Abstract
Variations in the estrogenic activity of the phytoestrogen-rich plant, Pueraria mirifica, were determined with yeast estrogen screen (YES) consisting of human estrogen receptors (hER) hERα and hERβ and human transcriptional intermediary factor 2 (hTIF2) or human steroid receptor coactivator 1 (hSRC1), respectively, together with the β-galactosidase expression cassette. Relative estrogenic potency was expressed by determining the β-galactosidase activity (EC50) of the tuber extracts in relation to 17β-estradiol. Twenty-four and 22 of the plant tuber ethanolic extracts interacted with hERα and hERβ, respectively, with a higher relative estrogenic potency with hERβ than with hERα. Antiestrogenic activity of the plant extracts was also determined by incubation of plant extracts with 17β-estradiol prior to YES assay. The plant extracts tested exhibited antiestrogenic activity. Both the estrogenic and the antiestrogenic activity of the tuber extracts were metabolically activated with the rat liver S9-fraction prior to the assay indicating the positive influence of liver enzymes. Correlation analysis between estrogenic potency and the five major isoflavonoid contents within the previously HPLC-analyzed tuberous samples namely puerarin, daidzin, genistin, daidzein, and genistein revealed a negative result.
YES assay; Estrogen receptor α (ERα); Estrogen receptor β (ERβ); Phytoestrogen; Isoflavonoid; Pueraria mirifica
Braz J Med Biol Res, February 2010, Volume 43(2) 195-200
Differential binding with ERα and ERβ of the phytoestrogen-rich plant Pueraria mirifica
C. Boonchird1, T. Mahapanichkul1 and  Correspondence and Footnotes
Correspondence and Footnotes
 W. Cherdshewasart2
W. Cherdshewasart2
1Department of Biotechnology, Faculty of Science, Mahidol University, Ratchathewi, Bangkok, Thailand
2Department of Biology, Faculty of Science, Chulalongkorn University, Patumwan, Bangkok, Thailand
 Correspondence and Footnotes
Correspondence and Footnotes
Correspondence and Footnotes
Correspondence and Footnotes
Correspondence and Footnotes
Correspondence and Footnotes
Abstract
Variations in the estrogenic activity of the phytoestrogen-rich plant, Pueraria mirifica, were determined with yeast estrogen screen (YES) consisting of human estrogen receptors (hER) hERα and hERβ and human transcriptional intermediary factor 2 (hTIF2) or human steroid receptor coactivator 1 (hSRC1), respectively, together with the β-galactosidase expression cassette. Relative estrogenic potency was expressed by determining the β-galactosidase activity (EC50) of the tuber extracts in relation to 17β-estradiol. Twenty-four and 22 of the plant tuber ethanolic extracts interacted with hERα and hERβ, respectively, with a higher relative estrogenic potency with hERβ than with hERα. Antiestrogenic activity of the plant extracts was also determined by incubation of plant extracts with 17β-estradiol prior to YES assay. The plant extracts tested exhibited antiestrogenic activity. Both the estrogenic and the antiestrogenic activity of the tuber extracts were metabolically activated with the rat liver S9-fraction prior to the assay indicating the positive influence of liver enzymes. Correlation analysis between estrogenic potency and the five major isoflavonoid contents within the previously HPLC-analyzed tuberous samples namely puerarin, daidzin, genistin, daidzein, and genistein revealed a negative result.
Key words: YES assay; Estrogen receptor α (ERα); Estrogen receptor β (ERβ); Phytoestrogen; Isoflavonoid; Pueraria mirifica
Introduction
Phytoestrogens are of biological interest because they exhibit estrogenic and antiestrogenic activities both in vitro and in vivo. The estrogenic and antiestrogenic activity of isoflavones, some of the major phytoestrogens, has been reported on the uterus of rats and mice (1,2), on mammalian cells (3), and yeast (4). There are various in vivo methods to measure the estrogenic activity of phytoestrogens, including an uterotropic assay (5) and vaginal cornification assay in ovariectomized rats (6-8). The MCF-7 screening test based on in vitro proliferation of the human mammary adenocarcinoma estrogen receptor (ER) α+ cell line is able to demonstrate the biphasic (proliferative effect at low dose and antiproliferative effect at high dose) estrogenic response of phytoestrogen-rich plant extracts (9,10). However, testing on animal models may not be practical for large-scale screening of the estrogenic activity of plant samples due to the cost and time-consuming procedures involved. In addition, the differential interactions of the plant extract with ERα and ERβ cannot be easily monitored in in vivo and in some in vitro assay. These cellular models often express other endogenous receptors such as progesterone receptor and glucocorticoid receptor (11,12), which could interfere with the accurate determination of the estrogenic effects of plant extracts.
