Abstract
Primary biliary cirrhosis (PBC) is a chronic and slowly progressive cholestatic liver disease of autoimmune etiology. A number of questions regarding its etiology are unclear. CD4+CD25+ regulatory T cells (Tregs) play a critical role in self-tolerance and, for unknown reasons, their relative number is reduced in PBC patients. B-cell-activating factor (BAFF) is a key survival factor during B-cell maturation and its concentration is increased in peripheral blood of PBC patients. It has been reported that activated B cells inhibit Treg cell proliferation and there are no BAFF receptors on Tregs. Therefore, we speculated that excessive BAFF may result in Treg reduction via B cells. To prove our hypothesis, we isolated Tregs and B cells from PBC and healthy donors. BAFF and IgM concentrations were then analyzed by ELISA and CD40, CD80, CD86, IL-10, and TGF-β expression in B cells and Tregs were measured by flow cytometry. BAFF up-regulated CD40, CD80, CD86, and IgM expression in B cells. However, BAFF had no direct effect on Treg cell apoptosis and cytokine secretion. Nonetheless, we observed that BAFF-activated B cells could induce Treg cell apoptosis and reduce IL-10 and TGF-β expression. We also showed that BAFF-activated CD4+ T cells had no effect on Treg apoptosis. Furthermore, we verified that bezafibrate, a hypolipidemic drug, can inhibit BAFF-induced Treg cell apoptosis. In conclusion, BAFF promotes Treg cell apoptosis and inhibits cytokine production by activating B cells in PBC patients. The results of this study suggest that inhibition of BAFF activation is a strategy for PBC treatment.
Primary biliary cirrhosis; Regulatory T cells; BAFF; B cells; Bezafibrate
Introduction
Primary biliary cirrhosis (PBC) is a chronic and slowly progressive cholestatic liver disease of autoimmune etiology characterized by injury to the intrahepatic bile ducts that may eventually lead to liver failure 1. Kumahgi T, Heathcote EJ. Primary biliary cirrhosis. Orphanet J Rare Dis 2008; 3: 1, doi: 10.1186/1750-1172-3-1.
https://doi.org/10.1186/1750-1172-3-1...
1,22. Kaplan MM, Gershwin ME. Primary biliary cirrhosis. N Engl J Med 2005; 353: 1261-1273, doi: 10.1056/NEJMra043898.
https://doi.org/10.1056/NEJMra043898...
. The immunological approach to PBC has provided much critical information regarding its pathogenesis. The breakdown of self-tolerance in both B and T cells is evident. However, a number of questions regarding its etiology are unclear 33. Ueno Y, Moritoki Y, Shimosegawa T, Gershwin ME. Primary biliary cirrhosis: what we know and what we want to know about human PBC and spontaneous PBC mouse models. J Gastroenterol 2007; 42: 189-195, doi: 10.1007/s00535-007-2019-y.
https://doi.org/10.1007/s00535-007-2019-...
.
CD4+CD25+ regulatory T cells (Tregs) play a critical role in self-tolerance, as seen in murine autoimmunity. They are able to control the production of pro-inflammatory cytokines by activated immune cells during peripheral inflammation, and are being investigated clinically as potential therapeutic agents for the treatment of numerous immune-mediated diseases 44. Nguyen K, D'Mello C, Le T, Urbanski S, Swain MG. Regulatory T cells suppress sickness behaviour development without altering liver injury in cholestatic mice. J Hepatol 2012; 56: 626-631, doi: 10.1016/j.jhep.2011.09.014.
https://doi.org/10.1016/j.jhep.2011.09.0...
. Studies on Tregs in human autoimmunity have primarily focused on peripheral blood samples. Patients with PBC showed a relative reduction of Tregs compared to controls 55. Lan RY, Cheng C, Lian ZX, Tsuneyama K, Yang GX, Moritoki Y, et al. Liver-targeted and peripheral blood alterations of regulatory T cells in primary biliary cirrhosis. Hepatology 2006; 43: 729-737, doi: 10.1002/hep.21123.
https://doi.org/10.1002/hep.21123...
.
Autoimmune diseases are characterized by the production of autoantibodies against self-antigens via the loss of B-cell tolerance. Although the factors that promote the loss of tolerance are incompletely known, B-cell activating factor (BAFF) clearly plays a role in autoimmune diseases. BAFF, a recently identified member of the tumor necrosis factor (TNF) family, is a key survival factor during B-cell maturation and is essential for the development of B-cell tolerance. Breakdown of the regulation of BAFF expression results in excessive BAFF production that impairs B-cell tolerance and leads to autoimmune phenomena. Elevated levels of BAFF were thus demonstrated in patients with systemic autoimmune diseases such as systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), systemic sclerosis, mixed cryoglobulinemia, myasthenia gravis, and celiac disease, as well as in organ-specific autoimmune diseases such as autoimmune hepatitis, bullous pemphigoid, and localized scleroderma. Excess BAFF may contribute to the production of autoantibodies in PBC 66. Lied GA, Berstad A. Functional and clinical aspects of the B-cell-activating factor (BAFF): a narrative review. Scand J Immunol 2011; 73: 1-7, doi: 10.1111/j.1365-3083.2010.02470.x.
https://doi.org/10.1111/j.1365-3083.2010...
