Abstract
Malignant hyperthermia (MH) is a pharmacogenetic disease triggered by volatile anesthetics and succinylcholine. Deaths due to MH have been reported in Brazil. The first Malignant Hyperthermia Diagnostic and Research Center in Latin America was inaugurated in 1993 at the Federal University of Rio de Janeiro, Brazil. The center followed the diagnostic protocols of the North America MH Group, in which the contractures of biopsies from the vastus lateralis muscle are analyzed after exposure to caffeine and halothane (CHCT). CHCT was performed in individuals who survived, their relatives and those with signs/symptoms somewhat related to MH susceptibility (MHS). Here, we report data from 194 patients collected over 16 years. The Southeast (N = 110) and South (N = 71) represented the majority of patients. Median age was 25 (4-70) years, with similar numbers of males (104) and females (90). MHS was found in 90 patients and 104 patients were normal. Abnormal responses to both caffeine and halothane were observed in 59 patients and to caffeine or halothane in 20 and 11 patients, respectively. The contracture of biopsies from MHS exposed to caffeine and halothane was 1.027 ± 0.075 g (N = 285) and 4.021 ± 0.255 g (N = 226), respectively. MHS was found in patients with either low or high blood creatine kinase and also, with a low score on the clinical grading scale. Thus, these parameters cannot be used with certainty to predict MHS. We conclude that the CHCT protocol described by the North America MH Group contributed to identification of MHS in suspected individuals at an MH center in Brazil with 100% sensitivity and 65.7% specificity.
Malignant hyperthermia; Diagnosis; Skeletal muscle; Anesthesia
Braz J Med Biol Res, June 2010, Volume 43(6) 549-556
Use of the caffeine-halothane contracture test for the diagnosis of malignant hyperthermia in Brazil
 Correspondence and Footnotes
Correspondence and Footnotes
 R.T. Sudo1, L.B.P. Cunha2, P.L. Carmo1, A.R. Matos1, M.M. Trachez4, L.A.M. Cardoso3, M.I.S. Aguiar1, A.V. Abreu3 and G. Zapata-Sudo1
R.T. Sudo1, L.B.P. Cunha2, P.L. Carmo1, A.R. Matos1, M.M. Trachez4, L.A.M. Cardoso3, M.I.S. Aguiar1, A.V. Abreu3 and G. Zapata-Sudo1
1Programa de Desenvolvimento de Fármacos, Instituto de Ciências Biomédicas, 2Serviço de Anestesiologia, 3Departamento de Ortopedia, Universidade Federal do Rio de Janeiro, Rio de Janeiro, RJ, Brasil
4Disciplina de Anestesiologia, Universidade Federal Fluminense, Rio de Janeiro, RJ, Brasil
 Correspondence and Footnotes
Correspondence and Footnotes
Correspondence and Footnotes
Correspondence and Footnotes
Correspondence and Footnotes
Correspondence and Footnotes
Abstract
Malignant hyperthermia (MH) is a pharmacogenetic disease triggered by volatile anesthetics and succinylcholine. Deaths due to MH have been reported in Brazil. The first Malignant Hyperthermia Diagnostic and Research Center in Latin America was inaugurated in 1993 at the Federal University of Rio de Janeiro, Brazil. The center followed the diagnostic protocols of the North American MH Group, in which the contractures of biopsies from the vastus lateralis muscle are analyzed after exposure to caffeine and halothane (CHCT). CHCT was performed in individuals who survived, their relatives and those with signs/symptoms somewhat related to MH susceptibility (MHS). Here, we report data from 194 patients collected over 16 years. The Southeast (N = 110) and South (N = 71) represented the majority of patients. Median age was 25 (4-70) years, with similar numbers of males (104) and females (90). MHS was found in 90 patients and 104 patients were normal. Abnormal responses to both caffeine and halothane were observed in 59 patients and to caffeine or halothane in 20 and 11 patients, respectively. The contracture of biopsies from MHS exposed to caffeine and halothane was 1.027 ± 0.075 g (N = 285) and 4.021 ± 0.255 g (N = 226), respectively. MHS was found in patients with either low or high blood creatine kinase and also with a low score on the clinical grading scale. Thus, these parameters cannot be used with certainty to predict MHS. We conclude that the CHCT protocol described by the North American MH Group contributed to identification of MHS in suspected individuals at an MH center in Brazil with 100% sensitivity and 65.7% specificity.
Key words: Malignant hyperthermia; Diagnosis; Skeletal muscle; Anesthesia
Introduction
Malignant hyperthermia (MH) is an autosomal dominant pharmacogenetic disease, with crises triggered in susceptible individuals (MHS) by exposure to halogenated general anesthetics and/or succinylcholine, a depolarizing muscle relaxant (1). Hypermetabolic signs such as increased oxygen consumption and carbon dioxide production and tachycardia may be observed a few minutes after contact of MHS patient with these compounds, followed by severe muscle rigidity and rapid body temperature elevation, which can reach more than 44°C, rhabdomyolysis, bleeding, acute renal failure, and cardiac arrhythmias (1). Death occurs in approximately 75% of untreated patients (2).