Pueraria mirifica, family Leguminosae, a traditional herbal plant used for treatment of menopausal symptoms in Thailand, was selected for the present study. The plant is a rich source of phytoestrogens including mirestrol (13), deoxymirestrol (14) and isoflavonoids (15,16). The plant"s powdered tuber has been shown to improve the lipid profiles and biochemical markers of bone turnover rates (17) and the recovery of vaginal health (18) in clinical trials with menopausal Thai females and also to provide other health benefits as demonstrated in animal tests such as prevention of osteoporosis (19,20), breast cancer (21) and estrogen deficiency replacement (22,23). However, there is only one report about this plant that demonstrated the action of the plant phytoestrogens at the ERα and ERβ level (21). To help clarify the action of phytoestrogens from this plant on ERα and ERβ, a yeast estrogen screening (YES) test based on the yeast two-hybrid system was applied to the plant extracts of the same group of P. mirifica used in HPLC analysis of isoflavonoids (15) and in a vaginal cornification assay (6,7). This YES system was constructed by inserting the human estrogen receptor (hER) and coactivator into the yeast cells, which allowed specific binding of a ligand to the ER prior to interaction with the yeast transcription machinery. The estrogenic effect was then quantitatively evaluated by the level of expression of the reporter gene encoding the β-galactosidase enzyme (24). Since hER exists in two subtypes, hERα and hERβ (25), distributed within specific tissues, the YES system comprised of human transcriptional intermediary factor 2 (hTIF2) and human steroid receptor coactivator 1 (hSRC1), which exhibited the greatest effectiveness on hERs in the presence of 17β-estradiol (25,26) and flanked by hERα and hERβ, was used in the present investigation.
Material and Methods
Plant material
The tuberous roots of P. mirifica were collected in 27 of 76 provinces in Thailand (15). The plant was identified by the author with reference to the study of Kashemsanta et al. (27) and voucher herbarium specimen No. BCU 11045 (9). The tuberous roots were cleaned, sliced, dried in a hot-air oven at 70°C until completely dry and subsequently ground to a powder. The powder was extracted with absolute ethanol at the 1:10 (w/v) ratio, the aqueous phase was filtered through Whatman filter paper No. 3 and subsequently dried in vacuo. Stock solutions of the plant extracts were freshly prepared in dimethylsulfoxide (DMSO) at doses ranging from 1 µg to 1 mg/mL. The final concentration of DMSO in the test did not exceed 0.02%. The tuberous root extracts were tested in both YES systems.
Construction of yeast estrogen screening strains ERα + hTIF2 and ERβ + hSRC1
The yeast Saccharomyces cerevisiae strain Y190 (MATa, ura3-52, his3-200, lys2-80.1, ade2-101, trp1-901, leu2-3,112, gal4Δ, gal80Δ, cyhr2, LYS2::GAL1-HIS3, URA3::GAL1-LacZ; Clontech®, USA) was used as host for construction of 2 yeast strains, namely YES-hERα + hTIF2 and YES-hERβ + hSRC1, harboring plasmid pGBT9-hERαLBD and pGAD424-hTIF2 coactivator, and plasmid pGBT9-hERβLBD and pGAD424-hSRC1 coactivator, respectively. Plasmids pGBT9-hERαLBD, pGBT9-hERβLBD and pGAD424-hSRC1 were constructed using genes hERα/β LBD and hSRC1 kindly provided by Dr. A. Ohta, Department of Biotechnology, the University of Tokyo, Japan (26). Plasmid pGAD424-hTIF2 was a gift from Dr. Y. Masamune, Department of Cellular and Molecular Biology, Faculty of Pharmaceutical Science, Kanazawa University (28).
Yeast culture condition
Yeasts were grown in synthetic dextrose minimal medium supplemented with adenine (0.67% yeast nitrogen base without amino acids, 2% glucose, and 0.002% adenine sulfate) at 30°C with vigorous shaking overnight. The assay was performed by incubating 50 µL of the overnight culture and 2.5 µL of the plant extract at a final concentration of 1 µg to 1 mg/mL dissolved in DMSO as a negative control or 2.5 µL of 10 mM to 100 pM 17β-estradiol as a positive control in the tube containing 200 µL fresh synthetic dextrose minimal medium supplemented with adenine. After incubation at 30°C with shaking for 4 h, β-galactosidase activity was determined.
β-galactosidase assay
After incubation, 150 µL cultured cells was added to each well of a 96-well microplate for the measurement of cell density at 600 nm using a microplate reader (Spectral MAXplus, Molecular Devices, USA). Another 100 µL cultured cells was centrifuged at 8600 g and resuspended in 200 µL Z-buffer (0.1 M sodium phosphate, pH 7.0, 10 mM KCl, 1 mM MgSO4, and 3.5 mM β-mercaptoethanol) containing 1 mg/mL Zymolyase 20T, and incubated at 37°C for 15 min. The cell lysate was incubated with 40 µL substrate (4 mg/mL o-nitrophenyl β-d-galactopyranoside in 0.1 M sodium phosphate buffer, pH 7.0) at 30°C for 30 min. When the yellow color of o-nitrophenol developed, 100 µL 1 M Na2CO3 was added to stop the reaction. To remove all cell debris, the reaction tube was centrifuged at 8600 g for 5 min. The 150-µL supernatant was transferred to each well of a 96-well microplate and absorbance at 420 and 550 nm was measured with a microplate reader. β-galactosidase activity is reported in Miller units (24).