.
Thus, BAFF has become a very attractive target for the treatment of autoimmune diseases with an altered B-cell function. BAFF inhibitors in the treatment of RA, SLE and other autoimmune diseases are under intensive investigation. Although the biology of BAFF remains poorly understood, results of the ongoing studies may enable the development of a new generation of BAFF inhibitors with more selective efficacy and increased safety 77. Krivosikova M, Dallos T, Maslinski W, Buc M. B-cell activating factor, its role in autoimmunity, and targeting in autoimmune diseases. Bratisl Lek Listy 2009; 110: 137-145..
We speculated that BAFF may be the reason for Treg reduction. It is known that activated T cells can express the BAFF receptors BR3 and TACI. However, Tregs do not express TACI or BR3 8. Marino E, Villanueva J, Walters S, Liuwantara D, Mackay F, Grey ST. CD4(+)CD25(+) T-cells control autoimmunity in the absence of B-cells. Diabetes 2009; 58: 1568-1577, doi: 10.2337/db08-1504.
https://doi.org/10.2337/db08-1504...
8,99. Walters S, Webster KE, Sutherland A, Gardam S, Groom J, Liuwantara D, et al. Increased CD4+Foxp3+ T cells in BAFF-transgenic mice suppress T cell effector responses. J Immunol 2009; 182: 793-801.. Thus, BAFF may affect Tregs in an indirect way. B cells are important for the regulation of autoimmune responses. A previous study showed that B cells regulated the number of Tregs in the central nervous system during experimental autoimmune encephalomyelitis and B cells play a major role in immune tolerance required for the prevention of autoimmunity by maintenance of Tregs via their expression of the glucocorticoid-induced TNF receptor ligand 1010. Ray A, Basu S, Williams CB, Salzman NH, Dittel BN. A novel IL-10-independent regulatory role for B-cells in suppressing autoimmunity by maintenance of regulatory T cells via GITR ligand. J Immunol 2012; 188: 3188-3198, doi: 10.4049/jimmunol.1103354.
https://doi.org/10.4049/jimmunol.1103354...
.
Furthermore, it has been reported that, upon activation, B cells express less TGF-β3, which reduces their capacity to expand Tregs and which also results in increased Treg death. This may ensure that B cells can function as potent professional antigen-presenting cells during infections 1111. Shah S, Qiao L. Resting B-cells expand a CD4+CD25+Foxp3+ Treg population via TGF-beta3. Eur J Immunol 2008; 38: 2488-2498, doi: 10.1002/eji.200838201.
https://doi.org/10.1002/eji.200838201...
. In view of these considerations, we speculate that BAFF may activate B cells first and that activated B cells then induce Treg death.
Bezafibrate, as a hypolipidemic drug, has shown therapeutic efficacy in patients with PBC in some pilot studies. However, little is known regarding the mechanism of action of bezafibrate in PBC 12. Iwasaki S, Ohira H, Nishiguchi S, Zeniya M, Kaneko S, Onji M, et al. The efficacy of ursodeoxycholic acid and Bezafibrate combination therapy for primary biliary cirrhosis: A prospective, multicenter study. Hepatol Res 2008; 38: 557-564, doi: 10.1111/j.1872-034X.2007.00305.x.
https://doi.org/10.1111/j.1872-034X.2007...
12,1313. Rudic JS, Poropat G, Krstic MN, Bjelakovic G, Gluud C. Bezafibrate for primary biliary cirrhosis. Cochrane Database Syst Rev 2012; 1: CD009145.. In our previous study, we found that bezafibrate can inhibit the effect of BAFF on B cells. In the present study, our objective was to determine whether bezafibrate can inhibit Treg death in vitro, explaining its therapeutic efficacy in PBC.
Material and Methods
Determination of BAFF levels in PBC serum and B cells
Sera were obtained from blood collected from 30 PBC patients (19 females and 11 males; median age: 50 years) before therapy at the Hospital (Nanjing, China), and were immediately frozen and kept at -80°C until assay. Informed written consent was obtained from all patients before experiments for this study. For comparison, serum BAFF levels were determined in 30 healthy donors with a similar age distribution. The supernatant of isolated B cells was collected and analyzed with a BAFF ELISA kit (R&D Systems, USA). The study protocol was approved by the Institutional Ethics Committee of Wuxi Infectious Diseases Hospital, China. BAFF concentrations in serum of PBC patients and B cells are reported as pg/mL serum or cell supernatant.