The MH crisis enhances Ca2+ release from the sarcoplasmic reticulum (3) through the type 1 ryanodine receptor (RyR1). Mutations in the RyR1 gene are associated with 50-70% of the families affected by MH (4). RyR1 is one of the largest proteins in the body, is coded by a complex gene of 158 kb and 106 exons (5) and more than 100 different mutations associated with MH have been described (6). Mutations in sodium channels (7) and the alpha subunit of the dihydropyridine receptor (8,9) are associated with MH, but are very rare. Different mutations can produce different MH phenotypes. Recently, new mutations have been suggested to be associated with MH such as carnitine palmitoyltransferase II (CPT II) deficiency with heterozygous R506C mutation (10) and mutation in the calsequestrin 1 (CASQ-1) gene (11).
MH has a low incidence but a high mortality rate. The incidence of MH is 1:15,000 and 1:50,000-100,000 in children and adults receiving anesthetics, respectively (12). After the clinical introduction of dantrolene sodium to treat MH, mortality decreased from 75% to less than 5% (12). However, the mortality of MH is increasing in the United States (11.7%) (13), Japan (15%) (14) and Taiwan (28.7%) (15). No data regarding MH incidence or death have been reported in Brazil.
Early detection of susceptibility is valuable in preventing MH mortality. The gold standard method to diagnose MH susceptibility is based on measuring the intensity of muscle biopsy contracture in response to caffeine and halothane (caffeine and halothane contracture test, CHCT), compounds that activate Ca2+ release from the sarcoplasmic reticulum through RyR1 (16,17). There are two diagnostic protocols for MH from the North American MH Group (17) and the European MH Group (18). The North American protocol consists of exposing the muscle biopsy to incremental concentrations of caffeine (0.5-32 mM) and a single concentration of halothane (3%). The European protocol uses the same dose-response curve for caffeine, but increases halothane concentrations (0.5-3%). The North American protocol of CHCT has 97% sensitivity and 78% specificity (19).
Global MH centers can use genetic diagnostic methods for MH, which are less invasive for the patient than the CHCT. Once a causative mutation has been detected in the proband or index patient, it can be used to test relatives who have not yet been tested by CHCT. Mutation carriers should consequently be regarded as susceptible to MH. However, only 30 mutations have been shown to cause MH directly, with an increase of Ca2+ efflux trough RyR1 observed in in vitro studies. Family members who do not carry the mutation observed in the pedigree should still undergo CHCT investigation (20).
MH can also be predicted after the development of a hypermetabolic crisis during general anesthesia. Larach et al. (21) described a clinical grading scale based on clinical signs and symptoms associated with MH. This scale ranks the qualitative likelihood that an adverse anesthetic event indicates MH. Factors like MH family history and signs of MH crisis during anesthesia such as muscular rigidity, muscle breakdown, respiratory acidosis, increased temperature, and cardiac arrhythmias are used as indicators to diagnose MH. However, currently this scale cannot be used to determine the diagnosis or treatment during an MH-like episode because clinical symptoms of an MH crisis are nonspecific, and the diagnosis can be difficult to establish only with clinical signs (20). The final diagnosis is only confirmed by the CHCT.
Many case reports at the Brazilian National Society of Anesthesiologists meetings have reported lethal MH episodes. In 1993, the first Malignant Hyperthermia Diagnostic and Research Center in Latin America was created in Rio de Janeiro, Brazil. This MH Center has brought significant benefit to the families and anesthesiologists involved with patients who present unexplainable complications during general anesthesia. The purpose of this article is to present data from the Center for the past 16 years and compare them to international data.
Subjects and Methods
Subjects
The Institutional Ethics Committee of the Federal University of Rio de Janeiro, Brazil, approved the study.
Control CHCT was performed in 32 volunteers, and Drs. Thomas Nelson and Henry Rosenberg, members of the North American MH Group, evaluated and approved the viability of the Center in 1992. Since then, the Brazilian MH Center in Rio de Janeiro has performed 194 biopsies with an average of 11.6 biopsies per year.
The patient selection protocol for CHCT was based on that described by the Malignant Hyperthermia Association of the United States (MHAUS): people who survived an MH crisis or presented an incomplete MH episode during general anesthesia (i.e., muscular rigidity without hyperthermia), relatives of MHS individuals, including relatives who suffered unexplainable death during general anesthesia, and patients who had never undergone general anesthesia, but had signs/symptoms or laboratory exams indicative of MH (i.e., frequent cramps, congenital anomalies, or increased creatine kinase (CK) levels). Most of the patients were referred to the MH Brazilian Center by their doctors due to a suspected clinical episode of MH. Other patients were recommended by relatives when MH susceptibility was suspected due to family deaths, survival or susceptibility confirmed by the North American protocol of CHCT. Patients with muscle atrophy, weighing less than 20 kg, or less than 4 years old were excluded from the exam.
A written informed consent form to participate in the study was signed by all subjects in accordance with the North American protocol of CHCT. Patients submitted to the CHCT were admitted to the hospital one day before the biopsy was taken and their general health was evaluated during the pre-anesthesia visit. A blood sample for CK measurement was collected in the operating room before performing the biopsy.