Metabolic activation
In vitro metabolic activation with the aid of the rat liver S9 fraction (Wako Pure Chemical Industries, Ltd., Japan) was tested against plant samples that did not exhibit estrogenic activity in the normal YES assay according to the method of Takatori et al. (29). A 10-μL aliquot of plant extract dissolved in DMSO was incubated with a 990-µL aliquot of S9 mixture containing 0.5 mg S9 protein, 0.16 M MgCl2, 0.1 M NADP, 0.1 M G-6-P, 0.5 M sodium phosphate buffer, pH 7.4, and 1 M KCl at 37°C for 4 h. The negative control consisted of the heat-inactivated (95°C for 5 min) S9 fraction. The test compounds were stored at -80°C until use. The final concentration of S9-treated P. mirifica in the bioassay was 1000 μg/mL.
Evaluation of antiestrogenic activity
To assess the antiestrogenic activity of P. mirifica, plant extracts from sources that exerted no estrogenic activity were examined based on the inhibition of β-galactosidase induction by 17β-estradiol in YES-hERα + hTIF2 and YES-hERβ + hSRC1.
Calculation of EC50 and statistical analysis
Data of β-galactosidase unit and concentration of test compounds were fitted using a four-parameter logistic dose-response model with the aid of the SigmaPlot software, Version 9 for Windows (Systat Software, Inc., USA). The β-galactosidase unit, EC50, and relative potency value compared with 17β-estradiol are reported as the mean ± SEM of at least 3 independent experiments. All data were analyzed by the Student t-test using the SPSS software version 12.0 for Windows (SPSS Inc., USA).
The correlation between the estrogenic potency of the plant extracts and 5 major isoflavonoid contents of the plant tuberous powder of the same plant samples (15) was calculated with the aid of the SPSS software version 12.0 for Windows (SPSS Inc.).
Results
The dose response of 17β-estradiol in YES-hERα + hTIF2 and YES-hERβ + hSRC1
The dose-response curves for 17β-estradiol in YES-hERα + hTIF2 and YES-hERβ + hSRC1, presented as β-galactosidase unit against log concentration of 17β-estradiol, are shown in Figure 1. The EC50 values of 17β-estradiol for the YES-hERα + hTIF2 and YES-hERβ + hSRC1 systems were 1.44 x 10-2 and 9.08 x 10-2 µg/mL, respectively.
Estrogenic activity of P. mirifica extract in YES-hERα + hTIF2
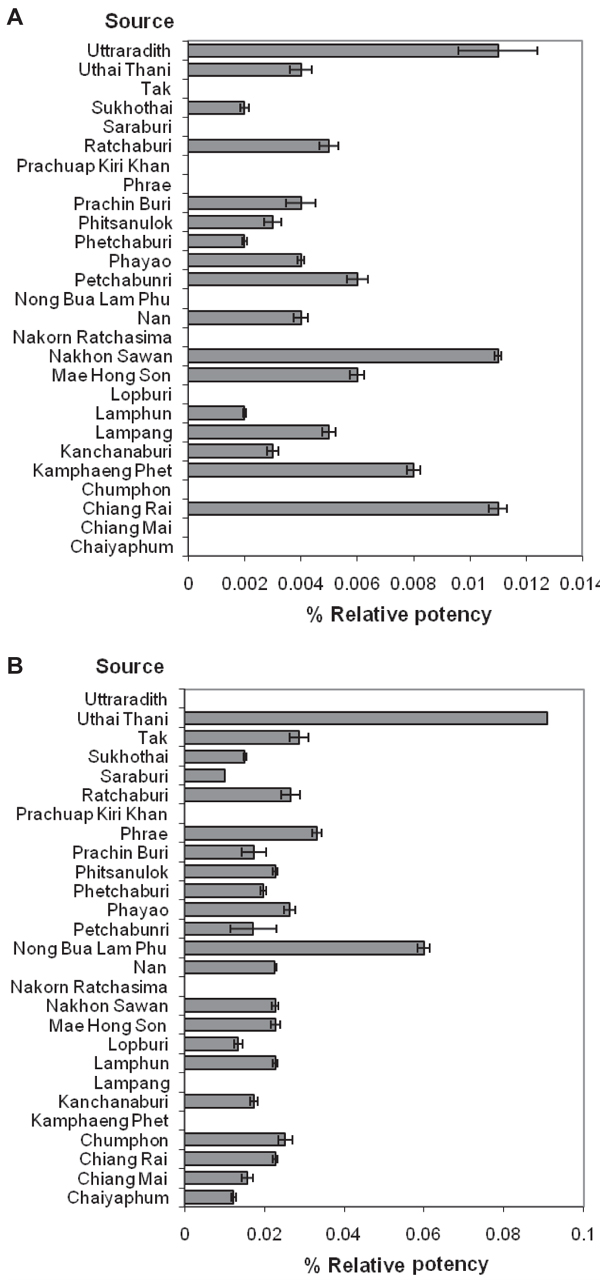
The relative potency values of P. mirifica extracts in YES-hERα + hTIF2 are shown in Figure 2A. Considering the relative potency value of the plant population, the estrogenic activity of the top five plant extracts was 0.006-0.011% of the activity of 17β-estradiol. Three plant samples had no estrogenic activity.
