisson test was used for comparison, the percentage of cells containing structural aberrations was significantly higher at certain DDT concentrations (0.20, 4.05 and 8.72 µg/mL) than in controls (9). There is also a report on the biomonitoring of populations occupationally and environmentally exposed to DDT, among which a positive correlation between the frequency of cells containing chromosomal aberrations and DDT plasma levels, as well as exposure time, had been found. Interestingly, direct or indirect contact with DDT was not always in correlation with the degree of contamination (11).
The results of the present study of the effect of DDT on the incidence of micronuclei are in agreement with the earlier observation (17). They indicated that, after exposure to p,p'-DDT at concentrations of 10 and 15 µg/mL, skin fibroblast culture of the Arctic beluga whale (Delphinapterus leucas) exhibits statistically significant increases in micronuclei, ranging from 1.7- to 5-fold compared to controls (17). However, there are no data about the micronucleus assay performed in human cells exposed to p,p'-DDT under in vitro conditions.
The present study reports the results of the frequencies of micronuclei, nucleoplasmic bridges and nuclear buds observed in cultured human lymphocytes treated with a very low concentration of p,p'-DDT, scored according to the criteria proposed by the HUMN project (15). Based on the results of the present study, it is clear that modified micronucleus assay is more sensitive to DNA damage than other cytogenetic methods and, as such, did show a statistically significant (P < 0.05) increase in the number of micronuclei, nucleoplasmic bridges and nuclear buds. These new criteria now indicate that this method can also be used to measure nucleoplasmic bridges, which are considered to be a biomarker of dicentric chromosomes resulting from telomere end-fusions or DNA mis-repair. This method is also capable of measuring nuclear buds, which are a biomarker able to detect gene amplification and altered gene dosage events by virtue of providing a measure for the extent of chromosome rearrangement which is otherwise not measured in this assay in case only micronuclei are scored (2,3,18,19).
Undoubtedly, the current results confirm the value of the CBMN assay as a sensitive endpoint that will have to be further evaluated and standardized for the assessment of micronuclei frequencies, nucleoplasmic bridges and nuclear buds, employed in comparing genetic damage caused by different mutagens under in vitro and in vivo conditions.
Total number and distribution of micronuclei, nucleoplasmic bridges and nuclear buds in peripheral blood samples exposed to 25 µg/L p,p'-DDT for 1, 2, 24, or 48 h.
References
1. Kirsch-Volders M, Sofuni T, Aardema M, Albertini S, Eastmond D, Fenech M, et al. Report from the in vitro micronucleus assay working group. Mutat Res 2003; 540: 153-163.
2. Fenech M. Cytokinesis-block micronucleus assay evolves into a "cytome" assay of chromosomal instability, mitotic dysfunction and cell death. Mutat Res 2006; 600: 58-66.
3. Crott JW, Mashiyama ST, Ames BN, Fenech M. The effect of folic acid deficiency and MTHFR C677T polymorphism on chromosome damage in human lymphocytes in vitro. Cancer Epidemiol Biomarkers Prev 2001; 10: 1089-1096.
4. ATSDR. Toxicological profile for DDT/DDD/DDE. Agency for Toxic Substances and Diseases Registry. Atlanta: US Public Health Service; 2002.
5. Turusov V, Rakitsky V, Tomatis L. Dichlorodiphenyltrichloroethane (DDT): ubiquity, persistence, and risks. Environ Health Perspect 2002; 110: 125-128.
6. Martin SA Jr, Harlow SD, Sowers MF, Longnecker MP, Garabrant D, Shore DL, et al. DDT metabolite and androgens in African-American farmers. Epidemiology 2002; 13: 454-458.
7. Perez-Maldonado IN, Athanasiadou M, Yanez L, Gonzalez-Amaro R, Bergman A, Diaz-Barriga F. DDE-induced apoptosis in children exposed to the DDT metabolite. Sci Total Environ 2006; 370: 343-351.