Caffeine-halothane contracture test
A biopsy of the vastus lateralis muscle approximately 3.5 cm long, 2.0 cm wide and 1.5 cm deep was performed under local anesthesia of the femoral and femoral-cutaneous nerves with a mixture of lidocaine (1.5%) and epinephrine (1:200,000) in adults, and under general anesthesia with a combination of propofol (1.5 mg·kg-1·min-1iv bolus followed by infusion of 100 µg·kg-1·min-1) and remifentanyl (2 µg/kg iv bolus) in children less than 14 years old. The muscle was immediately immersed in a temperature-controlled flask filled with Ringer-Krebs solution (118.1 mM NaCl, 3.4 mM KCl, 0.8 mM MgSO4.7H2O, 1.2 mM KH2PO4, 25 mM NaHCO3, 2.5 mM CaCl2.2H2O, 11.1 mM glucose), pH 7.4, oxygenated with a carbogen mixture (95% O2 plus 5% CO2). In the laboratory, 3-4 smaller fragments 1.5-2.0 cm long and 2.5-3.0 mm wide were dissected and positioned in vertical chambers (50 mL) in which one end of each muscle was attached to a fixed clamp and the other to an FT-03 force transducer (AstroMed Grass Instruments, USA) for isometric tension recording. The chambers were filled with oxygenated Ringer-Krebs solution maintained at 37 ± 0.1°C, pH 7.4 ± 0.02. Muscle twitches were elicited by electric field stimulation (50 V, 2-ms duration, 0.2 Hz). A Cyberamp (Axon Instruments, Inc., USA) conditioned the signals generated by the force transducer. These signals were digitalized (Digidata 1322, Axon Instruments, Inc., USA), displayed, and stored on a computer for further analysis using the Axoscope software (Axon Instruments, Inc., USA). The length-tension curve was constructed for each muscle fragment to obtain the maximal twitch response. After 30 min of adaptation, caffeine (Sigma-Aldrich, USA) was added to the solution in incremental concentrations (0.5, 1, 2, 4, 8, and 32 mM) at intervals of 4 min or after plateau contractions were reached. The stock solution of caffeine was freshly prepared in Ringer-Krebs solution. After the end of the caffeine contracture test, the chambers were carefully washed to eliminate contamination with caffeine and, using the same procedure, 3-4 additional muscle fragments were prepared for isometric tension recording. After a period of adaptation, a gas mixture containing 3% halothane (Cristália, Brazil) and carbogen was bubbled into the Ringer-Krebs solution for 10 min. A calibrated vaporizer (Fluovapor 1220, K. Takaoka, Brazil) was used to measure halothane concentration.
Muscle twitches (Pt; mean ± SEM = 2.928 ± 0.072 g, N = 1428) were used as a parameter of muscle viability but not for diagnostic purposes. Fragments with no twitches were discarded. The baseline tension for a diagnosis of MH was 0.2 and 0.7 g in response to caffeine (2 mM) and halothane (3%), respectively, measured in any muscle fragment independently of the type of protocol.
Clinical grading of patients
Patient information collected before biopsy included muscle rigidity, abnormal increase of CK, rhabdomyolysis-like signs, increased oxygen consumption and production of carbon dioxide by muscle metabolism, high temperature, tachycardia, arrhythmias, acidosis, response to dantrolene, and family history and was used to rank patients on a clinical scale to predict MH (21): 1 (almost never), 2 (unlikely), 3 (somewhat unlikely), 4 (somewhat likely), 5 (very likely), and 6 (almost certain). The MH susceptibility obtained from the North American protocol of CHCT was compared to that predicted by clinical grading.
Statistical analysis
Data are reported as means ± SEM. The average between two groups was compared by the Student t-test. Statistical significance was defined as P < 0.05. The GraphPad Prism version 4 software was used for statistical analysis.
Results
Demographic data
From February 1993 to October 2009, 194 patients were eligible for diagnosis by CHCT. Table 1 shows that 110 patients came from the Southeast region of Brazil, 71 from the South, 8 from the Central West, and 3 from the Northeast. Rio de Janeiro (30.4%), Santa Catarina (28.9%), and São Paulo (11.3%) were the Brazilian states that more contributed to the total number of patients submitted to the CHCT. No patient came from the North region. CHCT was also performed in 1 patient from Argentina and 1 from Portugal.
Similar numbers of males (104) and females (90) were submitted to the test. Patients were predominantly young, with 55.7% of them being less than 29 years old (Table 2). The youngest patient was 4, the oldest 70, and the median age was 25 years.
Caffeine-halothane contracture test
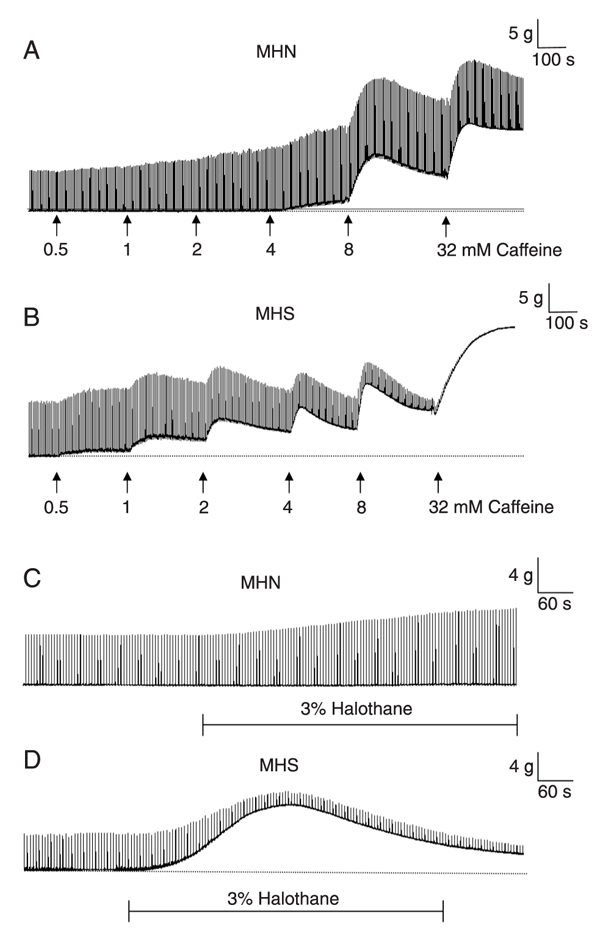
A typical recording of a CHCT is shown in Figure 1. The tension response of the muscle was dependent on caffeine concentration and MH susceptibility. While twitches were increased at concentrations lower than 4 mM, no increases of baseline (contracture) was noted when non-MHS (MHN) muscle was exposed to caffeine (Figure 1A). The intensity of muscle contracture increased in a dose-dependent manner at concentrations above the threshold. MHS muscle showed baseline contraction of more than 0.2 g after 2 mM caffeine (Figure 1B). MHN muscle was also less sensitive to halothane (3%), with increased muscle twitches but no increase in baseline tension (Figure 1C). In contrast, halothane increased MHS muscle tension above 0.7 g (Figure 1D).