Treg analysis
All blood samples were processed on the day of collection. Peripheral blood mononuclear cells (PBMCs) were isolated on Ficoll gradients (GE Healthcare, Sweden). For the detection of Tregs, PBMCs were stained with fluorescein isothyocyanate (FITC)-conjugated anti-CD4, phycoerythrin-Cy5 (PE-Cy5)-conjugated anti-CD25, and PE-conjugated anti-FOXP3 (eBiosciences, USA), according to the manufacturer protocol. After staining, cells were washed twice in fluorescence-activated cell sorting solution [phosphate-buffered saline (PBS) with 0.5% bovine serum albumin, and 0.02% sodium azide], fixed in PBS containing 1% paraformaldehyde, and collected on the same day using a FacsCalibur system (BD Biosciences, USA) followed by analysis with the FlowJo software (Tree Star, USA). CD25+ FOXP3+ Tregs were identified within gated CD4+ T cells.
Cell isolation
PBMCs were isolated by Ficoll-Hypaque density gradient centrifugation from heparinized blood. CD4+ CD25+ Tregs were isolated from PBMCs by CD4-negative selection followed by CD25-positive selection, using the CD4+ CD25+ T-cell isolation kit (Miltenyi Biotech, Germany), with MidiMACS and MiniMACS separator units (both from Miltenyi Biotech), according to manufacturer instructions. After CD25-positive selection, the cells that did not bind to MidiMACS beads were CD4+CD25- T cells. The homogeneity of Tregs was checked by CD4 and intracellular FOXP3 expression. B cells were isolated using CD19 MicroBeads, and the homogeneity of CD19+ B cells was indicated by flow cytometry. Isolated B cells were cultured with RPMI 1640 medium (Biochrome, Germany) supplemented with 10% FBS.
Analysis of B-cell activation
All antibodies used in flow cytometry were purchased from eBioscience, with the exception of PE-conjugated anti-human TGF-β1, 2 and 3 (R&D Systems). For the determination of B-cell activation, cells were stained with FITC-labeled monoclonal antibodies: anti-CD40, anti-CD80 and anti-CD86. For IgM analysis, the supernatant of isolated B cells treated or not with BAFF was collected and measured with an IgM ELISA kit (eBioscience).
Cytokine analysis in Tregs
For the staining of intracellular cytokines including TGF-β and IL-10, cells were stimulated with 25 ng/mL phorbol-12-myristate-13-acetate (Enzo, USA) and 1 µg/mL ionomycin (Enzo) in 1 mL RPMI 1640 medium supplemented with 10% FCS at 37°C for 6 h. Brefeldin A (1 µg/mL, Enzo) was added 1 h prior to cell harvesting. After labeling with surface antibodies, cells were permeabilized with a fix/perm solution (eBioscience) and stained with the appropriate intracellular antibodies according to manufacturer instructions. Isotype-matched control antibodies were used to determine the level of background staining and to help set the gate. In addition, the supernatant of isolated Tregs treated or not with BAFF was collected and measured with a Treg and IL-10 ELISA kit (eBioscience).
Assay of Treg cell apoptosis
Treg cell apoptosis was analyzed after treatment with BAFF or co-culture with B cells with or without bezafibrate. In addition, we also analyzed Treg cell apoptosis after treatment with BAFF or co-culture with CD4+CD25- T cells. Tregs were co-cultured with B cells or CD4+CD25- T cells in 12 wells (1 × 106/well, ratio = 1:1). Tregs were labeled with CFSE to distinguish them from B cells. Death of CFSE+ Tregs was determined based on staining with annexin-V and propidium iodide (PI). The number of cells in apoptosis includes prophase apoptosis (annexin V+PI-) and telophase apoptosis (annexin-V+PI+) cells.
Statistical analysis
Statistical analyses were performed using GraphPad Prism (USA). The two-tailed Student t-test was used for the pairwise comparison of experimental groups. Statistical significance was defined at the ≥95% confidence interval or P ≤ 0.05. In each figure, asterisks indicate results significantly different from control (P < 0.05). Data are reported as means ± SE for the number of independent experiments indicated in each figure legend.
Results
Reduction of circulating CD4+ CD25+FOXP3+ Tregs in PBC patients
Tregs play a key role in peripheral immune tolerance and prevent the occurrence of autoimmune diseases. In this study, Tregs were quantified by flow cytometry according to their CD4, CD25 and FOXP3 marker expression. The percentage of CD4+ CD25+ FOXP3+ T cells was significantly decreased in the 30 patients with PBC (2.194 ± 0.81%, P < 0.001) compared to the 30 healthy controls (3.751 ± 1.08%; Figure 1).
