Abstract
The objective of this study was to evaluate the effect of tamoxifen on the plasma concentration of NT-pro-B-type natriuretic peptide (NT-proBNP) in women undergoing chemotherapy for breast cancer and to correlate changes in NT-proBNP with the left ventricular ejection fraction (LVEF). Over a period of 12 months, we followed 60 women with a diagnosis of breast cancer. The patients were separated into a group that received only chemotherapy (n=23), a group that received chemotherapy + tamoxifen (n=21), and a group that received only tamoxifen (n=16). Plasma levels of NT-proBNP were assessed at 0 (T0), 6 (T6), and 12 (T12) months of treatment, and echocardiography data were assessed at T0 and T12. Plasma NT-proBNP levels were increased in the chemotherapy-only group at T6 and T12, whereas elevated NT-proBNP levels were only found at T6 in the chemotherapy + tamoxifen group. At T12, the chemotherapy + tamoxifen group exhibited a significant reduction in the peptide to levels similar to the group that received tamoxifen alone. The chemotherapy-only group exhibited a significant decrease in LVEF at T12, whereas the chemotherapy + tamoxifen and tamoxifen-only groups maintained levels similar to those at the beginning of treatment. Treatment with tamoxifen for 6 months after chemotherapy significantly reduced the plasma levels of NT-proBNP and did not change LVEF in women with breast cancer.
Cardiovascular risk profile; Adjuvant chemotherapy; Hormone therapy; NT-B-proBNP; Ecocardiography
Introduction
An increase in the incidence of cardiovascular disease (CVD) in women has been associated with a dramatic decrease in the secretion of ovarian hormones that occurs after menopause, owing to the loss of the cardioprotective action of estrogen (11. Yang XP, Reckelhoff JF. Estrogen, hormonal replacement therapy and cardiovascular disease. Curr Opin Nephrol Hypertens 2011; 20: 133-138, doi: 10.1097/MNH.0b013e3283431921.
https://doi.org/10.1097/MNH.0b013e328343...
). Hormone therapy with tamoxifen hydrochloride, a selective estrogen receptor modulator (SERM), interferes with the binding of estrogen to its receptor, thus acting as an estrogen agonist in some tissues, such as the heart, and antagonists in others, such as the breast (22. Jensen EV, Jordan VC. The estrogen receptor: a model for molecular medicine. Clin Cancer Res 2003; 9: 1980-1989.). In women with estrogen receptor (ER)-positive tumors, tamoxifen is recommended as an adjuvant systemic therapy that is typically administered after traditional surgery or chemotherapy treatments (33. Jordan VC. Tamoxifen (ICI46,474) as a targeted therapy to treat and prevent breast cancer. Br J Pharmacol 2006; 147 (Suppl 1): S269-S276.). Studies have demonstrated that the use of this drug in post-menopausal women with breast cancer not only reduces the risk of relapse but also has beneficial effects on the lipid profile, thereby exerting a cardioprotective effect by reducing serum levels of total cholesterol and low density lipoprotein (LDL) (44. Nordenskjold B, Rosell J, Rutqvist LE, Malmstrom PO, Bergh J, Bengtsson NO, et al. Coronary heart disease mortality after 5 years of adjuvant tamoxifen therapy: results from a randomized trial. J Natl Cancer Inst 2005; 97: 1609-1610, doi: 10.1093/jnci/dji342.
https://doi.org/10.1093/jnci/dji342...
,55. Ali S, Buluwela L, Coombes RC. Antiestrogens and their therapeutic applications in breast cancer and other diseases. Annu Rev Med 2011; 62: 217-232, doi: 10.1146/annurev-med-052209-100305.
https://doi.org/10.1146/annurev-med-0522...
).
In addition to hormone treatment, chemotherapy is also recommended in some cases of breast cancer. Studies have shown, however, that some agents, especially anthracyclines (e.g., doxorubicin), have short- and long-term cardiotoxic effects in addition to their cytotoxic and cytostatic effects (66. Jones LW, Haykowsky MJ, Swartz JJ, Douglas PS, Mackey JR. Early breast cancer therapy and cardiovascular injury. J Am Coll Cardiol 2007; 50: 1435-1441, doi: 10.1016/j.jacc.2007.06.037.
https://doi.org/10.1016/j.jacc.2007.06.0...
,77. Floyd JD, Nguyen DT, Lobins RL, Bashir Q, Doll DC, Perry MC. Cardiotoxicity of cancer therapy. J Clin Oncol 2005; 23: 7685-7696, doi: 10.1200/JCO.2005.08.789.
https://doi.org/10.1200/JCO.2005.08.789...
). Among those effects, increased production of reactive oxygen species (ROS) is particularly notable. ROS contribute to the development or aggravation of pre-existing CVD and can cause progressive myocardial damage in either a dose-dependent or a cumulative fashion (88. Mercuro G, Cadeddu C, Piras A, Dessi M, Madeddu C, Deidda M, et al. Early epirubicin-induced myocardial dysfunction revealed by serial tissue Doppler echocardiography: correlation with inflammatory and oxidative stress markers. Oncologist 2007; 12: 1124-1133, doi: 10.1634/theoncologist.12-9-1124.
https://doi.org/10.1634/theoncologist.12...
). Such damage can vary from an asymptomatic decrease in left ventricular ejection fraction (LVEF) to a risk of irreversible chronic cardiac failure (99. Barrett-Lee PJ, Dixon JM, Farrell C, Jones A, Leonard R, Murray N, et al. Expert opinion on the use of anthracyclines in patients with advanced breast cancer at cardiac risk. Ann Oncol 2009; 20: 816-827, doi: 10.1093/annonc/mdn728.
https://doi.org/10.1093/annonc/mdn728...
).