MHS was detected in 90 of 194 patients (46.4%) and not detected in 104 patients (53.6%) (Figure 2). Biopsies from 59 of 90 patients (65.6%) responded positively to caffeine and halothane, 20 only to caffeine, and 11 only to halothane. MHS patients were split between females (36) and males (54). The average baseline tension (contracture) of MHN muscle after 2 mM caffeine or 3% halothane was 0.007 ± 0.001 g (N = 432) and 0.050 ± 0.011 g (N = 485), respectively. The number of muscle specimens was higher than the number of patients because 3-4 specimens for each patient were used for the caffeine or halothane test. The average tension from MHS fragments was 1.027 ± 0.075 g (N = 285) and 4.021 ± 0.255 g (N = 226), respectively (P < 0.05 versus MHN; Table 3).
The CK levels measured in MHN (192.8 ± 65.3 U/L, N = 104) and MHS patients (216.3 ± 53.4 U/L, N = 90) were similar. Most MHN patients (91) had CK levels within the normal range (Figure 3). Surprisingly, 65 MHS patients (72.2%) showed normal CK levels (Figure 3), with only 25 patients (27.7%) showing above normal levels. Most MHS patients (67.8%) that positively responded to both caffeine and halothane had normal CK levels, with 19 patients (32.2%) showing above normal levels. Most MHS patients that responded to caffeine or halothane had normal CK levels (Figure 3).
Correlation between CHCT and clinical grading
We next correlated CHCT and the MH rank described by Larach et al. (21) (Table 4). Of 59 patients at low risk to develop MH (ranks 1 and 2), 40 (67.8%) were diagnosed as MHN and 19 (32.2%) as MHS. One hundred and twenty-five patients were distributed in the median rank (ranks 3 and 4), with a mix of MHN (N = 62, 49.6%) and MHS (N = 63; 50.4%). Twenty of 90 MHS patients (22.2%) and 41 of 104 MHN patients (39.4%) suffered a suspected MH crisis during general anesthesia. All grade 6 patients (N = 4) who survived a complete clinically detected MH crisis presented a positive response to CHCT. Eighty percent of grade 5 and 6 patients presented a positive response.
The sensitivity and specificity obtained from CHCT was calculated based on subjects ranked 6 from the scale ("almost certain" to be MHS), and from subjects ranked 1 (unlikely to be MHS). The calculated specificity and sensitivity from this center were 100% and 65.7%, respectively.
Typical isometric tension recording of the vastus lateralis muscle of MHN (A and C) and MHS (B and D) patients in the presence of caffeine (0.5, 1, 2, 4, 8, and 32 mM) and halothane (3%). Note that after adding caffeine to the solution (arrows) the height of twitches increased in a dose-dependent manner. The threshold to increase baseline tension (contracture) was lower for MHS (B) than for MHN muscle fragments (A). Halothane was bubbled into the solution for 10 min. The threshold of MHS muscle response (D) to halothane was lower than that of MHN muscle (C). MHN = malignant hyperthermia non-susceptible patient; MHS = malignant hyperthermia susceptible patient.
Malignant hyperthermia susceptibility of patients submitted to the caffeine (Caff) and halothane (Hal) contracture test. The patients were divided into malignant hyperthermia non-susceptible (MHN) and malignant hyperthermia susceptible (MHS) subjects. The MHS group of patients was divided into 3 subgroups representing those who responded positively to both caffeine and halothane, to caffeine only and to halothane only. Patient distribution according to MH susceptibility is shown in parentheses.
Creatine kinase (CK) levels of patients submitted to the malignant hyperthermia susceptibility test. Patient distribution according to MH susceptibility is shown in parentheses. The dashed line represents the threshold of abnormal CK level (170 U/L). MHN = malignant hyperthermia non-susceptible patient; MHS = malignant hyperthermia susceptible patient.
Distribution of 192 Brazilian patients submitted to the North American caffeine halothane contracture test (CHCT) for the diagnosis of malignant hyperthermia from 1993 to 2009.
Age distribution of 194 patients submitted to the North American caffeine halothane contracture test (CHCT) for the diagnosis of malignant hyperthermia.
Vastus lateralis muscle contracture in response to exposure to 2 mM caffeine and 3% halothane (CHCT).