Percentage of Tregs in PBMCs of healthy donors and PBC patients. PBMCs were collected from 30 PBC patients and 30 healthy donors and stained with FITC-conjugated anti-CD4, PE-Cy5-conjugated anti-CD25, and PE-conjugated anti-FOXP3, according to the manufacturer protocol. CD25+ FOXP3+ Tregs of donors (mean ± SE = 3.751 ± 0.106) and patients (mean ± SE = 2.914 ± 0.089) were identified within gated CD4+ T cells. Tregs = regulatory T cells; PBMCs = peripheral blood mononuclear cells; PBC = primary biliary cirrhosis. *P < 0.05vs normal (two-tailed Studentt-test).
BAFF levels are elevated in PBC patients and in B cells from PBC patients
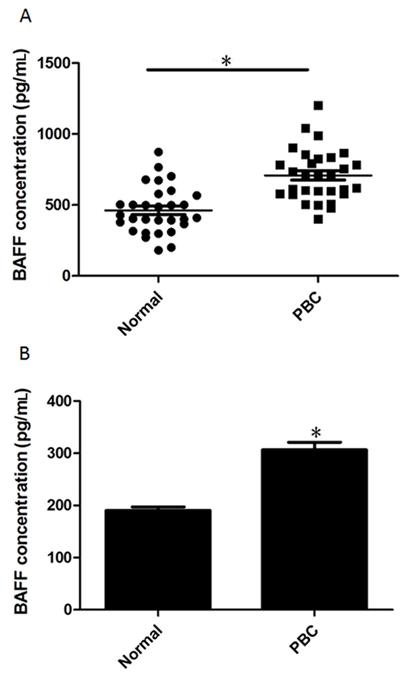
Serum levels of BAFF were significantly increased in all PBC patients (median 708 pg/mL, P < 0.001; Figure 2A) compared to all control donors (median 462 pg/mL). Furthermore, we isolated B cells from healthy donors and PBC patients and cultured them on 48-well plates. The homogeneity of B cells was first indicated by flow cytometry. As shown in Figure S1A, the contamination by other cell types was less than 3.5%. After 48 h, the concentration of BAFF secreted by B cells was assessed with an ELISA kit. As shown in Figure 2B, B cells from PBC secreted more BAFF than B cells from healthy donors.
B-cell-activating factor (BAFF) expression in peripheral blood and isolated B cells from healthy donors and primary biliary cirrhosis (PBC) patients. A, Sera were obtained from blood collected from 30 PBC patients (mean ± SE = 708.4 ± 33.2) and 30 healthy donors (mean ± SE = 462.1 ± 30.05) and assessed with a BAFF ELISA kit. B, B cells were isolated using CD19 MicroBeads and isolated B cells were cultured with RPMI 1640 medium supplemented with 10% FBS. The supernatant of isolated B cells was collected and assessed with a BAFF ELISA kit. Data are reported as means ± SE from 30 different donors. *P < 0.05 vs normal (two-tailed Studentt-test).
BAFF can activate B cells but has no effect on Tregs
BAFF is a key survival factor during B-cell maturation. However, excessive BAFF production can impair B-cell tolerance. We treated isolated B cells with 20 and 40 ng/mL BAFF for 48 h, and then measured the B-cell activation markers CD40, CD80, and CD86 by flow cytometry. As shown in Figure 3A, BAFF can significantly activate B cells. Furthermore, we found that BAFF could promote IgM secretion in B cells (Figure 3B).
B-cell-activating factor (BAFF) can activate B cells but has no direct effect on regulatory T cells (Tregs). A, Purified B cells were treated with 20 and 40 ng/mL BAFF for 24 h and CD40, CD80 and CD86 were then measured by flow cytometry. B, Purified B cells were treated with 20 and 40 ng/mL BAFF for 72 h and IgM was then tested by ELISA.C, Purified Tregs were treated with 20 and 40 ng/mL BAFF for 48 h and Treg apoptosis was then analyzed by annexin-V-PI (propidium iodide) staining.D, The percentage of cell apoptosis was analyzed statistically. E, Purified Tregs were treated with 20 and 40 ng/mL BAFF for 48 h and IL-10 and TGF-β were then assessed by ELISA. Data are reported as means ± SE of replicates from a sample of an individual donor for three representative independent experiments. *P < 0.05 vs control (one-way ANOVA followed by the Student-Newman-Keuls multiple comparisons test). ns = not significant.