Estrogenic activity of P. mirifica extract in YES-hERβ + hSRC1
The estrogenic activity presented as the relative potency value of P. mirifica extracts in YES-hERβ + hSRC1 is shown in Figure 2B. The estrogenic activity of the top five plant extracts was 0.027-0.091% of the activity of 17β-estradiol. Five plant samples had no estrogenic activity.
Comparison of the estrogenic activity of P. mirifica extract in YES systems
In order to compare the estrogenic activity of P. mirifica extracts between YES-hERα + hTIF2 and YES-hERβ + hSRC1, the relative potency values presented in Figure 2A and Figure 2B were compared. The maximum relative potency value of P. mirifica in YES-hERβ + hSRC1 was 8.3-fold the relative potency of YES-hERα + hTIF2.
Antiestrogenic activity of P. mirifica extracts
The plant extracts with no expression of estrogenic activity in YES-hERα + hTIF2 and YES-hERβ + hSRC1 (Nakorn Ratchasima, Prachuap Kiri Khan), in YES-hERα + hTIF2 (Saraburi) and in YES-hERβ + hSRC1 (Uttraradith), at the concentration of 1000 µg/mL, were examined by incubating them with yeast cells in the presence of 1 µM 17β-estradiol. The antiestrogenic effects of P. mirifica plant extracts are illustrated in Figure 3. Some plant extracts could significantly decreased 17β-estradiol-dependent β-galactosidase activity in both YES systems compared to the cells incubated with 17β-estradiol alone (P < 0.05). The plant extract from Nakorn Ratchasima and Uttraradith had the highest antiestrogenic activity in YES-hERα + hTIF2 and YES-hERβ + hSRC1, respectively. Furthermore, the antiestrogenic effect of the plant extracts tested in YES-hERβ/hSRC1 was 2-fold higher than that of YES-hERα + hTIF2 (P < 0.05). The result implies that the P. mirifica plant extract had antiestrogenic activity in YES systems and was more potent in YES-hERβ + hSRC1 than in YES-hERα + hTIF2.
Estrogenic activity of S9-treated P. mirifica extract
P. mirifica extracts that exhibited no estrogenic activity by the YES assay were activated with the S9 fraction and the reaction mixtures were then assayed on each YES system. The estrogenic activities of P. mirifica extracts from Nakorn Ratchasima, Prachuap Kiri Khan and Saraburi treated with the S9 fraction in YES-hERα + hTIF2 (Figure 4A) and YES-hERβ + hSRC1 (Figure 4B) are shown. The plant extracts from the first 3 sources had a significant estrogenic activity in YES-hERα + hTIF2 but not in YES-hERβ + hSRC1 after treatment with the active S9 fraction compared with the heat-inactivated sample (P < 0.05). The S9-treated plant extract from Uttraradith did not show any estrogenic activity.
Correlation analysis
Analysis of the correlation between percent relative estrogenic potency analyzed by YES with ERα or ERβ and the 5 major isoflavonoids within the plant tubers including puerarin, daidzin, genistin, daidzein, and genistein analyzed by HPLC of individual plant samples (15) showed no correlation.
Estrogenic activity of 17β-estradiol evaluated by YES-hERα + hTIF2 and YES-hERβ + hSRC1. Each value is the mean ± SEM of three independent experiments.
Percent relative potency of Pueraria mirifica plant extract collected from 27 provinces in Thailand determined by the YES-hERα + hTIF2 (A) and YES-hERβ + hSRC1 (B) systems. Each value is the mean ± SEM of three independent experiments.
Antiestrogenic activity of Pueraria mirifica plant extracts determined by YES systems. NR = Nakorn Ratchasima; PK = Prachuap Kiri Khan; S = Saraburi; U = Uttraradith; E2 = 17β-estradiol. Each column is the mean ± SEM of three independent experiments. *P < 0.05 significantly different from 17β-estradiol (Student t-test).
Estrogenic activity of Pueraria mirifica after treatment with the rat liver S9 fraction determined by the YES-hERα + hTIF2 (A) and YES-hERβ + hSRC1 (B) systems. NR = Nakorn Ratchasima; PK = Prachuap Kiri Khan; S = Saraburi; U = Uttraradith; S9-inactive = heat-inactivated S9 fraction (negative control); S9-active = active S9 fraction. Each value is the mean ± SEM of three independent experiments. A final concentration of S9-treated P. mirifica plant extract at 1000 µg/mL was tested in YES system. *P < 0.05 significantly different from S9-inactive treatment (Student t-test).
Discussion
In this study, the estrogenic activity of P. mirifica tuberous extracts was determined by a YES two-hybrid system harboring coactivators to establish similarity to the human estrogen gene regulation system. Based on the relative potency of the plant extracts to induce receptor-dependent transcription, P. mirifica extracts were found to be more potent in YES-hERβ + hSRC1 than in YES-hERα + hTIF2. The advantage of the YES two-hybrid assay is a clear-cut estrogenic response initiated by ERα or Erβ, which is not possible to detect in the animal test that comprises both ERα and ERβ in some organs. Thus, YES is a useful assay to demonstrate differences of phytoestrogens with respect to ERβ over ERα.