For the detection and prevention of cardiotoxicity at an early stage, the use of NT-pro-B-type natriuretic peptide (NT-proBNP) has also been investigated as a marker of clinical and subclinical cardiac failure. It is secreted by the ventricles in response to an increase in wall stress or ventricular dilatation owing to stretching of myocardial fibers in response to a volume or pressure overload (1010. Schnabel R, Lubos E, Rupprecht HJ, Espinola-Klein C, Bickel C, Lackner KJ, et al. B-type natriuretic peptide and the risk of cardiovascular events and death in patients with stable angina: results from the AtheroGene study. J Am Coll Cardiol 2006; 47: 552-558, doi: 10.1016/j.jacc.2005.09.039.
https://doi.org/10.1016/j.jacc.2005.09.0...
).
Thus, although the cardiotoxic effects of chemotherapy are well known, the influence of hormone therapy with tamoxifen on the deleterious effects of chemotherapy on the cardiovascular system have not been demonstrated. The objective of the present study was, therefore, to evaluate the effects of tamoxifen on an important marker of cardiac damage (NT-proBNP) and on ventricular cardiac function, and to examine possible correlations between NT-proBNP plasma levels and LVEF after chemotherapy in women with breast cancer.
Patients and Methods
Patients
This study was conducted between February 2008 and December 2011 in a group of 60 women with a confirmed history of breast cancer and who were undergoing chemotherapy and/or hormone therapy with tamoxifen. Patients between the ages of 40 and 60 years with early-stage breast cancer without metastases were eligible for inclusion in the study. Selected patients were clinically monitored by the same oncologist to ensure that there were no differences between the types of treatment. All women agreed to participate in the study by signing a free and informed consent form. Women who were smokers, had hypertension, diabetes or pre-existing CVD, and those with an LVEF <60% on the first echocardiogram (ECO) were excluded from the study. The study was approved by the Human Research Ethics Committee of Universidade Federal do Espírito Santo under registration No. 23/2008 and conducted according to the principles of the Declaration of Helsinki.
Study protocol
At the beginning and the end of the study, blood pressure (BP), heart rate (HR; HEM, 742, automatic, Omron, Japan), body weight and height (Welmy¯ scale, Brazil, with readings of 0.1 kg and 0.5 cm, respectively) were measured. Blood samples were collected at 0 (T0), 6 (T6), and 12 (T12) months of treatment to measure serum levels of NT-proBNP. Initiation of treatment (T0) was defined as the period between the diagnosis of breast cancer and the start of chemotherapy or hormone therapy. The participants were divided into 3 groups (Figure 1): a) women who received chemotherapy treatment for 6 months (n=23), b) women who received chemotherapy for 6 months followed by an additional 6 months of tamoxifen (n=21), and c) women who received tamoxifen only for 12 months (n=16). The daily doses of tamoxifen were 20 mg (tamoxifen hydrochloride, Sanofi-Aventis, France).
Group division: women who received chemotherapy treatment for 6 months; women who received chemotherapy for 6 months followed by an additional 6 months of tamoxifen; and women who received tamoxifen for 12 months. proBNP: pro-B-type natriuretic peptide; LVEF: left ventricular ejection fraction.
Biochemical analysis
All samples included in the first biochemical analysis (T0) were from patients who had not received any treatment for breast cancer or other cancers. After 12 h of fasting, 20 mL blood was collected from the antecubital vein using Vacutainer tubes (Becton Dickinson, USA). Plasma was separated by centrifugation at 2158 g for 10 min at 4°C. Blood for the NT-proBNP assay was collected into EDTA tubes and a validated, commercially available immunoassay (Elecsys¯ NT-proBNP, Roche Diagnostics, USA) was used according to the manufacturer's instructions.
Mean arterial pressure and body mass index
After measuring BP at T0 and T12, mean arterial pressure (MAP) was calculated as follows: MAP=(systolic BP+2×diastolic BP)/3. Body mass was also assessed at T0 and T12 using the body mass index (BMI), which was calculated as weight (kg)×height (m)-2.
Echocardiogram
Cardiac performance was assessed by ECO and LVEF at T0 and T12. The study participants laid on a medical stretcher in the supine position for approximately 10 min in a quiet, temperature-controlled room (20-22°C). All ECOs were performed and evaluated by the same echocardiography physician using a high-resolution device (Toshiba Aplio CV ultrasound system-SSA 790A/CV; Toshiba Corp., Japan). LVEF was obtained from the apical four- and two-chamber views according to Simpson's rule and was considered abnormal if <55%.
Statistical analysis
We used one-way analysis of variance (ANOVA) followed by a post-hoc Bonferroni test to compare means, with P<0.05 indicating statistical significance. Data are reported as means±SD. The correlation between variables (NT-proBNP and LVEF) was determined by Pearson's correlation coefficient (r), and r values were assigned according to Dancey and Reidy's (2005) classification (1111. Dancey CP, Reidy J. Statistics without maths for psychology: using SPSS for Windows. 3rd ed. London: Prentice Hall; 2005.). Statistical analyses were performed using the GB-Stat software (Version 6.5, Dynamic Microsystems Inc., USA).
Results
Patient characteristics
There were no differences in the average age and BMI among the chemotherapy-only (48±7 years and 25.6±4.17 kg/m2), chemotherapy + tamoxifen (47±7 years and 25.8±3.9 kg/m2), and tamoxifen-only (48±4 and 26.9±3.13 kg/m2) groups. Forty-three women (72%) had tumors smaller than 2 cm, 10 (17%) had tumors between 2 and 5 cm, and only 4 (11%) had tumors greater than 5 cm. Most women (65%) had received 5 chemotherapy sessions, and the most frequently used chemotherapy (73%) was a combination of 50-60 mg/m2 doxorubicin and 500-600 mg/m2 cyclophosphamide (Table 1).
Cardiovascular profile
There were no significant differences in MAP at T0 and T12 in the chemotherapy-only (92±6 and 93±10 mmHg, respectively), chemotherapy + tamoxifen (94±8 and 91±9 mmHg, respectively) and tamoxifen-only (93±9 and 91±6 mmHg, respectively) groups.