To determine if BAFF affects Treg apoptosis and cytokine secretion, isolated Tregs were treated with 20 and 40 ng/mL BAFF. The homogeneity of Tregs was determined with CD4 and FOXP3 antibodies before treatment with BAFF. As shown in Figure S1B, more than 90% of the CD4+ cells were Tregs. After 48 h, cell apoptosis, TGF-β and IL-10 concentration were determined. The results showed that BAFF had no effects on Treg cell apoptosis (Figure 3C and D) or cytokine secretion (Figure 3E). These results indicate that BAFF may affect Tregs in an indirect way.
BAFF indirectly inhibits Tregs through B cells and bezafibrate can inhibit Treg suppression by BAFF-activated B cells
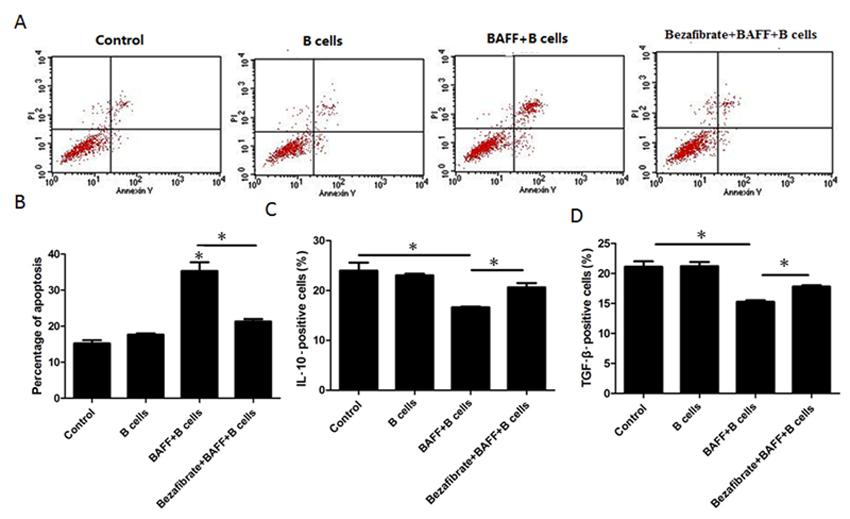
To determine if BAFF-activated B cells inhibit Treg proliferation and cytokine secretion, Tregs and B cells were co-cultured with or without BAFF. We found that BAFF could cause B cells to induce Treg cell apoptosis (Figure 4A and B). Additionally, BAFF-activated B cells reduced IL-10 and TGF-β secretion in Tregs (Figure 4C and D). To investigate if BAFF-treated CD4+CD25- T cells, which also express BAFF receptors, were able to modulate Treg survival, Tregs and CD4+CD25- cells were co-cultured with or without 40 ng/mL BAFF for 48 h and we found that BAFF-treated CD4+CD25- cells had no effects on Treg cell apoptosis. These results further indicate that B cells mediated the effects of BAFF on Tregs.
B-cell-activating factor (BAFF) induces regulatory T cell (Treg) apoptosis and cytokine expression through B cells. A, CFSE-labeled Tregs were co-cultured with B cells with or without 40 ng/mL BAFF and 40 mg/mL bezafibrate for 48 h and Treg apoptosis was then analyzed by annexin-V-PI (propidium iodide) staining. B, The percentage of cell apoptosis was analyzed statistically. C, CFSE-labeled Tregs were co-cultured with B cells with or without 40 ng/mL BAFF and 40 mg/mL bezafibrate for 48 h and IL-10 was then tested by flow cytometry. D, CFSE-labeled Tregs were co-cultured with B cells with or without 40 ng/mL BAFF and 40 mg/mL bezafibrate for 48 h and TGF-β was then tested by flow cytometry. Data are reported as means ± SE of replicates from a sample of an individual donor for three representative independent experiments. *P < 0.05 vs control (one-way ANOVA followed by the Student-Newman-Keuls multiple comparisons test).
Bezafibrate is effective for the treatment of PBC, although little is known regarding the mechanism of action involved. Here, we pretreated Tregs and B cells with 40 mg/mL bezafibrate before treatment with BAFF. We found that bezafibrate could reduce BAFF-stimulated B-cell-induced Treg cell apoptosis (Figure 4A and B) and promote TGF-β and IL-10 expression in Tregs (Figure 4C and D).
Discussion
CD4+ CD25+ Tregs were reduced in peripheral blood of PBC patients. Tregs play a critical role in self-tolerance, as seen in murine autoimmunity. They are able to control the production of pro-inflammatory cytokines by activated immune cells during peripheral inflammation, and are being investigated clinically as potential therapeutic agents for the treatment of numerous immune-mediated diseases1414. Hsieh CS, Lee HM, Lio CW. Selection of regulatory T cells in the thymus. Nat Rev Immunol 2012; 12: 157-167.. In our study, we found that PBC patients showed a relative reduction of Tregs compared to healthy donors, in agreement with data reported by Wei et al. 1515. Wei HX, Chuang YH, Li B, Wei H, Sun R, Moritoki Y, et al. CD4+ CD25+ Foxp3+ regulatory T cells protect against T cell-mediated fulminant hepatitis in a TGF-beta-dependent manner in mice. J Immunol 2008; 181: 7221-7229..