Discussion
Malignant hyperthermia is a potentially lethal pharmacogenetic disorder of skeletal muscle induced by exposure to the inhalation of general anesthetics and/or succinylcholine (1). The high mortality of MH (ca. 75%) has been reduced by research on the disease, development of diagnostic protocols, and specific drug treatments for acute MH symptoms. The CHCT is still a reliable and highly sensitive test for detecting MH susceptibility. We present here data obtained for 16 years using the CHCT protocol approved by the North American MH Group at the MH Diagnostic Center of the Federal University of Rio de Janeiro.
The distribution of 194 patients submitted to the CHCT was not homogeneous from the 11 Brazilian states, probably because of the center’s location. Most patients (N = 110, 56.7%) came from the Southeast, including the State of Rio de Janeiro, where the diagnostic center is located. However, 71 patients (36.6%) were from the South, which is located farther than the Southeast. Santa Catarina, with 56 patients, is more than twice as far as São Paulo from the diagnostic center, and São Paulo State is approximately 6.9 times larger than Santa Catarina. Thus, geographic localization is not the only factor that explains the high MH density. In fact, patients from Santa Catarina are related to three families of German origin, and the mutation could have resulted from European ancestors. This is in agreement with another large family of Italian heritage from São Paulo showing a severe MH crisis with death during general anesthesia. Additionally, an important explanation for the reduced number of patients from São Paulo in comparison to Santa Catarina is related to the development of an MH Diagnosic Center in that state at the Federal University of São Paulo (UNIFESP). Families from the Northeast, where colonization was from Holland, can show MH crises as well. No native Brazilian Indians received the test. Absence of an MH Diagnostic Center in Argentina brought one patient with family episodes of MH from that country, and relatives living in Brazil recommended another patient, also with a family history of MH, from Portugal. MH susceptibility was confirmed only in the Argentinian patient.
Most (75.3%) patients were less than 40 years old, with a median age of 25 years, indicating that MH is more prevalent in young individuals.
MH susceptibility was found in 46.4% of the patients (90/194), in agreement with rates from laboratories from Denmark and Toronto, 51.8 and 31.8% (22), respectively, where 1082/2214 patients were found to be susceptible to MH (48.9%) (23). MH susceptibility was lower (21.1%) in 10 laboratories from the North American MH Association (19). We detected muscle contracture to both caffeine and halothane in 59 of 90 MHS patients, and a selective response to caffeine or halothane in 20 and 11 patients, respectively. This apparent discrepancy in the reaction of the muscle to caffeine or halothane may occur because of the diversity of RyR1 mutations (24). According to the North American MH group (17), patients with abnormal reactions to caffeine or halothane are considered to be MHS. According to the European MH group (18), which has some differences in the protocol, one positive test means the individual is considered to be MH equivocal (MHE). Thus, 34.4% of the Brazilian MHS patients could be classified as MHE, similar to two Danish laboratories, with 34.8 and 34.5% (25) and to 22 centers of the European MH group, with 33.5% (26).
The hypermetabolism and breakdown of muscle membranes induced by increased Ca2+ concentration during an MH crisis may cause hyperkalemia, hyperphosphatemia, hypocalcemia, and increased serum levels of CK. Increased CK levels correlate with MHS incidence but are not sufficient as a screening test (27). Weglinski et al. (28) reported that 49% of 49 patients with high CK levels had a positive CHCT. However, Malandrini et al. (29) described only one MHS and one MHE patient of 37 with idiopathic hyperkalemia, or elevated serum CK levels without weaknesses or other neuromuscular symptoms. CK levels above normal (25-170 U/L) were observed in 13/104 (12.5%) and 25/90 (27.8%) of MHN and MHS patients, respectively, indicating a lack of correlation of CK levels with MH susceptibility.
We correlated the clinical signs and symptoms suggested by the North American MH Registry (21) to our in vitro test. A high correlation (>80%) was found in high-risk patients (rank 4-6), but a CHCT above the normal level was observed in 32.2% of rank 1-2 patients (low risk). We concluded that the clinically based score cannot predict that patients in the low probability rank are not susceptible to MH crises. MH clinical grading may therefore underestimate the likelihood of an MH event if an anesthetic was aborted early in the course of a reaction or in the absence of complete monitoring with capnography and arterial blood gas analysis (30). Recently, at the 3rd International Symposium on MH, Larach et al. (31) showed a similar lack of correlation between clinical grading and CHCT in North American patients. The clinical presentation of MH is highly variable and clinical data were frequently incomplete, making the MH risk difficult to measure using a clinical scale (32).
Genetic testing for detecting MH susceptibility (20) requires the consideration of mutations specific to a population, such as North America (33). However, not all mutations show a similar prevalence, and most of them are found in a single family. Our MH Center has already described a specific mutation in two Brazilian families (34).
In summary, the recommended CHCT described by the North American Malignant Hyperthermia Group was successfully applied at the Brazilian MH Center in Rio de Janeiro for the identification of MH susceptibility in 194 patients recommended for the test over 16 years of existence of the center.
References
1. Denborough M. Malignant hyperthermia. Lancet 1998; 352: 1131-1136.
2. Ellis FR, Halsall PJ, Christian AS. Clinical presentation of suspected malignant hyperthermia during anaesthesia in 402 probands. Anaesthesia 1990; 45: 838-841.
3. Britt BA, Kalow W. Malignant hyperthermia: a statistical review. Can Anaesth Soc J 1970; 17: 293-315.
4. Rueffert H, Olthoff D, Deutrich C, Meinecke CD, Froster UG. Mutation screening in the ryanodine receptor 1 gene (RYR1) in patients susceptible to malignant hyperthermia who show definite IVCT results: identification of three novel mutations. Acta Anaesthesiol Scand 2002; 46: 692-698.