Likewise, there were no significant differences in HR between the study groups at T0 and T12 (74±5 and 73±4 bpm, in the chemotherapy group; 73±4 and 72±4 bpm in the chemotherapy + tamoxifen group; 72±4 and 73±5 bpm in the tamoxifen group).
Cardiovascular risk markers
Pro-B-type natriuretic peptide
Figure 2 shows the plasma levels of NT-proBNP at T0, T6 and T12. NT-proBNP levels were significantly increased in the chemotherapy group at T6 and T12 (69.06±11.7 and 42.35±12.9 pg/mL, respectively, P<0.01) relative to T0 (8.98±2.1 pg/mL). NT-proBNP levels were also increased in the chemotherapy + tamoxifen group at T6 (67.35±11.9 pg/mL, P<0.01) relative to T0 (9.48±1.79 pg/mL); however, at T12 the levels in this group were similar to those at T0 (11.39±2.74 and 9.48±1.79 pg/mL, respectively). No differences were detected in the plasma concentrations of NT-proBNP in the women in the tamoxifen-only group.
Plasma concentrations of pro-B-type natriuretic peptide (proBNP) in the groups treated with chemotherapy alone, chemotherapy followed by tamoxifen, and tamoxifen alone. Data are reported as means±SD. *P<0.01 compared to T0; #P<0.01 compared to T6 (Bonferroni test).
When we evaluated whether the different treatments differentially influenced NT-proBNP levels, we found that the chemotherapy and chemotherapy + tamoxifen groups had higher plasma levels of NT-proBNP (69.06±11.7 and 67.35±11.86 pg/mL, respectively, P<0.01) than the tamoxifen-only group (10.21±2.49 pg/mL) at T6. At T12, plasma levels of NT-proBNP remained high in the chemotherapy group (42.35±12.93 pg/mL, P<0.01), but were significantly decreased in the chemotherapy + tamoxifen group (11.39±2.74 pg/mL), in which the levels were similar to those of the tamoxifen-only group (8.35±1.66 pg/mL).
LVEF
Figure 3 shows cardiac performance as assessed by ECO, which was used to assess LVEF at T0 and T12. In the chemotherapy group, LVEF was significantly reduced at T12 (63.3±4.3%, P<0.01) relative to T0 (74.7±4.8%). In contrast, the LVEF values for the chemotherapy + tamoxifen and tamoxifen-only groups at T12 (76.2±3.7 and 75.6±4.1%, respectively) were similar to those at T0 (73.7±4.2 and 76.8±4.82%, respectively).
Cardiac performance assessed by left ventricular ejection fraction (LVEF) in the groups treated with chemotherapy alone, chemotherapy followed by tamoxifen, and tamoxifen alone. Data are reported as means±SD. *P<0.01 compared to T0; #P<0.01 compared to T12 in the group treated with chemotherapy alone (Bonferroni test).
Assessing the effects of treatment on cardiac performance revealed that the LVEF values were similar among the chemotherapy (74.7±4.79%), chemotherapy + tamoxifen (73.7±4.23%) and tamoxifen-only (76.8±4.8%) groups at T0. However, there was a reduction in LVEF in the chemotherapy group (63.3±4.3%, P<0.01) relative to the chemotherapy + tamoxifen (76.2±3.7%) and the tamoxifen-only (75.6±4.1%) groups at T12.
Correlation between NT-proBNP and LVEF
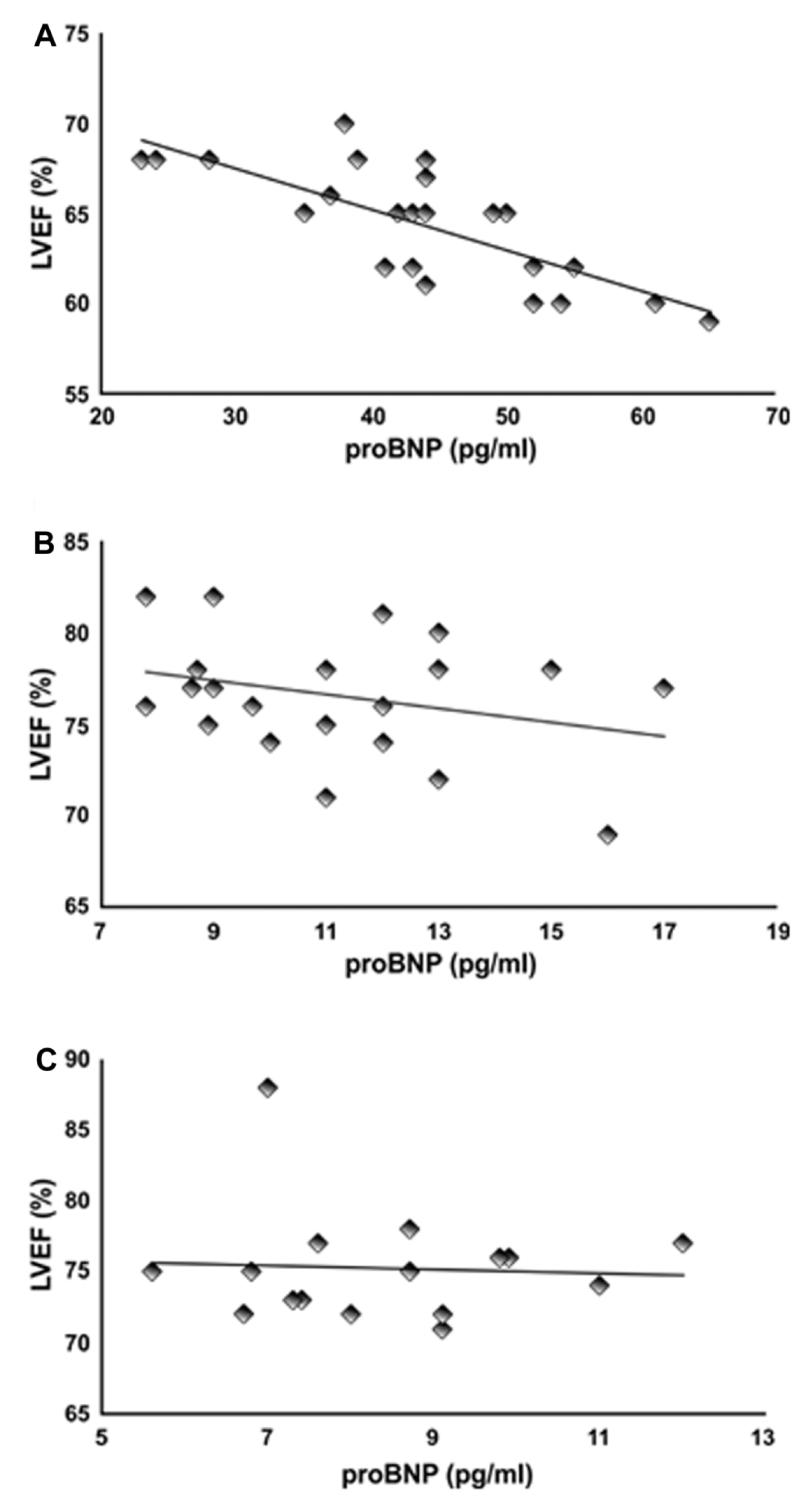
Pearson's correlation analysis showed a strong negative correlation (-0.77, P<0.01) between LVEF and plasma NT-proBNP levels in the chemotherapy group (Figure 4A) at T12. However, the negative correlation between LVEF and NT-proBNP levels in the chemotherapy + tamoxifen (Figure 4B) and tamoxifen-only (Figure 4C) groups was weak (-0.29 and -0.05, respectively), which is consistent with the finding that neither LVEF nor NT-proBNP differed significantly in those groups at T12.
Pearson's correlation (r) between measures of left ventricular ejection fraction (LVEF) and pro-B-type natriuretic peptide (proBNP) in the groups treated with chemotherapy alone (A), chemotherapy followed by tamoxifen (B), and tamoxifen alone (C).