BAFF was elevated in PBC patients. Autoimmune diseases are characterized by the production of autoantibodies against self-antigens via the loss of B-cell tolerance. Although the factors that promote the loss of tolerance are still insufficiently known, BAFF clearly plays a role in autoimmune diseases 1616. Liu Z, Davidson A. BAFF and selection of autoreactive B-cells. Trends Immunol 2011; 32: 388-394, doi: 10.1016/j.it.2011.06.004.
https://doi.org/10.1016/j.it.2011.06.004...
. Several autoimmune diseases such as SLE, Sjögren's syndrome, RA, systemic sclerosis, mixed cryoglobulinemia, myasthenia gravis, and celiac disease, as well as organ-specific autoimmune diseases such as autoimmune hepatitis, bullous pemphigoid and localized scleroderma, have shown elevated BAFF levels 66. Lied GA, Berstad A. Functional and clinical aspects of the B-cell-activating factor (BAFF): a narrative review. Scand J Immunol 2011; 73: 1-7, doi: 10.1111/j.1365-3083.2010.02470.x.
https://doi.org/10.1111/j.1365-3083.2010...
. In the present study, we confirmed that BAFF concentration was increased in peripheral blood of patients and we found that BAFF could induce B cells to secrete more IgM. These results are consistent with the fact that PBC patients have more IgM-type autoantibodies.
BAFF promoted Treg cell apoptosis and blocked cytokine secretion via B cells. In order to investigate if and how excessive BAFF reduces the number of Tregs, we first treated Tregs with BAFF. As reported in a study 88. Marino E, Villanueva J, Walters S, Liuwantara D, Mackay F, Grey ST. CD4(+)CD25(+) T-cells control autoimmunity in the absence of B-cells. Diabetes 2009; 58: 1568-1577, doi: 10.2337/db08-1504.
https://doi.org/10.2337/db08-1504...
showing that Tregs do not express BAFF receptors, BAFF had no effect on Treg cell apoptosis. Thus, BAFF may affect Tregs in an indirect way. BAFF receptors were mainly expressed on B cells and it was reported that resting B cells can expand and maintain specific Tregs 17. Chen LC, Delgado JC, Jensen PE, Chen X. Direct expansion of human allospecific FoxP3+CD4+ regulatory T cells with allogeneic B-cells for therapeutic application. J Immunol 2009; 183: 4094-4102, doi: 10.4049/jimmunol.0901081.
https://doi.org/10.4049/jimmunol.0901081...
17,1818. Hamel KM, Cao Y, Ashaye S, Wang Y, Dunn R, Kehry MR, et al. B-cell depletion enhances T regulatory cell activity essential in the suppression of arthritis. J Immunol 2011; 187: 4900-4906, doi: 10.4049/jimmunol.1101844.
https://doi.org/10.4049/jimmunol.1101844...
, while activated B cells may involve inhibition of Treg function in an IL-2-dependent way 19. Olson TS, Bamias G, Naganuma M, Rivera-Nieves J, Burcin TL, Ross W, et al. Expanded B-cell population blocks regulatory T cells and exacerbates ileitis in a murine model of Crohn disease. J Clin Invest 2004; 114: 389-398.
19,2020. Tretter T, Venigalla RK, Eckstein V, Saffrich R, Sertel S, Ho AD, et al. Induction of CD4+ T-cell anergy and apoptosis by activated human B-cells. Blood 2008; 112: 4555-4564, doi: 10.1182/blood-2008-02-140087.
https://doi.org/10.1182/blood-2008-02-14...
. Moreover, activated T cells also express the BAFF receptors TACI 2121. von Bulow GU, Bram RJ. NF-AT activation induced by a CAML-interacting member of the tumor necrosis factor receptor superfamily. Science 1997; 278: 138-141, doi: 10.1126/science.278.5335.138.
https://doi.org/10.1126/science.278.5335...
and BR322. Ng LG, Sutherland AP, Newton R, Qian F, Cachero TG, Scott ML, et al. B-cell-activating factor belonging to the TNF family (BAFF)-R is the principal BAFF receptor facilitating BAFF costimulation of circulating T and B-cells. J Immunol 2004; 173: 807-817.
22,2323. Ye Q, Wang L, Wells AD, Tao R, Han R, Davidson A, et al. BAFF binding to T cell-expressed BAFF-R costimulates T cell proliferation and alloresponses. Eur J Immunol 2004; 34: 2750-2759, doi: 10.1002/eji.200425198.
https://doi.org/10.1002/eji.200425198...