8. Wojtowicz AK, Gregoraszczuk EL, Ptak A, Falandysz J. Effect of single and repeated in vitro exposure of ovarian follicles to o,p'-DDT and p,p'-DDT and their metabolites. Pol J Pharmacol 2004; 56: 465-472.
9. Perez-Maldonado IN, Herrera C, Batres LE, Gonzalez-Amaro R, Diaz-Barriga F, Yanez L. DDT-induced oxidative damage in human blood mononuclear cells. Environ Res 2005; 98: 177-184.
10. Lessa JM, Becak W, Nazareth RM, Pereira CA, Ungaro MT. Cytogenetic study of DDT on human lymphocytes in vitro. Mutat Res 1976; 40: 131-138.
11. Rabello MN, Dealmeida WF, Pigati P, Ungaro MT, Murata T, Perira CA, et al. Cytogenetic study on individuals occupationally exposed to DDT. Mutat Res 1975; 28: 449-454.
12. Yanez L, Ortiz-Perez D, Batres LE, Borja-Aburto VH, Diaz-Barriga F. Levels of dichlorodiphenyltrichloroethane and deltamethrin in humans and environmental samples in malarious areas of Mexico. Environ Res 2002; 88: 174-181.
13. Fenech M, Morley AA. Measurement of micronuclei in lymphocytes. Mutat Res 1985; 147: 29-36.
14. Fenech M. The in vitro micronucleus technique. Mutat Res 2000; 455: 81-95.
15. Fenech M, Chang WP, Kirsch-Volders M, Holland N, Bonassi S, Zeiger E. HUMN project: detailed description of the scoring criteria for the cytokinesis-block micronucleus assay using isolated human lymphocyte cultures. Mutat Res 2003; 534: 65-75.
16. Amer SM, Fahmy MA, Donya SM. Cytogenetic effect of some insecticides in mouse spleen. J Appl Toxicol 1996; 16: 1-3.
17. Gauthier JM, Dubeau H, Rassart E. Induction of micronuclei in vitro by organochlorine compounds in beluga whale skin fibroblasts. Mutat Res 1999; 439: 87-95.
18. Umegaki K, Fenech M. Cytokinesis-block micronucleus assay in WIL2-NS cells: a sensitive system to detect chromosomal damage induced by reactive oxygen species and activated human neutrophils. Mutagenesis 2000; 15: 261-269.
19. Thomas P, Umegaki K, Fenech M. Nucleoplasmic bridges are a sensitive measure of chromosome rearrangement in the cytokinesis-block micronucleus assay. Mutagenesis 2003; 18: 187-194.
 Address for correspondence: V. Garaj-Vrhovac, Institute for Medical Research and Occupational Health, Mutagenesis Unit, Ksaverska c. 2, 10 000 Zagreb, Croatia. Fax: +385-1-4673-303. E-mail: vgaraj@imi.hr
Address for correspondence: V. Garaj-Vrhovac, Institute for Medical Research and Occupational Health, Mutagenesis Unit, Ksaverska c. 2, 10 000 Zagreb, Croatia. Fax: +385-1-4673-303. E-mail: vgaraj@imi.hr
Research supported by the Croatian Ministry of Science, Education and Sports (#0022-0222148-2125). Received August 27, 2007. Accepted May 21, 2008.
- 1. Kirsch-Volders M, Sofuni T, Aardema M, Albertini S, Eastmond D, Fenech M, et al. Report from the in vitro micronucleus assay working group. Mutat Res 2003; 540: 153-163.
- 2. Fenech M. Cytokinesis-block micronucleus assay evolves into a "cytome" assay of chromosomal instability, mitotic dysfunction and cell death. Mutat Res 2006; 600: 58-66.