Discussion
This study showed that tamoxifen treatment promotes significant changes both in the plasma concentration of an important marker associated with an increased risk of CVD (NT-proBNP) and in functional parameters of cardiac performance (LVEF) in women with breast cancer who have previously undergone chemotherapy. Previous studies had confirmed the cardiotoxic effects of chemotherapy (88. Mercuro G, Cadeddu C, Piras A, Dessi M, Madeddu C, Deidda M, et al. Early epirubicin-induced myocardial dysfunction revealed by serial tissue Doppler echocardiography: correlation with inflammatory and oxidative stress markers. Oncologist 2007; 12: 1124-1133, doi: 10.1634/theoncologist.12-9-1124.
https://doi.org/10.1634/theoncologist.12...
,99. Barrett-Lee PJ, Dixon JM, Farrell C, Jones A, Leonard R, Murray N, et al. Expert opinion on the use of anthracyclines in patients with advanced breast cancer at cardiac risk. Ann Oncol 2009; 20: 816-827, doi: 10.1093/annonc/mdn728.
https://doi.org/10.1093/annonc/mdn728...
), and we are not aware of any data in the literature on the cardiac actions of tamoxifen following chemotherapy.
Broeyer et al. (1212. Broeyer FJ, Osanto S, Ritsema van Eck HJ, van Steijn AQ, Ballieux BE, Schoemaker RC, et al. Evaluation of biomarkers for cardiotoxicity of anthracyclin-based chemotherapy. J Cancer Res Clin Oncol 2008; 134: 961-968, doi: 10.1007/s00432-008-0372-8.
https://doi.org/10.1007/s00432-008-0372-...
) demonstrated that patients who underwent anthracycline-based chemotherapy had higher levels of NT-proBNP combined with a decrease in myocardial contractility relative to the control population. An increase in plasma concentrations of this peptide is a reliable marker of ventricular dysfunction, because it is produced and secreted by the heart in response to wall stress or ventricular dilatation (1010. Schnabel R, Lubos E, Rupprecht HJ, Espinola-Klein C, Bickel C, Lackner KJ, et al. B-type natriuretic peptide and the risk of cardiovascular events and death in patients with stable angina: results from the AtheroGene study. J Am Coll Cardiol 2006; 47: 552-558, doi: 10.1016/j.jacc.2005.09.039.
https://doi.org/10.1016/j.jacc.2005.09.0...
). Corroborating those results, our data showed an increase in plasma concentrations of NT-proBNP combined with a reduction in LVEF 6 months after the cessation of chemotherapy. Furthermore, we found a strong negative correlation (-0.8; P<001) between LVEF and NT-proBNP in this group at T12.
No correlation was found between LVEF and NT-proBNP at T12 in the chemotherapy + tamoxifen and tamoxifen groups, where levels were similar to those at the beginning of treatment. We have shown that although chemotherapy promoted significant changes in plasma concentrations of NT-proBNP, tamoxifen treatment resulted in a decrease in plasma concentrations of this peptide and an improvement in LVEF 12 months after treatment (T12), thus lowering important risk factors for CVD after chemotherapy. In a recent study, we showed the effects of tamoxifen on other important markers of cardiovascular risk, such as an increase in apolipoprotein-A1 and a decrease in apolipoprotein-B100 and C-reactive protein in women with breast cancer after treatment with chemotherapy (1313. Romero WG, Da Silva FB, Borgo MV, Bissoli NS, Gouvea SA, Abreu GR. Tamoxifen alters the plasma concentration of molecules associated with cardiovascular risk in women with breast cancer undergoing chemotherapy. Oncologist 2012; 17: 499-507, doi: 10.1634/theoncologist.2011-0369.
https://doi.org/10.1634/theoncologist.20...
