Abstracts
Cardiovascular diseases (CVD) are the main causes of death in the Western world. Among the risk factors that are modifiable by diet, for reducing cardiovascular disease risks, the total plasma concentrations of cholesterol, triglycerides, LDL-C, and HDL-C are the most important. Dietary measures can balance these components of the lipid profile thus reducing the risk of cardiovascular diseases. The main food components that affect the lipid profile and can be modified by diet are the saturated and trans fats, unsaturated fats, cholesterol, phytosterols, plant protein, and soluble fiber. A wealth of evidence suggests that saturated and trans fats and cholesterol in the diet raise the total plasma cholesterol and LDL-C. Trans fats also reduce HDL-C, an important lipoprotein for mediating the reverse cholesterol transport. On the other hand, phytosterols, plant proteins, isoflavones, and soluble fiber are protective diet factors against cardiovascular diseases by modulating plasma lipoprotein levels. These food components at certain concentrations are able to reduce the total cholesterol, TG, and LDL-C and raise the plasma levels of HDL-C. Therefore, diet is an important tool for the prevention and control of cardiovascular diseases, and should be taken into account as a whole, i.e., not only the food components that modulate plasma concentrations of lipoproteins, but also the diet content of macro nutrients and micronutrients should be considered.
cholesterol; lipoproteins; vegetable protein; fiber; fat; phytosterols
As doenças cardiovasculares (DCV) são a principal causa de morte no mundo ocidental. Entre os fatores de risco modificáveis pela dieta para reduzir os riscos de doenças cardiovasculares destacam-se as concentrações plasmáticas de colesterol total, triglicérides, LDL-C e HDL-C. Medidas dietéticas podem ser adotadas para equilibrar estes componentes do perfil lipídico e, assim, prevenir doenças cardiovasculares. Os principais componentes dos alimentos que afetam o perfil lipídico e cuja ingestão pode ser modificada pela dieta são as gorduras saturadas e gorduras trans, gorduras insaturadas, colesterol, fitosteróis, proteínas vegetais e fibras solúveis. Há uma riqueza de evidências de que gorduras saturadas e trans e colesterol da dieta aumentam as concentrações do colesterol plasmático total e do LDL-C. As gorduras trans também reduzem o HDL-C, uma lipoproteína importante para mediar o transporte reverso do colesterol. Por outro lado, fitosteróis, proteínas vegetais, isoflavonas e fibras solúveis são fatores da dieta protetores na doença cardiovascular, modulando os níveis plasmáticos de lipoproteína. Estes componentes dos alimentos em determinadas concentrações são capazes de reduzir o colesterol total, TG e LDL-C e elevar os níveis plasmáticos de HDL-C. A dieta é, portanto, uma importante ferramenta para a prevenção e controle das doenças cardiovasculares, sendo importante considerá-la como um todo, não apenas os componentes dos alimentos que modulam as concentrações plasmáticas de lipoproteínas, mas também o conteúdo da dieta de macronutrientes e micronutrientes.
colesterol; lipoproteínas; proteína vegetal; fibra; gorduras; fitoesteróis
REVIEW
Influence of food components on lipid metabolism: scenarios and perspective on the control and prevention of dyslipidemias
Influência de componentes dos alimentos no metabolismo lipídico: cenários e perspectiva no controle e prevenção de dislipidemias
Karoline de Macedo Gonçalves FrotaI; Andrea Carvalheiro Guerra MatiasII; José Alfredo Gomes ArêasIII,* * A quem a correspondência deve ser enviada
IDepartamento de Nutrição, Universidade Federal do Piauí - UFPI, Rua Cícero Eduardo, S/N, CEP 64600-000, Picos - PI, Brasil
IIInstituto de Ciências da Saúde , Universidade Paulista - UNIP, Campus Tatuapé, Rua Antônio de Macedo, 505,CEP 03087-040, São Paulo - SP, Brasil
IIIDepartamento de Nutrição, Faculdade de Saúde Pública, Universidade de São Paulo - USP, Av. Dr. Arnaldo, 715, CEP 01246-904, São Paulo - SP, Brasil, E-mail: jagareas@usp.br
ABSTRACT
Cardiovascular diseases (CVD) are the main causes of death in the Western world. Among the risk factors that are modifiable by diet, for reducing cardiovascular disease risks, the total plasma concentrations of cholesterol, triglycerides, LDL-C, and HDL-C are the most important. Dietary measures can balance these components of the lipid profile thus reducing the risk of cardiovascular diseases. The main food components that affect the lipid profile and can be modified by diet are the saturated and trans fats, unsaturated fats, cholesterol, phytosterols, plant protein, and soluble fiber. A wealth of evidence suggests that saturated and trans fats and cholesterol in the diet raise the total plasma cholesterol and LDL-C. Trans fats also reduce HDL-C, an important lipoprotein for mediating the reverse cholesterol transport. On the other hand, phytosterols, plant proteins, isoflavones, and soluble fiber are protective diet factors against cardiovascular diseases by modulating plasma lipoprotein levels. These food components at certain concentrations are able to reduce the total cholesterol, TG, and LDL-C and raise the plasma levels of HDL-C. Therefore, diet is an important tool for the prevention and control of cardiovascular diseases, and should be taken into account as a whole, i.e., not only the food components that modulate plasma concentrations of lipoproteins, but also the diet content of macro nutrients and micronutrients should be considered.