5. Phillips MS, Fujii J, Khanna VK, DeLeon S, Yokobata K, de Jong PJ, et al. The structural organization of the human skeletal muscle ryanodine receptor (RYR1) gene. Genomics 1996; 34: 24-41.
6. Robinson R, Carpenter D, Shaw MA, Halsall J, Hopkins P. Mutations in RYR1 in malignant hyperthermia and central core disease. Hum Mutat 2006; 27: 977-989.
7. Vita GM, Olckers A, Jedlicka AE, George AL, Heiman-Patterson T, Rosenberg H, et al. Masseter muscle rigidity associated with glycine1306-to-alanine mutation in the adult muscle sodium channel alpha-subunit gene. Anesthesiology 1995; 82: 1097-1103.
8. Monnier N, Kozak-Ribbens G, Krivosic-Horber R, Nivoche Y, Qi D, Kraev N, et al. Correlations between genotype and pharmacological, histological, functional, and clinical phenotypes in malignant hyperthermia susceptibility. Hum Mutat 2005; 26: 413-425.
9. Brooks C, Robinson RL, Halsall PJ, Hopkins PM. No evidence of mutations in the CACNA1S gene in the UK malignant hyperthermia population. Br J Anaesth 2002; 88: 587-589.
10. Hogan KJ, Vladutiu GD. Malignant hyperthermia-like syndrome and carnitine palmitoyltransferase II deficiency with heterozygous R503C mutation. Anesth Analg 2009; 109: 1070-1072.
11. Protasi F, Paolini C, Dainese M. Calsequestrin-1: a new candidate gene for malignant hyperthermia and exertional/environmental heat stroke. J Physiol 2009; 587: 3095-3100.
12. Ording H. Investigation of malignant hyperthermia susceptibility in Denmark. Dan Med Bull 1996; 43: 111-125.
13. Rosero EB, Adesanya AO, Timaran CH, Joshi GP. Trends and outcomes of malignant hyperthermia in the United States, 2000 to 2005. Anesthesiology 2009; 110: 89-94.
14. Migita T, Mukaida K, Kawamoto M, Kobayashi M, Yuge O. Fulminant-type malignant hyperthermia in Japan: cumulative analysis of 383 cases. J Anesth 2007; 21: 285-288.
15. Yip WH, Mingi CL, Ooi SJ, Chen SC, Chiang YY. A survey for prevention and treatment of malignant hyperthermia in Taiwan. Acta Anaesthesiol Taiwan 2004; 42: 147-151.
16. Ellis FR, Harriman DG. A new screening test for susceptibility to malignant hyperpyrexia. Br J Anaesth 1973; 45: 638.
17. Larach MG. Standardization of the caffeine halothane muscle contracture test. North American Malignant Hyperthermia Group. Anesth Analg 1989; 69: 511-515.
18. European MH Group. A protocol for the investigation of malignant hyperpyrexia (MH) susceptibility. The European Malignant Hyperpyrexia Group. Br J Anaesth 1984; 56: 1267-1269.
19. Allen GC, Larach MG, Kunselman AR. The sensitivity and specificity of the caffeine-halothane contracture test: a report from the North American Malignant Hyperthermia Registry. The North American Malignant Hyperthermia Registry of MHAUS. Anesthesiology 1998; 88: 579-588.
20. Urwyler A, Deufel T, McCarthy T, West S. Guidelines for molecular genetic detection of susceptibility to malignant hyperthermia. Br J Anaesth 2001; 86: 283-287.
21. Larach MG, Localio AR, Allen GC, Denborough MA, Ellis FR, Gronert GA, et al. A clinical grading scale to predict malignant hyperthermia susceptibility. Anesthesiology 1994; 80: 771-779.
22. Islander G, Ording H, Bendixen D, Ranklev TE. Reproducibility of in vitro contracture test results in patients tested for malignant hyperthermia susceptibility. Acta Anaesthesiol Scand 2002; 46: 1144-1149.
23. Carr AS, Lerman J, Cunliffe M, McLeod ME, Britt BA. Incidence of malignant hyperthermia reactions in 2,214 patients undergoing muscle biopsy. Can J Anaesth 1995; 42: 281-286.
24. Fiege M, Wappler F, Weisshorn R, Ulrich GM, Steinfath M, Schulte Am Esch J. Results of contracture tests with halothane, caffeine, and ryanodine depend on different malignant hyperthermia-associated ryanodine receptor gene mutations. Anesthesiology 2002; 97: 345-350.
25. Urwyler A, Censier K, Seeberger MD, Drewe J, Rothenbuhler JM, Frei F. [Diagnosis of susceptibility for malignant hyperthermia using in-vitro muscle contraction testing in Switzerland]. Schweiz Med Wochenschr 1991; 121: 566-571.
26. Ording H, Brancadoro V, Cozzolino S, Ellis FR, Glauber V, Gonano EF, et al. In vitro contracture test for diagnosis of malignant hyperthermia following the protocol of the European MH Group: results of testing patients surviving fulminant MH and unrelated low-risk subjects. The European Malignant Hyperthermia Group. Acta Anaesthesiol Scand 1997; 41: 955-966.
27. McPherson E, Taylor CA Jr. The genetics of malignant hyperthermia: evidence for heterogeneity. Am J Med Genet 1982; 11: 273-285.