The estrogenic activity of P. mirifica extracts evaluated by YES systems obviously varied. However, the YES assay revealed a much lower estrogenic activity of the plant extracts compared to 17β-estradiol than observed in the vaginal cornification assay (6-8). There was a difference between the two assays, i.e., there was no metabolic activation in the ordinary YES assay while metabolic activation was initiated by both intestinal microbes (29,30) and liver enzymes (31-33). Note that the results of metabolic activation of the plant samples with the S9 fraction were not in the same direction as the test without activation in which the plant extracts exhibited stronger estrogenic activity in YES-hERα + hTIF2 (Figure 4A) than in YES-hERβ + hSRC1 (Figure 2B). This demonstrates that phytoestrogens in the native form bind more strongly to ERβ than to ERα but the metabolized form may bind more strongly to ERα than to ERβ. The result was in the same direction as tested in MCF-7 cells (33).
The present data show that the P. mirifica extracts also had antiestrogenic effects at the highest dose tested of 1000 μg/mL in both YES systems. This revealed that plant samples with no estrogenic activity in the YES assay may show antiestrogenic activity in the presence of estrogen. A similar result was obtained in the previous evaluation of the estrogenic/antiestrogenic activity of a plant sample collected from Chiang Mai province when the plant extract was incubated with 17β-estradiol in a test with MCF-7 cells. The P. mirifica EtOH extract showed a significant antagonistic effect on 17β-estradiol at medium (100 µg/mL) and high (1000 µg/mL) doses (7). These two results demonstrate that antiestrogenic activity may be one of the key biological activities of P. mirifica extracts and might be related to its potential role in the prevention of breast cancer in rats (21). Previous studies have shown that isoflavones and medicinal plants have an estrogenic action and antiestrogenic activity in the in vitro tests (34).
Even though the YES assay is rapid and can be used as a quantitative assay, it may not exhibit the high level of estrogenic activity observed with the vaginal cornification and uterotropic assays. Note that the vaginal cornification assay of the same plant samples elicited a delayed response which, however, was comparable to the response to 17β-estradiol. Furthermore, it may not be possible to apply the results of the tests of the same plant materials to animal and human consumption mainly due to the lack of metabolic activation.
The lack of correlation between the 5 major isoflavonoid contents of the plants and their estrogenic activity evaluated by the YES assay revealed that the major plant constituents that exhibited significant binding with ERα or ERβ may not be these isoflavonoids but other chemical such as miroestrol, which had been shown to have strong binding to ERα in MCF-7 cells (35).
We demonstrated that YES systems can provide useful information in the screening of potential phytoestrogen-rich plant material, especially P. mirifica, for further development of phytoestrogen-rich products.
References
1. Katzenellenbogen BS, Ferguson ER, Lan NC. Fundamental differences in the action of estrogens and antiestrogens on the uterus: comparison between compounds with similar duration of action. Endocrinology 1977; 100: 1252-1259.
2. Folman Y, Pope GS. The interaction in the immature mouse of potent oestrogens with coumestrol, genistein and other utero-vaginotrophic compounds of low potency. J Endocrinol 1966; 34: 215-225.
3. Wang C, Kurzer MS. Phytoestrogen concentration determines effects on DNA synthesis in human breast cancer cells. Nutr Cancer 1997; 28: 236-247.
4. Collins BM, McLachlan JA, Arnold SF. The estrogenic and antiestrogenic activities of phytochemicals with the human estrogen receptor expressed in yeast. Steroids 1997; 62: 365-372.
5. Benson GK, Cowie AT, Hosking ZD. Mammogenic activity of miroestrol. J Endocrinol 1961; 21: 401-409.
6. Malaivijitnond S, Chansri K, Kijkuokul P, Urasopon N, Cherdshewasart W. Using vaginal cytology to assess the estrogenic activity of phytoestrogen-rich herb. J Ethnopharmacol 2006; 107: 354-360.
7. Cherdshewasart W, Kitsamai Y, Malaivijitnond S. Evaluation of the estrogenic activity of the wild Pueraria mirifica by vaginal cornification assay. J Reprod Dev 2007; 53: 385-393.
8. Cherdshewasart W, Sriwatcharakul S, Malaivijitnond S. Variance of estrogenic activity of the phytoestrogen-rich plant. Maturitas 2008; 61: 350-357.
9. Cherdshewasart W, Cheewasopit W, Picha P. The differential anti-proliferation effect of white (Pueraria mirifica), red (Butea superba), and black (Mucuna collettii) Kwao Krua plants on the growth of MCF-7 cells. J Ethnopharmacol 2004; 93: 255-260.
10. Cherdshewasart W, Traisup V, Picha P. Determination of the estrogenic activity of wild phytoestrogen-rich Pueraria mirifica by MCF-7 proliferation assay. J Reprod Dev 2008; 54: 63-67.