).
Cardiotoxicity induced by chemotherapy has been demonstrated by experimental studies in which a single dose of anthracycline induced the apoptosis of cardiac myocytes (1414. Arola OJ, Saraste A, Pulkki K, Kallajoki M, Parvinen M, Voipio-Pulkki LM. Acute doxorubicin cardiotoxicity involves cardiomyocyte apoptosis. Cancer Res 2000; 60: 1789-1792.). Moreover, increased plasma levels of natriuretic peptides and a subsequent reduction in myocardial contractility were observed in women with breast cancer given anthracycline-based chemotherapy followed by thoracic wall radiotherapy (1515. Heidenreich PA, Hancock SL, Vagelos RH, Lee BK, Schnittger I. Diastolic dysfunction after mediastinal irradiation. Am Heart J 2005; 150: 977-982, doi: 10.1016/j.ahj.2004.12.026.
https://doi.org/10.1016/j.ahj.2004.12.02...
,1616. Tassan-Mangina S, Codorean D, Metivier M, Costa B, Himberlin C, Jouannaud C, et al. Tissue Doppler imaging and conventional echocardiography after anthracycline treatment in adults: early and late alterations of left ventricular function during a prospective study. Eur J Echocardiogr 2006; 7: 141-146, doi: 10.1016/j.euje.2005.04.009.
https://doi.org/10.1016/j.euje.2005.04.0...
). Studies have shown that 20-51% of patients who undergo anthracycline-based chemotherapy develop some type of subclinical cardiac dysfunction (1717. Perez EA, Suman VJ, Davidson NE, Kaufman PA, Martino S, Dakhil SR, et al. Effect of doxorubicin + cyclophosphamide on left ventricular ejection fraction in patients with breast cancer in the North Central Cancer Treatment Group N9831 Intergroup Adjuvant Trial. J Clin Oncol 2004; 22: 3700-3704, doi: 10.1200/JCO.2004.03.516.
https://doi.org/10.1200/JCO.2004.03.516...
).
No changes in left ventricular mass were observed in any of the groups in our study during the entire treatment period, most likely because the 12-month duration of the study was not long enough to allow for the expression of structural changes that could be detected by echocardiography.
One hypothesis to explain the cardiovascular changes that occur with the use of chemotherapeutics is that their metabolism by hepatic mixed-function monooxygenases (cytochrome P-450 oxidase/reductase) leads to the generation of free radicals. The hydrogen peroxide (H2O2) produced binds to stable adriamycin-iron complexes, which are then degraded by catalases. Catalase, also known as hydroperoxidase, is stored in peroxisomes, which are present at low levels in human myocardial cells but not in other tissues, such as the kidney and liver (1818. Simmons TW, Jamall IS. Relative importance of intracellular glutathione peroxidase and catalase in vivo for prevention of peroxidation to the heart. Cardiovasc Res 1989; 23: 774-779, doi: 10.1093/cvr/23.9.774.
https://doi.org/10.1093/cvr/23.9.774...
). Additionally, the myocardium does not express glycoprotein P-170, which is active in the presence of a Ca/Mg-dependent ATPase (modulated by calmodulin) and is responsible for the rapid cellular elimination of chemotherapy drugs. The absence of such mechanisms contributes to myocardial injury following chemotherapy (1919. Dhalla NS, Saini HK, Tappia PS, Sethi R, Mengi SA, Gupta SK. Potential role and mechanisms of subcellular remodeling in cardiac dysfunction due to ischemic heart disease. J Cardiovasc Med 2007; 8: 238-250, doi: 10.2459/01.JCM.0000263489.13479.68.
https://doi.org/10.2459/01.JCM.000026348...
). The initial subcellular damage induces the remodeling of cell structures such as the sarcolemma, sarcoplasmatic reticulum, extracellular matrix, myofibrils, and mitochrondia. The subsequent contractile dysfunction and cardiac arrhythmia are strongly associated with cardiac remodeling and hypertrophy (1919. Dhalla NS, Saini HK, Tappia PS, Sethi R, Mengi SA, Gupta SK. Potential role and mechanisms of subcellular remodeling in cardiac dysfunction due to ischemic heart disease. J Cardiovasc Med 2007; 8: 238-250, doi: 10.2459/01.JCM.0000263489.13479.68.
https://doi.org/10.2459/01.JCM.000026348...
20. Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 2007; 39: 44-84, doi: 10.1016/j.biocel.2006.07.001.
https://doi.org/10.1016/j.biocel.2006.07...
-2121. Shiomi T, Tsutsui H, Matsusaka H, Murakami K, Hayashidani S, Ikeuchi M, et al. Overexpression of glutathione peroxidase prevents left ventricular remodeling and failure after myocardial infarction in mice. Circulation 2004; 109: 544-549, doi: 10.1161/01.CIR.0000109701.77059.E9.
https://doi.org/10.1161/01.CIR.000010970...
). Thus, the heart becomes vulnerable to lesions caused by ROS, with significant damage to myocardial cells and subsequent cardiac dysfunction during the process of cardiac injury (2020. Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 2007; 39: 44-84, doi: 10.1016/j.biocel.2006.07.001.
https://doi.org/10.1016/j.biocel.2006.07...
).
Another mechanism that might be responsible for reduced LVEF in the group treated with chemotherapy alone is a change in intracellular Ca2+, which regulates cardiomyocyte contractility. The generation of ROS and H2O2 in ischemic cardiomyocytes in the presence of intracellular iron and impaired intracellular Ca2+ homeostasis leads to a decrease in myocardial function due to Ca2+ uptake through L-type voltage-dependent Ca2+ channels and the release of large amounts of Ca2+ by the sarcoplasmic reticulum through ryanodine receptors (2222. Thorburn A, Frankel AE. Apoptosis and anthracycline cardiotoxicity. Mol Cancer Ther 2006; 5: 197-199, doi: 10.1158/1535-7163.MCT-06-CO1.
https://doi.org/10.1158/1535-7163.MCT-06...