- 3. Crott JW, Mashiyama ST, Ames BN, Fenech M. The effect of folic acid deficiency and MTHFR C677T polymorphism on chromosome damage in human lymphocytes in vitro Cancer Epidemiol Biomarkers Prev 2001; 10: 1089-1096.
- 4. ATSDR. Toxicological profile for DDT/DDD/DDE. Agency for Toxic Substances and Diseases Registry Atlanta: US Public Health Service; 2002.
- 5. Turusov V, Rakitsky V, Tomatis L. Dichlorodiphenyltrichloroethane (DDT): ubiquity, persistence, and risks. Environ Health Perspect 2002; 110: 125-128.
- 6. Martin SA Jr, Harlow SD, Sowers MF, Longnecker MP, Garabrant D, Shore DL, et al. DDT metabolite and androgens in African-American farmers. Epidemiology 2002; 13: 454-458.
- 7. Perez-Maldonado IN, Athanasiadou M, Yanez L, Gonzalez-Amaro R, Bergman A, Diaz-Barriga F. DDE-induced apoptosis in children exposed to the DDT metabolite. Sci Total Environ 2006; 370: 343-351.
- 8. Wojtowicz AK, Gregoraszczuk EL, Ptak A, Falandysz J. Effect of single and repeated in vitro exposure of ovarian follicles to o,p'-DDT and p,p'-DDT and their metabolites. Pol J Pharmacol 2004; 56: 465-472.
- 9. Perez-Maldonado IN, Herrera C, Batres LE, Gonzalez-Amaro R, Diaz-Barriga F, Yanez L. DDT-induced oxidative damage in human blood mononuclear cells. Environ Res 2005; 98: 177-184.
- 10. Lessa JM, Becak W, Nazareth RM, Pereira CA, Ungaro MT. Cytogenetic study of DDT on human lymphocytes in vitro Mutat Res 1976; 40: 131-138.
- 11. Rabello MN, Dealmeida WF, Pigati P, Ungaro MT, Murata T, Perira CA, et al. Cytogenetic study on individuals occupationally exposed to DDT. Mutat Res 1975; 28: 449-454.
- 12. Yanez L, Ortiz-Perez D, Batres LE, Borja-Aburto VH, Diaz-Barriga F. Levels of dichlorodiphenyltrichloroethane and deltamethrin in humans and environmental samples in malarious areas of Mexico. Environ Res 2002; 88: 174-181.
- 13. Fenech M, Morley AA. Measurement of micronuclei in lymphocytes. Mutat Res 1985; 147: 29-36.
- 14. Fenech M. The in vitro micronucleus technique. Mutat Res 2000; 455: 81-95.
- 15. Fenech M, Chang WP, Kirsch-Volders M, Holland N, Bonassi S, Zeiger E. HUMN project: detailed description of the scoring criteria for the cytokinesis-block micronucleus assay using isolated human lymphocyte cultures. Mutat Res 2003; 534: 65-75.
- 16. Amer SM, Fahmy MA, Donya SM. Cytogenetic effect of some insecticides in mouse spleen. J Appl Toxicol 1996; 16: 1-3.
- 17. Gauthier JM, Dubeau H, Rassart E. Induction of micronuclei in vitro by organochlorine compounds in beluga whale skin fibroblasts. Mutat Res 1999; 439: 87-95.
- 18. Umegaki K, Fenech M. Cytokinesis-block micronucleus assay in WIL2-NS cells: a sensitive system to detect chromosomal damage induced by reactive oxygen species and activated human neutrophils. Mutagenesis 2000; 15: 261-269.
- 19. Thomas P, Umegaki K, Fenech M. Nucleoplasmic bridges are a sensitive measure of chromosome rearrangement in the cytokinesis-block micronucleus assay. Mutagenesis 2003; 18: 187-194.
Correspondence and Footnotes
Publication Dates
-
Publication in this collection
31 May 2008 -
Date of issue
June 2008
History
-
Received
27 Aug 2007 -
Accepted
21 May 2008