11. Harmsen S, Meijerman I, Beijnen JH, Schellens JH. The role of nuclear receptors in pharmacokinetic drug-drug interactions in oncology. Cancer Treat Rev 2007; 33: 369-380.
12. Bitter GA. Regulation of human estrogen receptor alpha-mediated gene transactivation in Saccharomyces cerevisiae by human coactivator and corepressor proteins. J Steroid Biochem Mol Biol 2007; 103: 189-195.
13. Cain JC. Miroestrol: an oestrogen from the plant Pueraria mirifica. Nature 1960; 188: 774-777.
14. Chansakaow S, Ishikawa T, Seki H, Sekine Y, Okada M, Chaichantipyuth C. Identification of deoxymiroestrol as the actual rejuvenating principle of "Kwao Keur", Pueraria mirifica. The known miroestrol may be an artifact. J Nat Prod 2000; 63: 173-175.
15. Cherdshewasart W, Subtang S, Dahlan W. Major isoflavonoid contents of the phytoestrogen rich-herb Pueraria mirifica in comparison with Pueraria lobata. J Pharm Biomed Anal 2007; 43: 428-434.
16. Cherdshewasart W, Sriwatcharakul S. Major isoflavonoid contents of the 1-year-cultivated phytoestrogen-rich herb, Pueraria mirifica. Biosci Biotechnol Biochem 2007; 71: 2527-2533.
17. Manonai J, Chittacharoen A, Udomsubpayakul U, Theppisai H, Theppisai U. Effects and safety of Pueraria mirifica on lipid profiles and biochemical markers of bone turnover rates in healthy postmenopausal women. Menopause 2008; 15: 530-535.
18. Manonai J, Chittacharoen A, Theppisai U, Theppisai H. Effect of Pueraria mirifica on vaginal health. Menopause 2007; 14: 919-924.
19. Urasopon N, Hamada Y, Asaoka K, Cherdshewasart W, Malaivijitnond S. Pueraria mirifica, a phytoestrogen-rich herb, prevents bone loss in orchidectomized rats. Maturitas 2007; 56: 322-331.
20. Urasopon N, Hamada Y, Cherdshewasart W, Malaivijitnond S. Preventive effects of Pueraria mirifica on bone loss in ovariectomized rats. Maturitas 2008; 59: 137-148.
21. Cherdshewasart W, Panriansaen R, Picha P. Pretreatment with phytoestrogen-rich plant decreases breast tumor incidence and exhibits lower profile of mammary ERα and ERβ. Maturitas 2007; 58: 174-181.
22. Trisomboon H, Malaivijitnond S, Cherdshewasart W, Watanabe G, Taya K. Effect of Pueraria mirifica on the sexual skin coloration of aged menopausal cynomolgus monkeys. J Reprod Dev 2006; 52: 537-542.
23. Jaroenporn S, Malaivijitnond S, Wattanasirmkit K, Watanabe G, Taya K, Cherdshewasart W. Assessment of fertility and reproductive toxicity in adult female mice after long-term exposure to Pueraria mirifica herb. J Reprod Dev 2007; 53: 995-1005.
24. Nishikawa J, Saito K, Goto J, Dakeyama F, Matsuo M, Nishihara T. New screening methods for chemicals with hormonal activities using interaction of nuclear hormone receptor with coactivator. Toxicol Appl Pharmacol 1999; 154: 76-83.
25. Morani A, Warner M, Gustafsson JA. Biological functions and clinical implications of oestrogen receptors alfa and beta in epithelial tissues. J Intern Med 2008; 264: 128-142.
26. Lee HS, Miyauchi K, Nagata Y, Fukuda R, Sasagawa S, Endoh H, et al. Employment of the human estrogen receptor beta ligand-binding domain and co-activator SRC1 nuclear receptor-binding domain for the construction of a yeast two-hybrid detection system for endocrine disrupters. J Biochem 2002; 131: 399-405.
27. Kashemsanta MLC, Suvatabhandu K, Airy SHK. A new species of Pueraria (Leguminosae) from Thailand, yielding an oestrogenic principle. Kew Bull 1952; 7: 263-266.
28. Morito K, Hirose T, Kinjo J, Hirakawa T, Okawa M, Nohara T, et al. Interaction of phytoestrogens with estrogen receptors alpha and beta. Biol Pharm Bull 2001; 24: 351-356.
29. Takatori S, Kitagawa Y, Oda H, Miwa G, Nishikawa J-I, Nishihara T, et al. Estrogenicity of metabolites of benzophenone derivatives examined by a yeast two-hybrid assay. J Health Sci 2003; 49: 91-98.
30. Hur HG, Lay JO Jr, Beger RD, Freeman JP, Rafii F. Isolation of human intestinal bacteria metabolizing the natural isoflavone glycosides daidzin and genistin. Arch Microbiol 2000; 174: 422-428.
31. Bowey E, Adlercreutz H, Rowland I. Metabolism of isoflavones and lignans by the gut microflora: a study in germ-free and human flora associated rats. Food Chem Toxicol 2003; 41: 631-636.
32. Lee YS, Park JS, Cho SD, Son JK, Cherdshewasart W, Kang KS. Requirement of metabolic activation for estrogenic activity of Pueraria mirifica. J Vet Sci 2002; 3: 273-277.