). The resulting high cytoplasmic concentrations of Ca2+ in the cardiomyocytes contribute to ventricular dysfunction caused by chemotherapy (66. Jones LW, Haykowsky MJ, Swartz JJ, Douglas PS, Mackey JR. Early breast cancer therapy and cardiovascular injury. J Am Coll Cardiol 2007; 50: 1435-1441, doi: 10.1016/j.jacc.2007.06.037.
https://doi.org/10.1016/j.jacc.2007.06.0...
).
Our study shows that the use of tamoxifen in the therapeutic regimen promoted normalization of LVEF at T12, and that it remained similar to the LVEF in the group using only tamoxifen for the treatment of breast cancer. This drug may be acting through estrogen receptor beta (ERβ) to stimulate endothelial nitric oxide synthase (eNOS) activity (2323. Pedram A, Razandi M, Aitkenhead M, Levin ER. Estrogen inhibits cardiomyocyte hypertrophy in vitro. Antagonism of calcineurin-related hypertrophy through induction of MCIP1. J Biol Chem 2005; 280: 26339-26348, doi: 10.1074/jbc.M414409200.
https://doi.org/10.1074/jbc.M414409200...
) and promote antioxidant effects by increasing catalase activity, which is responsible for the degradation of H2O2 into H2O and O2 (2424. Day BJ. Catalase and glutathione peroxidase mimics. Biochem Pharmacol 2009; 77: 285-296, doi: 10.1016/j.bcp.2008.09.029.
https://doi.org/10.1016/j.bcp.2008.09.02...
). Tamoxifen treatment also promotes a significant increase in the antioxidant activity of glutathione and glutathione peroxidase, which are two cellular antioxidants involved in defense after cellular damage due to H2O2 and lipid peroxidation during myocardial injury (2525. Ek RO, Yildiz Y, Cecen S, Yenisey C, Kavak T. Effects of tamoxifen on myocardial ischemia-reperfusion injury model in ovariectomized rats. Mol Cell Biochem 2008; 308: 227-235, doi: 10.1007/s11010-007-9633-0.
https://doi.org/10.1007/s11010-007-9633-...
). These antioxidants are most likely the greatest contributors to the cardioprotective effects of tamoxifen in humans. Tamoxifen may also mimic the action of estrogen through ERβ present mainly in the mitochondria of cardiomyocytes, and mediate antihypertrophic effects through the inhibition of calcineurin activity, stimulation of MCIP1 via PI3 kinase, and inhibition of interstitial fibrosis (2626. Yang SH, Liu R, Perez EJ, Wen Y, Stevens SM Jr, Valencia T, et al. Mitochondrial localization of estrogen receptor beta. Proc Natl Acad Sci U S A 2004; 101: 4130-4135, doi: 10.1073/pnas.0306948101.
https://doi.org/10.1073/pnas.0306948101...
). The evaluation of NT-proBNP levels and LVEF before and after chemotherapy would be of great clinical relevance because it would reveal subclinical cardiac damage at an early stage (2727. Maisel A. The coming of age of natriuretic peptides: the emperor does have clothes! J Am Coll Cardiol 2006; 47: 61-64, doi: 10.1016/j.jacc.2005.10.011.
https://doi.org/10.1016/j.jacc.2005.10.0...
). The cardiotoxic potential of drugs used for breast cancer treatment is indicated by increases in plasma NT-proBNP peptide and prolonged QT intervals that have been found 24 h after the administration of anthracycline chemotherapy (1212. Broeyer FJ, Osanto S, Ritsema van Eck HJ, van Steijn AQ, Ballieux BE, Schoemaker RC, et al. Evaluation of biomarkers for cardiotoxicity of anthracyclin-based chemotherapy. J Cancer Res Clin Oncol 2008; 134: 961-968, doi: 10.1007/s00432-008-0372-8.
https://doi.org/10.1007/s00432-008-0372-...
).
Experimental studies in our laboratory have also demonstrated increased adenosine-induced vasodilatation in the coronary beds of spontaneously hypertensive female rats treated with tamoxifen, which was later associated with an improvement in cardiac performance (2828. Borgo MV, Lopes AB, Gouvea SA, Romero WG, Moyses MR, Bissoli NS, et al. Effect of tamoxifen on the coronary vascular reactivity of spontaneously hypertensive female rats. Braz J Med Biol Res 2011; 44: 786-792, doi: 10.1590/S0100-879X2011007500099.
https://doi.org/10.1590/S0100-879X201100...
).
In this clinical study, we have shown a clear correlation between high NT-proBNP levels and reduced cardiac performance due to the action of chemotherapeutic agents, a result that was reversed by the action of tamoxifen. Our results corroborate data from other studies showing that plasma levels of NT-proBNP are also elevated in patients with asymptomatic ventricular dysfunction (2929. Lerman A, Gibbons RJ, Rodeheffer RJ, Bailey KR, McKinley LJ, Heublein DM, et al. Circulating N-terminal atrial natriuretic peptide as a marker for symptomless left-ventricular dysfunction. Lancet 1993; 341: 1105-1109, doi: 10.1016/0140-6736(93)93125-K.
https://doi.org/10.1016/0140-6736(93)931...
), reduced LVEF, ventricular dysfunction, or coronary artery disease (3030. Davidson NC, Naas AA, Hanson JK, Kennedy NS, Coutie WJ, Struthers AD. Comparison of atrial natriuretic peptide B-type natriuretic peptide, and N-terminal proatrial natriuretic peptide as indicators of left ventricular systolic dysfunction. Am J Cardiol 1996; 77: 828-831, doi: 10.1016/S0002-9149(97)89176-X.
https://doi.org/10.1016/S0002-9149(97)89...