28. Weglinski MR, Wedel DJ, Engel AG. Malignant hyperthermia testing in patients with persistently increased serum creatine kinase levels. Anesth Analg 1997; 84: 1038-1041.
29. Malandrini A, Orrico A, Gaudiano C, Gambelli S, Galli L, Berti G, et al. Muscle biopsy and in vitro contracture test in subjects with idiopathic hyperCKemia. Anesthesiology 2008; 109: 625-628.
30. Larach MG. Should we reassess the susceptibility of MH patients? Can J Anaesth 1997; 44: 685-688.
31. Larach MG, Fuhrmann LJ, Allen GC. The epidemiology of malignant hyperthermia events in North America. In: Morio M, Kikuchi H, Yuge O (Editors), Malignant hyperthermia. Proceedings of 3rd International Symposium on Malignant Hyperthermia. Tokyo: Springer; 2010. p 39-41.
32. Girard T, Treves S, Voronkov E, Siegemund M, Urwyler A. Molecular genetic testing for malignant hyperthermia susceptibility. Anesthesiology 2004; 100: 1076-1080.
33. Sambuughin N, Nelson TE, Jankovic J, Xin C, Meissner G, Mullakandov M, et al. Identification and functional characterization of a novel ryanodine receptor mutation causing malignant hyperthermia in North American and South American families. Neuromuscul Disord 2001; 11: 530-537.
34. McWilliams S, Nelson T, Sudo RT, Zapata-Sudo G, Batti M, Sambuughin N. Novel skeletal muscle ryanodine receptor mutation in a large Brazilian family with malignant hyperthermia. Clin Genet 2002; 62: 80-83.
Acknowledgments
Research supported by CNPq, CAPES, Fundação Universitária José Bonifácio (FUJB), FAPERJ, and Sistema Único de Saúde (SUS).
 Address for correspondence: R.T. Sudo, Programa de Desenvolvimento de Fármacos, Instituto de Ciências Biomédicas, Universidade Federal do Rio de Janeiro, Av. Carlos Chagas, 373, Sala 14, Bloco J, CCS, 21941-902 Rio de Janeiro, RJ, Brasil. Fax: +55-21-2562-6505. E-mail: rtsudo@farmaco.ufrj.br
Address for correspondence: R.T. Sudo, Programa de Desenvolvimento de Fármacos, Instituto de Ciências Biomédicas, Universidade Federal do Rio de Janeiro, Av. Carlos Chagas, 373, Sala 14, Bloco J, CCS, 21941-902 Rio de Janeiro, RJ, Brasil. Fax: +55-21-2562-6505. E-mail: rtsudo@farmaco.ufrj.br
Received November 7, 2009. Accepted April 30, 2010. Available online May 14, 2010. Published June 11, 2010.
The Brazilian Journal of Medical and Biological Research is partially financed by
- 1. Denborough M. Malignant hyperthermia. Lancet 1998; 352: 1131-1136.
- 2. Ellis FR, Halsall PJ, Christian AS. Clinical presentation of suspected malignant hyperthermia during anaesthesia in 402 probands. Anaesthesia 1990; 45: 838-841.
- 3. Britt BA, Kalow W. Malignant hyperthermia: a statistical review. Can Anaesth Soc J 1970; 17: 293-315.
- 4. Rueffert H, Olthoff D, Deutrich C, Meinecke CD, Froster UG. Mutation screening in the ryanodine receptor 1 gene (RYR1) in patients susceptible to malignant hyperthermia who show definite IVCT results: identification of three novel mutations. Acta Anaesthesiol Scand 2002; 46: 692-698.
- 5. Phillips MS, Fujii J, Khanna VK, DeLeon S, Yokobata K, de Jong PJ, et al. The structural organization of the human skeletal muscle ryanodine receptor (RYR1) gene. Genomics 1996; 34: 24-41.
- 6. Robinson R, Carpenter D, Shaw MA, Halsall J, Hopkins P. Mutations in RYR1 in malignant hyperthermia and central core disease. Hum Mutat 2006; 27: 977-989.
- 7. Vita GM, Olckers A, Jedlicka AE, George AL, Heiman-Patterson T, Rosenberg H, et al. Masseter muscle rigidity associated with glycine1306-to-alanine mutation in the adult muscle sodium channel alpha-subunit gene. Anesthesiology 1995; 82: 1097-1103.
- 8. Monnier N, Kozak-Ribbens G, Krivosic-Horber R, Nivoche Y, Qi D, Kraev N, et al. Correlations between genotype and pharmacological, histological, functional, and clinical phenotypes in malignant hyperthermia susceptibility. Hum Mutat 2005; 26: 413-425.
- 9. Brooks C, Robinson RL, Halsall PJ, Hopkins PM. No evidence of mutations in the CACNA1S gene in the UK malignant hyperthermia population. Br J Anaesth 2002; 88: 587-589.
- 10. Hogan KJ, Vladutiu GD. Malignant hyperthermia-like syndrome and carnitine palmitoyltransferase II deficiency with heterozygous R503C mutation. Anesth Analg 2009; 109: 1070-1072.
- 11. Protasi F, Paolini C, Dainese M. Calsequestrin-1: a new candidate gene for malignant hyperthermia and exertional/environmental heat stroke. J Physiol 2009; 587: 3095-3100.
- 12. Ording H. Investigation of malignant hyperthermia susceptibility in Denmark. Dan Med Bull 1996; 43: 111-125.