33. Cherdshewasart W, Sriwatcharakul S. Metabolic activation promotes estrogenic activity of the phytoestrogen-rich plant. Maturitas 2008; 59: 128-136.
34. Kim G, Kang SC, Kim KC, Choung ES, Zee OP. Screening of estrogenic and antiestrogenic activities from medicinal plants. Environ Toxicol Pharm 2008; 25: 75-82.
35. Matsumura A, Ghosh A, Pope GS, Darbre PD. Comparative study of oestrogenic properties of eight phytoestrogens in MCF7 human breast cancer cells. J Steroid Biochem Mol Biol 2005; 94: 431-443.
Acknowledgments
This research was partially supported by the Center of Excellence on Agricultural Biotechnology, Postgraduate Education and Research Development Office, Commission on Higher Education, the Ministry of Education. We would like to thank Prof. Dr. Tsutomo Nishihara and Prof. Dr. Akinori Ohta for their generosity providing yeast strains and plasmids, respectively.
 Address for correspondence: W. Cherdshewasart, Department of Biology, Faculty of Science, Chulalongkorn University, Phyathai Road, Bangkok 10330, Thailand. Fax: +66-0-2218-5386. E-mail: cwichai@sc.chula.ac.th
Address for correspondence: W. Cherdshewasart, Department of Biology, Faculty of Science, Chulalongkorn University, Phyathai Road, Bangkok 10330, Thailand. Fax: +66-0-2218-5386. E-mail: cwichai@sc.chula.ac.th
Received April 19, 2009. Accepted November 27, 2009. Available online December 18, 2009. Published February 1, 2010.
The Brazilian Journal of Medical and Biological Research is partially financed by
- 1. Katzenellenbogen BS, Ferguson ER, Lan NC. Fundamental differences in the action of estrogens and antiestrogens on the uterus: comparison between compounds with similar duration of action. Endocrinology 1977; 100: 1252-1259.
- 2. Folman Y, Pope GS. The interaction in the immature mouse of potent oestrogens with coumestrol, genistein and other utero-vaginotrophic compounds of low potency. J Endocrinol 1966; 34: 215-225.
- 3. Wang C, Kurzer MS. Phytoestrogen concentration determines effects on DNA synthesis in human breast cancer cells. Nutr Cancer 1997; 28: 236-247.
- 4. Collins BM, McLachlan JA, Arnold SF. The estrogenic and antiestrogenic activities of phytochemicals with the human estrogen receptor expressed in yeast. Steroids 1997; 62: 365-372.
- 5. Benson GK, Cowie AT, Hosking ZD. Mammogenic activity of miroestrol. J Endocrinol 1961; 21: 401-409.
- 6. Malaivijitnond S, Chansri K, Kijkuokul P, Urasopon N, Cherdshewasart W. Using vaginal cytology to assess the estrogenic activity of phytoestrogen-rich herb. J Ethnopharmacol 2006; 107: 354-360.
- 7. Cherdshewasart W, Kitsamai Y, Malaivijitnond S. Evaluation of the estrogenic activity of the wild Pueraria mirifica by vaginal cornification assay. J Reprod Dev 2007; 53: 385-393.
- 8. Cherdshewasart W, Sriwatcharakul S, Malaivijitnond S. Variance of estrogenic activity of the phytoestrogen-rich plant. Maturitas 2008; 61: 350-357.
- 9. Cherdshewasart W, Cheewasopit W, Picha P. The differential anti-proliferation effect of white (Pueraria mirifica), red (Butea superba), and black (Mucuna collettii) Kwao Krua plants on the growth of MCF-7 cells. J Ethnopharmacol 2004; 93: 255-260.
- 10. Cherdshewasart W, Traisup V, Picha P. Determination of the estrogenic activity of wild phytoestrogen-rich Pueraria mirifica by MCF-7 proliferation assay. J Reprod Dev 2008; 54: 63-67.
- 11. Harmsen S, Meijerman I, Beijnen JH, Schellens JH. The role of nuclear receptors in pharmacokinetic drug-drug interactions in oncology. Cancer Treat Rev 2007; 33: 369-380.
- 12. Bitter GA. Regulation of human estrogen receptor alpha-mediated gene transactivation in Saccharomyces cerevisiae by human coactivator and corepressor proteins. J Steroid Biochem Mol Biol 2007; 103: 189-195.
- 13. Cain JC. Miroestrol: an oestrogen from the plant Pueraria mirifica Nature 1960; 188: 774-777.
- 14. Chansakaow S, Ishikawa T, Seki H, Sekine Y, Okada M, Chaichantipyuth C. Identification of deoxymiroestrol as the actual rejuvenating principle of "Kwao Keur", Pueraria mirifica The known miroestrol may be an artifact. J Nat Prod 2000; 63: 173-175.
- 15. Cherdshewasart W, Subtang S, Dahlan W. Major isoflavonoid contents of the phytoestrogen rich-herb Pueraria mirifica in comparison with Pueraria lobata J Pharm Biomed Anal 2007; 43: 428-434.