). Meinardi et al. (3131. Meinardi MT, van Veldhuisen DJ, Gietema JA, Dolsma WV, Boomsma F, van den Berg MP, et al. Prospective evaluation of early cardiac damage induced by epirubicin-containing adjuvant chemotherapy and locoregional radiotherapy in breast cancer patients. J Clin Oncol 2001; 19: 2746-2753.) also reported that plasma levels of NT-proBNP in breast cancer patients were elevated relative to the pre-treatment period when measured 1 year after anthracycline chemotherapy and thoracic wall radiotherapy.
This study demonstrated that the chronic use of tamoxifen can significantly reduce plasma concentrations of a biomarker of cardiac lesions (NT-proBNP) and does not change cardiac function (LVEF) after chemotherapy in women with breast cancer. Although structural changes were not detected during the study period, the positive correlation between elevated NT-proBNP levels and reduced LVEF indicates the presence of significant functional changes in cardiac performance. We suggest careful monitoring and clinical evaluation of cardiac function in women receiving anthracycline chemotherapy for breast cancer to reduce morbidity and mortality from cardiovascular complications.
Acknowledgments
The authors would like to thank Santa Rita de Cassia Hospital, Vitoria, ES, Brazil. Research supported by grants and fellowship from FAPES (Vitoria, ES, Brazil).
References
-
1Yang XP, Reckelhoff JF. Estrogen, hormonal replacement therapy and cardiovascular disease. Curr Opin Nephrol Hypertens 2011; 20: 133-138, doi: 10.1097/MNH.0b013e3283431921.
» https://doi.org/10.1097/MNH.0b013e3283431921 -
2Jensen EV, Jordan VC. The estrogen receptor: a model for molecular medicine. Clin Cancer Res 2003; 9: 1980-1989.
-
3Jordan VC. Tamoxifen (ICI46,474) as a targeted therapy to treat and prevent breast cancer. Br J Pharmacol 2006; 147 (Suppl 1): S269-S276.
-
4Nordenskjold B, Rosell J, Rutqvist LE, Malmstrom PO, Bergh J, Bengtsson NO, et al. Coronary heart disease mortality after 5 years of adjuvant tamoxifen therapy: results from a randomized trial. J Natl Cancer Inst 2005; 97: 1609-1610, doi: 10.1093/jnci/dji342.
» https://doi.org/10.1093/jnci/dji342 -
5Ali S, Buluwela L, Coombes RC. Antiestrogens and their therapeutic applications in breast cancer and other diseases. Annu Rev Med 2011; 62: 217-232, doi: 10.1146/annurev-med-052209-100305.
» https://doi.org/10.1146/annurev-med-052209-100305 -
6Jones LW, Haykowsky MJ, Swartz JJ, Douglas PS, Mackey JR. Early breast cancer therapy and cardiovascular injury. J Am Coll Cardiol 2007; 50: 1435-1441, doi: 10.1016/j.jacc.2007.06.037.
» https://doi.org/10.1016/j.jacc.2007.06.037 -
7Floyd JD, Nguyen DT, Lobins RL, Bashir Q, Doll DC, Perry MC. Cardiotoxicity of cancer therapy. J Clin Oncol 2005; 23: 7685-7696, doi: 10.1200/JCO.2005.08.789.
» https://doi.org/10.1200/JCO.2005.08.789 -
8Mercuro G, Cadeddu C, Piras A, Dessi M, Madeddu C, Deidda M, et al. Early epirubicin-induced myocardial dysfunction revealed by serial tissue Doppler echocardiography: correlation with inflammatory and oxidative stress markers. Oncologist 2007; 12: 1124-1133, doi: 10.1634/theoncologist.12-9-1124.
» https://doi.org/10.1634/theoncologist.12-9-1124 -
9Barrett-Lee PJ, Dixon JM, Farrell C, Jones A, Leonard R, Murray N, et al. Expert opinion on the use of anthracyclines in patients with advanced breast cancer at cardiac risk. Ann Oncol 2009; 20: 816-827, doi: 10.1093/annonc/mdn728.
» https://doi.org/10.1093/annonc/mdn728 -
10Schnabel R, Lubos E, Rupprecht HJ, Espinola-Klein C, Bickel C, Lackner KJ, et al. B-type natriuretic peptide and the risk of cardiovascular events and death in patients with stable angina: results from the AtheroGene study. J Am Coll Cardiol 2006; 47: 552-558, doi: 10.1016/j.jacc.2005.09.039.
» https://doi.org/10.1016/j.jacc.2005.09.039 -
11Dancey CP, Reidy J. Statistics without maths for psychology: using SPSS for Windows. 3rd ed. London: Prentice Hall; 2005.
-
12Broeyer FJ, Osanto S, Ritsema van Eck HJ, van Steijn AQ, Ballieux BE, Schoemaker RC, et al. Evaluation of biomarkers for cardiotoxicity of anthracyclin-based chemotherapy. J Cancer Res Clin Oncol 2008; 134: 961-968, doi: 10.1007/s00432-008-0372-8.
» https://doi.org/10.1007/s00432-008-0372-8 -
13Romero WG, Da Silva FB, Borgo MV, Bissoli NS, Gouvea SA, Abreu GR. Tamoxifen alters the plasma concentration of molecules associated with cardiovascular risk in women with breast cancer undergoing chemotherapy. Oncologist 2012; 17: 499-507, doi: 10.1634/theoncologist.2011-0369.
» https://doi.org/10.1634/theoncologist.2011-0369 -
14Arola OJ, Saraste A, Pulkki K, Kallajoki M, Parvinen M, Voipio-Pulkki LM. Acute doxorubicin cardiotoxicity involves cardiomyocyte apoptosis. Cancer Res 2000; 60: 1789-1792.
-
15Heidenreich PA, Hancock SL, Vagelos RH, Lee BK, Schnittger I. Diastolic dysfunction after mediastinal irradiation. Am Heart J 2005; 150: 977-982, doi: 10.1016/j.ahj.2004.12.026.