. Therefore, BAFF may inhibit Tregs by activating B cells or T cells. Subsequently, Tregs were co-cultured with BAFF-activated B cells or T cells and we found that B cells mediated the inhibition of BAFF on Tregs. It was confirmed that B cells inhibited both the expansion and function of Tregs in proteoglycan-induced arthritis 1010. Ray A, Basu S, Williams CB, Salzman NH, Dittel BN. A novel IL-10-independent regulatory role for B-cells in suppressing autoimmunity by maintenance of regulatory T cells via GITR ligand. J Immunol 2012; 188: 3188-3198, doi: 10.4049/jimmunol.1103354.
https://doi.org/10.4049/jimmunol.1103354...
.
Bezafibrate can inhibit Treg suppression by BAFF-activated B cells. Bezafibrate is a fibrate drug used for the treatment of hyperlipidemia. The therapeutic efficacy of bezafibrate, a hypolipidemic drug, has been shown in patients with PBC in some pilot studies. Down-regulation of nitrite production by dendritic cells may have some relationship with the therapeutic efficacy of bezafibrate for PBC 2424. Akbar SM, Furukawa S, Nakanishi S, Abe M, Horiike N, Onji M. Therapeutic efficacy of decreased nitrite production by Bezafibrate in patients with primary biliary cirrhosis. J Gastroenterol 2005; 40: 157-163, doi: 10.1007/s00535-004-1518-3.
https://doi.org/10.1007/s00535-004-1518-...
. In our study, we found that bezafibrate could reduce BAFF-stimulated B-cell-induced Treg cell apoptosis and promote TGF-β and IL-10 expression in Tregs.
Tregs were reduced in PBC patients while BAFF expression was up-regulated in peripheral blood and B cells from PBC patients. BAFF had no direct effect on Treg apoptosis or cytokine secretion. However, BAFF can induce Treg cell apoptosis and reduce IL-10 and TGF-β expression via B-cell activation. Bezafibrate can inhibit Treg suppression by BAFF-activated B cells. Our study could provide some suggestions for PBC treatment.
Supplemental Material
References
-
1Kumahgi T, Heathcote EJ. Primary biliary cirrhosis. Orphanet J Rare Dis 2008; 3: 1, doi: 10.1186/1750-1172-3-1.
» https://doi.org/10.1186/1750-1172-3-1 -
2Kaplan MM, Gershwin ME. Primary biliary cirrhosis. N Engl J Med 2005; 353: 1261-1273, doi: 10.1056/NEJMra043898.
» https://doi.org/10.1056/NEJMra043898 -
3Ueno Y, Moritoki Y, Shimosegawa T, Gershwin ME. Primary biliary cirrhosis: what we know and what we want to know about human PBC and spontaneous PBC mouse models. J Gastroenterol 2007; 42: 189-195, doi: 10.1007/s00535-007-2019-y.
» https://doi.org/10.1007/s00535-007-2019-y -
4Nguyen K, D'Mello C, Le T, Urbanski S, Swain MG. Regulatory T cells suppress sickness behaviour development without altering liver injury in cholestatic mice. J Hepatol 2012; 56: 626-631, doi: 10.1016/j.jhep.2011.09.014.
» https://doi.org/10.1016/j.jhep.2011.09.014 -
5Lan RY, Cheng C, Lian ZX, Tsuneyama K, Yang GX, Moritoki Y, et al. Liver-targeted and peripheral blood alterations of regulatory T cells in primary biliary cirrhosis. Hepatology 2006; 43: 729-737, doi: 10.1002/hep.21123.
» https://doi.org/10.1002/hep.21123 -
6Lied GA, Berstad A. Functional and clinical aspects of the B-cell-activating factor (BAFF): a narrative review. Scand J Immunol 2011; 73: 1-7, doi: 10.1111/j.1365-3083.2010.02470.x.
» https://doi.org/10.1111/j.1365-3083.2010.02470.x -
7Krivosikova M, Dallos T, Maslinski W, Buc M. B-cell activating factor, its role in autoimmunity, and targeting in autoimmune diseases. Bratisl Lek Listy 2009; 110: 137-145.
-
8Marino E, Villanueva J, Walters S, Liuwantara D, Mackay F, Grey ST. CD4(+)CD25(+) T-cells control autoimmunity in the absence of B-cells. Diabetes 2009; 58: 1568-1577, doi: 10.2337/db08-1504.
» https://doi.org/10.2337/db08-1504 -
9Walters S, Webster KE, Sutherland A, Gardam S, Groom J, Liuwantara D, et al. Increased CD4+Foxp3+ T cells in BAFF-transgenic mice suppress T cell effector responses. J Immunol 2009; 182: 793-801.