- 16. Cherdshewasart W, Sriwatcharakul S. Major isoflavonoid contents of the 1-year-cultivated phytoestrogen-rich herb, Pueraria mirifica Biosci Biotechnol Biochem 2007; 71: 2527-2533.
- 17. Manonai J, Chittacharoen A, Udomsubpayakul U, Theppisai H, Theppisai U. Effects and safety of Pueraria mirifica on lipid profiles and biochemical markers of bone turnover rates in healthy postmenopausal women. Menopause 2008; 15: 530-535.
- 18. Manonai J, Chittacharoen A, Theppisai U, Theppisai H. Effect of Pueraria mirifica on vaginal health. Menopause 2007; 14: 919-924.
- 19. Urasopon N, Hamada Y, Asaoka K, Cherdshewasart W, Malaivijitnond S. Pueraria mirifica, a phytoestrogen-rich herb, prevents bone loss in orchidectomized rats. Maturitas 2007; 56: 322-331.
- 20. Urasopon N, Hamada Y, Cherdshewasart W, Malaivijitnond S. Preventive effects of Pueraria mirifica on bone loss in ovariectomized rats. Maturitas 2008; 59: 137-148.
- 21. Cherdshewasart W, Panriansaen R, Picha P. Pretreatment with phytoestrogen-rich plant decreases breast tumor incidence and exhibits lower profile of mammary ERα and ERβ. Maturitas 2007; 58: 174-181.
- 22. Trisomboon H, Malaivijitnond S, Cherdshewasart W, Watanabe G, Taya K. Effect of Pueraria mirifica on the sexual skin coloration of aged menopausal cynomolgus monkeys. J Reprod Dev 2006; 52: 537-542.
- 23. Jaroenporn S, Malaivijitnond S, Wattanasirmkit K, Watanabe G, Taya K, Cherdshewasart W. Assessment of fertility and reproductive toxicity in adult female mice after long-term exposure to Pueraria mirifica herb. J Reprod Dev 2007; 53: 995-1005.
- 24. Nishikawa J, Saito K, Goto J, Dakeyama F, Matsuo M, Nishihara T. New screening methods for chemicals with hormonal activities using interaction of nuclear hormone receptor with coactivator. Toxicol Appl Pharmacol 1999; 154: 76-83.
- 25. Morani A, Warner M, Gustafsson JA. Biological functions and clinical implications of oestrogen receptors alfa and beta in epithelial tissues. J Intern Med 2008; 264: 128-142.
- 26. Lee HS, Miyauchi K, Nagata Y, Fukuda R, Sasagawa S, Endoh H, et al. Employment of the human estrogen receptor beta ligand-binding domain and co-activator SRC1 nuclear receptor-binding domain for the construction of a yeast two-hybrid detection system for endocrine disrupters. J Biochem 2002; 131: 399-405.
- 27. Kashemsanta MLC, Suvatabhandu K, Airy SHK. A new species of Pueraria (Leguminosae) from Thailand, yielding an oestrogenic principle. Kew Bull 1952; 7: 263-266.
- 28. Morito K, Hirose T, Kinjo J, Hirakawa T, Okawa M, Nohara T, et al. Interaction of phytoestrogens with estrogen receptors alpha and beta. Biol Pharm Bull 2001; 24: 351-356.
- 29. Takatori S, Kitagawa Y, Oda H, Miwa G, Nishikawa J-I, Nishihara T, et al. Estrogenicity of metabolites of benzophenone derivatives examined by a yeast two-hybrid assay. J Health Sci 2003; 49: 91-98.
- 30. Hur HG, Lay JO Jr, Beger RD, Freeman JP, Rafii F. Isolation of human intestinal bacteria metabolizing the natural isoflavone glycosides daidzin and genistin. Arch Microbiol 2000; 174: 422-428.
- 31. Bowey E, Adlercreutz H, Rowland I. Metabolism of isoflavones and lignans by the gut microflora: a study in germ-free and human flora associated rats. Food Chem Toxicol 2003; 41: 631-636.
- 32. Lee YS, Park JS, Cho SD, Son JK, Cherdshewasart W, Kang KS. Requirement of metabolic activation for estrogenic activity of Pueraria mirifica J Vet Sci 2002; 3: 273-277.
- 33. Cherdshewasart W, Sriwatcharakul S. Metabolic activation promotes estrogenic activity of the phytoestrogen-rich plant. Maturitas 2008; 59: 128-136.
- 34. Kim G, Kang SC, Kim KC, Choung ES, Zee OP. Screening of estrogenic and antiestrogenic activities from medicinal plants. Environ Toxicol Pharm 2008; 25: 75-82.
- 35. Matsumura A, Ghosh A, Pope GS, Darbre PD. Comparative study of oestrogenic properties of eight phytoestrogens in MCF7 human breast cancer cells. J Steroid Biochem Mol Biol 2005; 94: 431-443.
Correspondence and Footnotes
Publication Dates
-
Publication in this collection
22 Feb 2010 -
Date of issue
Feb 2010
History
-
Accepted
27 Nov 2009 -
Received
19 Apr 2009