» https://doi.org/10.1016/j.ahj.2004.12.026 -
16Tassan-Mangina S, Codorean D, Metivier M, Costa B, Himberlin C, Jouannaud C, et al. Tissue Doppler imaging and conventional echocardiography after anthracycline treatment in adults: early and late alterations of left ventricular function during a prospective study. Eur J Echocardiogr 2006; 7: 141-146, doi: 10.1016/j.euje.2005.04.009.
» https://doi.org/10.1016/j.euje.2005.04.009 -
17Perez EA, Suman VJ, Davidson NE, Kaufman PA, Martino S, Dakhil SR, et al. Effect of doxorubicin + cyclophosphamide on left ventricular ejection fraction in patients with breast cancer in the North Central Cancer Treatment Group N9831 Intergroup Adjuvant Trial. J Clin Oncol 2004; 22: 3700-3704, doi: 10.1200/JCO.2004.03.516.
» https://doi.org/10.1200/JCO.2004.03.516 -
18Simmons TW, Jamall IS. Relative importance of intracellular glutathione peroxidase and catalase in vivo for prevention of peroxidation to the heart. Cardiovasc Res 1989; 23: 774-779, doi: 10.1093/cvr/23.9.774.
» https://doi.org/10.1093/cvr/23.9.774 -
19Dhalla NS, Saini HK, Tappia PS, Sethi R, Mengi SA, Gupta SK. Potential role and mechanisms of subcellular remodeling in cardiac dysfunction due to ischemic heart disease. J Cardiovasc Med 2007; 8: 238-250, doi: 10.2459/01.JCM.0000263489.13479.68.
» https://doi.org/10.2459/01.JCM.0000263489.13479.68 -
20Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 2007; 39: 44-84, doi: 10.1016/j.biocel.2006.07.001.
» https://doi.org/10.1016/j.biocel.2006.07.001 -
21Shiomi T, Tsutsui H, Matsusaka H, Murakami K, Hayashidani S, Ikeuchi M, et al. Overexpression of glutathione peroxidase prevents left ventricular remodeling and failure after myocardial infarction in mice. Circulation 2004; 109: 544-549, doi: 10.1161/01.CIR.0000109701.77059.E9.
» https://doi.org/10.1161/01.CIR.0000109701.77059.E9 -
22Thorburn A, Frankel AE. Apoptosis and anthracycline cardiotoxicity. Mol Cancer Ther 2006; 5: 197-199, doi: 10.1158/1535-7163.MCT-06-CO1.
» https://doi.org/10.1158/1535-7163.MCT-06-CO1 -
23Pedram A, Razandi M, Aitkenhead M, Levin ER. Estrogen inhibits cardiomyocyte hypertrophy in vitro Antagonism of calcineurin-related hypertrophy through induction of MCIP1. J Biol Chem 2005; 280: 26339-26348, doi: 10.1074/jbc.M414409200.
» https://doi.org/10.1074/jbc.M414409200 -
24Day BJ. Catalase and glutathione peroxidase mimics. Biochem Pharmacol 2009; 77: 285-296, doi: 10.1016/j.bcp.2008.09.029.
» https://doi.org/10.1016/j.bcp.2008.09.029 -
25Ek RO, Yildiz Y, Cecen S, Yenisey C, Kavak T. Effects of tamoxifen on myocardial ischemia-reperfusion injury model in ovariectomized rats. Mol Cell Biochem 2008; 308: 227-235, doi: 10.1007/s11010-007-9633-0.
» https://doi.org/10.1007/s11010-007-9633-0 -
26Yang SH, Liu R, Perez EJ, Wen Y, Stevens SM Jr, Valencia T, et al. Mitochondrial localization of estrogen receptor beta. Proc Natl Acad Sci U S A 2004; 101: 4130-4135, doi: 10.1073/pnas.0306948101.
» https://doi.org/10.1073/pnas.0306948101 -
27Maisel A. The coming of age of natriuretic peptides: the emperor does have clothes! J Am Coll Cardiol 2006; 47: 61-64, doi: 10.1016/j.jacc.2005.10.011.
» https://doi.org/10.1016/j.jacc.2005.10.011 -
28Borgo MV, Lopes AB, Gouvea SA, Romero WG, Moyses MR, Bissoli NS, et al. Effect of tamoxifen on the coronary vascular reactivity of spontaneously hypertensive female rats. Braz J Med Biol Res 2011; 44: 786-792, doi: 10.1590/S0100-879X2011007500099.
» https://doi.org/10.1590/S0100-879X2011007500099 -
29Lerman A, Gibbons RJ, Rodeheffer RJ, Bailey KR, McKinley LJ, Heublein DM, et al. Circulating N-terminal atrial natriuretic peptide as a marker for symptomless left-ventricular dysfunction. Lancet 1993; 341: 1105-1109, doi: 10.1016/0140-6736(93)93125-K.
» https://doi.org/10.1016/0140-6736(93)93125-K -
30Davidson NC, Naas AA, Hanson JK, Kennedy NS, Coutie WJ, Struthers AD. Comparison of atrial natriuretic peptide B-type natriuretic peptide, and N-terminal proatrial natriuretic peptide as indicators of left ventricular systolic dysfunction. Am J Cardiol 1996; 77: 828-831, doi: 10.1016/S0002-9149(97)89176-X.
» https://doi.org/10.1016/S0002-9149(97)89176-X -
31Meinardi MT, van Veldhuisen DJ, Gietema JA, Dolsma WV, Boomsma F, van den Berg MP, et al. Prospective evaluation of early cardiac damage induced by epirubicin-containing adjuvant chemotherapy and locoregional radiotherapy in breast cancer patients. J Clin Oncol 2001; 19: 2746-2753.
-
First published online.
Publication Dates
-
Publication in this collection
21 Nov 2014 -
Date of issue
Feb 2015
History
-
Received
15 June 2014 -
Accepted
17 Sept 2014