- 13. Rosero EB, Adesanya AO, Timaran CH, Joshi GP. Trends and outcomes of malignant hyperthermia in the United States, 2000 to 2005. Anesthesiology 2009; 110: 89-94.
- 14. Migita T, Mukaida K, Kawamoto M, Kobayashi M, Yuge O. Fulminant-type malignant hyperthermia in Japan: cumulative analysis of 383 cases. J Anesth 2007; 21: 285-288.
- 15. Yip WH, Mingi CL, Ooi SJ, Chen SC, Chiang YY. A survey for prevention and treatment of malignant hyperthermia in Taiwan. Acta Anaesthesiol Taiwan 2004; 42: 147-151.
- 16. Ellis FR, Harriman DG. A new screening test for susceptibility to malignant hyperpyrexia. Br J Anaesth 1973; 45: 638.
- 17. Larach MG. Standardization of the caffeine halothane muscle contracture test. North American Malignant Hyperthermia Group. Anesth Analg 1989; 69: 511-515.
- 18. European MH Group. A protocol for the investigation of malignant hyperpyrexia (MH) susceptibility. The European Malignant Hyperpyrexia Group. Br J Anaesth 1984; 56: 1267-1269.
- 19. Allen GC, Larach MG, Kunselman AR. The sensitivity and specificity of the caffeine-halothane contracture test: a report from the North American Malignant Hyperthermia Registry. The North American Malignant Hyperthermia Registry of MHAUS. Anesthesiology 1998; 88: 579-588.
- 20. Urwyler A, Deufel T, McCarthy T, West S. Guidelines for molecular genetic detection of susceptibility to malignant hyperthermia. Br J Anaesth 2001; 86: 283-287.
- 21. Larach MG, Localio AR, Allen GC, Denborough MA, Ellis FR, Gronert GA, et al. A clinical grading scale to predict malignant hyperthermia susceptibility. Anesthesiology 1994; 80: 771-779.
- 22. Islander G, Ording H, Bendixen D, Ranklev TE. Reproducibility of in vitro contracture test results in patients tested for malignant hyperthermia susceptibility. Acta Anaesthesiol Scand 2002; 46: 1144-1149.
- 23. Carr AS, Lerman J, Cunliffe M, McLeod ME, Britt BA. Incidence of malignant hyperthermia reactions in 2,214 patients undergoing muscle biopsy. Can J Anaesth 1995; 42: 281-286.
- 24. Fiege M, Wappler F, Weisshorn R, Ulrich GM, Steinfath M, Schulte Am Esch J. Results of contracture tests with halothane, caffeine, and ryanodine depend on different malignant hyperthermia-associated ryanodine receptor gene mutations. Anesthesiology 2002; 97: 345-350.
- 25. Urwyler A, Censier K, Seeberger MD, Drewe J, Rothenbuhler JM, Frei F. [Diagnosis of susceptibility for malignant hyperthermia using in-vitro muscle contraction testing in Switzerland]. Schweiz Med Wochenschr 1991; 121: 566-571.
- 26. Ording H, Brancadoro V, Cozzolino S, Ellis FR, Glauber V, Gonano EF, et al. In vitro contracture test for diagnosis of malignant hyperthermia following the protocol of the European MH Group: results of testing patients surviving fulminant MH and unrelated low-risk subjects. The European Malignant Hyperthermia Group. Acta Anaesthesiol Scand 1997; 41: 955-966.
- 27. McPherson E, Taylor CA Jr. The genetics of malignant hyperthermia: evidence for heterogeneity. Am J Med Genet 1982; 11: 273-285.
- 28. Weglinski MR, Wedel DJ, Engel AG. Malignant hyperthermia testing in patients with persistently increased serum creatine kinase levels. Anesth Analg 1997; 84: 1038-1041.
- 29. Malandrini A, Orrico A, Gaudiano C, Gambelli S, Galli L, Berti G, et al. Muscle biopsy and in vitro contracture test in subjects with idiopathic hyperCKemia. Anesthesiology 2008; 109: 625-628.
- 30. Larach MG. Should we reassess the susceptibility of MH patients? Can J Anaesth 1997; 44: 685-688.
- 31. Larach MG, Fuhrmann LJ, Allen GC. The epidemiology of malignant hyperthermia events in North America. In: Morio M, Kikuchi H, Yuge O (Editors), Malignant hyperthermia. Proceedings of 3rd International Symposium on Malignant Hyperthermia Tokyo: Springer; 2010. p 39-41.
- 32. Girard T, Treves S, Voronkov E, Siegemund M, Urwyler A. Molecular genetic testing for malignant hyperthermia susceptibility. Anesthesiology 2004; 100: 1076-1080.
- 33. Sambuughin N, Nelson TE, Jankovic J, Xin C, Meissner G, Mullakandov M, et al. Identification and functional characterization of a novel ryanodine receptor mutation causing malignant hyperthermia in North American and South American families. Neuromuscul Disord 2001; 11: 530-537.
- 34. McWilliams S, Nelson T, Sudo RT, Zapata-Sudo G, Batti M, Sambuughin N. Novel skeletal muscle ryanodine receptor mutation in a large Brazilian family with malignant hyperthermia. Clin Genet 2002; 62: 80-83.
Correspondence and Footnotes
Publication Dates
-
Publication in this collection
07 June 2010 -
Date of issue
June 2010
History
-
Accepted
30 Apr 2010 -
Received
07 Nov 2009