-
10Ray A, Basu S, Williams CB, Salzman NH, Dittel BN. A novel IL-10-independent regulatory role for B-cells in suppressing autoimmunity by maintenance of regulatory T cells via GITR ligand. J Immunol 2012; 188: 3188-3198, doi: 10.4049/jimmunol.1103354.
» https://doi.org/10.4049/jimmunol.1103354 -
11Shah S, Qiao L. Resting B-cells expand a CD4+CD25+Foxp3+ Treg population via TGF-beta3. Eur J Immunol 2008; 38: 2488-2498, doi: 10.1002/eji.200838201.
» https://doi.org/10.1002/eji.200838201 -
12Iwasaki S, Ohira H, Nishiguchi S, Zeniya M, Kaneko S, Onji M, et al. The efficacy of ursodeoxycholic acid and Bezafibrate combination therapy for primary biliary cirrhosis: A prospective, multicenter study. Hepatol Res 2008; 38: 557-564, doi: 10.1111/j.1872-034X.2007.00305.x.
» https://doi.org/10.1111/j.1872-034X.2007.00305.x -
13Rudic JS, Poropat G, Krstic MN, Bjelakovic G, Gluud C. Bezafibrate for primary biliary cirrhosis. Cochrane Database Syst Rev 2012; 1: CD009145.
-
14Hsieh CS, Lee HM, Lio CW. Selection of regulatory T cells in the thymus. Nat Rev Immunol 2012; 12: 157-167.
-
15Wei HX, Chuang YH, Li B, Wei H, Sun R, Moritoki Y, et al. CD4+ CD25+ Foxp3+ regulatory T cells protect against T cell-mediated fulminant hepatitis in a TGF-beta-dependent manner in mice. J Immunol 2008; 181: 7221-7229.
-
16Liu Z, Davidson A. BAFF and selection of autoreactive B-cells. Trends Immunol 2011; 32: 388-394, doi: 10.1016/j.it.2011.06.004.
» https://doi.org/10.1016/j.it.2011.06.004 -
17Chen LC, Delgado JC, Jensen PE, Chen X. Direct expansion of human allospecific FoxP3+CD4+ regulatory T cells with allogeneic B-cells for therapeutic application. J Immunol 2009; 183: 4094-4102, doi: 10.4049/jimmunol.0901081.
» https://doi.org/10.4049/jimmunol.0901081 -
18Hamel KM, Cao Y, Ashaye S, Wang Y, Dunn R, Kehry MR, et al. B-cell depletion enhances T regulatory cell activity essential in the suppression of arthritis. J Immunol 2011; 187: 4900-4906, doi: 10.4049/jimmunol.1101844.
» https://doi.org/10.4049/jimmunol.1101844 -
19Olson TS, Bamias G, Naganuma M, Rivera-Nieves J, Burcin TL, Ross W, et al. Expanded B-cell population blocks regulatory T cells and exacerbates ileitis in a murine model of Crohn disease. J Clin Invest 2004; 114: 389-398.
-
20Tretter T, Venigalla RK, Eckstein V, Saffrich R, Sertel S, Ho AD, et al. Induction of CD4+ T-cell anergy and apoptosis by activated human B-cells. Blood 2008; 112: 4555-4564, doi: 10.1182/blood-2008-02-140087.
» https://doi.org/10.1182/blood-2008-02-140087 -
21von Bulow GU, Bram RJ. NF-AT activation induced by a CAML-interacting member of the tumor necrosis factor receptor superfamily. Science 1997; 278: 138-141, doi: 10.1126/science.278.5335.138.
» https://doi.org/10.1126/science.278.5335.138 -
22Ng LG, Sutherland AP, Newton R, Qian F, Cachero TG, Scott ML, et al. B-cell-activating factor belonging to the TNF family (BAFF)-R is the principal BAFF receptor facilitating BAFF costimulation of circulating T and B-cells. J Immunol 2004; 173: 807-817.
-
23Ye Q, Wang L, Wells AD, Tao R, Han R, Davidson A, et al. BAFF binding to T cell-expressed BAFF-R costimulates T cell proliferation and alloresponses. Eur J Immunol 2004; 34: 2750-2759, doi: 10.1002/eji.200425198.
» https://doi.org/10.1002/eji.200425198 -
24Akbar SM, Furukawa S, Nakanishi S, Abe M, Horiike N, Onji M. Therapeutic efficacy of decreased nitrite production by Bezafibrate in patients with primary biliary cirrhosis. J Gastroenterol 2005; 40: 157-163, doi: 10.1007/s00535-004-1518-3.
» https://doi.org/10.1007/s00535-004-1518-3
Publication Dates
-
Publication in this collection
10 May 2013 -
Date of issue
May 2013
History
-
Received
15 Sept 2012 -
Accepted
11 Mar 2013