Keywords: cholesterol; lipoproteins; vegetable protein; fiber; fat; phytosterols.
RESUMO
As doenças cardiovasculares (DCV) são a principal causa de morte no mundo ocidental. Entre os fatores de risco modificáveis pela dieta para reduzir os riscos de doenças cardiovasculares destacam-se as concentrações plasmáticas de colesterol total, triglicérides, LDL-C e HDL-C. Medidas dietéticas podem ser adotadas para equilibrar estes componentes do perfil lipídico e, assim, prevenir doenças cardiovasculares. Os principais componentes dos alimentos que afetam o perfil lipídico e cuja ingestão pode ser modificada pela dieta são as gorduras saturadas e gorduras trans, gorduras insaturadas, colesterol, fitosteróis, proteínas vegetais e fibras solúveis. Há uma riqueza de evidências de que gorduras saturadas e trans e colesterol da dieta aumentam as concentrações do colesterol plasmático total e do LDL-C. As gorduras trans também reduzem o HDL-C, uma lipoproteína importante para mediar o transporte reverso do colesterol. Por outro lado, fitosteróis, proteínas vegetais, isoflavonas e fibras solúveis são fatores da dieta protetores na doença cardiovascular, modulando os níveis plasmáticos de lipoproteína. Estes componentes dos alimentos em determinadas concentrações são capazes de reduzir o colesterol total, TG e LDL-C e elevar os níveis plasmáticos de HDL-C. A dieta é, portanto, uma importante ferramenta para a prevenção e controle das doenças cardiovasculares, sendo importante considerá-la como um todo, não apenas os componentes dos alimentos que modulam as concentrações plasmáticas de lipoproteínas, mas também o conteúdo da dieta de macronutrientes e micronutrientes.
Palavras-chave: colesterol; lipoproteínas; proteína vegetal; fibra; gorduras; fitoesteróis.
1 Introduction
Cardiovascular diseases (CVD) are the main causes of death in the Western world. High concentrations of low-density lipoprotein (LDL) and high density lipoprotein (HDL) are major risk factors for the development of these diseases (NCEP, 2001). High levels of triglycerides or lipoprotein of very low density (VLDL) also represent risk factors, and the extent of this risk depends on low HDL-C and other interrelated risk factors, such as smoking, visceral obesity, hypertension, and insulin resistance (KRAUSS et al., 2000; NCEP, 2001).
Cardiovascular diseases are caused by atherosclerosis, a process characterized by the endothelial dysfunction and deposit of cholesterol into macrophages and smooth muscle cells in the endothelial wall due to high levels of LDL-C, lipoprotein (a), remnant lipoprotein, and low levels of HDL-C (SCHAEFER, 2002). Hypercholesterolemia is critical to the formation of atherosclerosis. The presence of heart disease in populations with average total cholesterol less than 180 mg.dL-1 (SCHAEFER, 2002) is unusual.
According to the report of strategies for reducing blood cholesterol, the National Cholesterol Education Program (2001) estimated that a 1% reduction in serum cholesterol reduces the risk of cardiovascular disease in 2 to 3%. This document recognizes that the dietary therapy is an important tool to reduce and control cholesterol levels. Diet can promote a gradual decrease in LDL cholesterol of about 10 to 13%. A small reduction, estimated in 1.8 mg.dL-1, in LDL-C reduces the risk of cardiovascular events (heart attack, stroke, hospitalization for unstable angina, or revascularization) in 1% (CANNON et al., 2006).
The purpose of this review is to present objectively the main food components that are involved in the modulation of blood cholesterol and lipoproteins and the consequent prevention or control of cardiovascular disease by dietary means.
2 Dietary Components
2.1 Dietary lipids
Fatty acids
Fatty acids are saponifiable organic substances, which can be classified as saturated or unsaturated depending on the absence or presence of double bond in the carbon chain, respectively. Unsaturated fatty acids are classified as monounsaturated or polyunsaturated depending on the number of unsaturations in the molecule. Most fats contain different proportions of each of these fatty acids, but they are usually classified according to the predominant type. Unsaturated fatty acids are predominantly of plant origin, whose oils are extracted from cereals, legumes, and fruits while the saturated fatty acids are predominantly of animal origin. However, coconut, cocoa, and palm oils are predominantly constituted of saturated fatty acids, whereas fish oil is rich in unsaturated fatty acids.
The concentration of plasma lipoproteins is modified by both the quantity and the quality of lipid consumed (saturated fatty acids, monounsaturated, polyunsaturated, and trans) and the balance of these fatty acids that is more relevant than the total amount of dietary fat (KASIM-KARAKAS et al., 2000; HU et al., 2001a).
Unsaturated fatty acids
Unsaturated fatty acids are represented by n-3 families: α-linolenic acid (ALA 18:3 n-3), eicosapentaenoic acid (EPA 20:5 n-3), and docosahexaenoic acid (DHA 22:6 n-3); n-6 families: linoleic acid (LA 18:2 n-6) and arachidonic acid (20:4 n-6); and n-9 families: oleic acid (cis18: 1n-9). Polyunsaturated fatty acids (PUFA), precursors of n-3 and n-6 families, linoleic, and linolenic acid, respectively, are defined as essential fatty acids because they are not synthesized endogenously by humans due to lack of desaturase enzymes, which are capable of inserting double bonds between carbon 3-4 and 6-7, as well as hydrogenase enzymes capable of removing such unsaturation.
The main sources of EPA and DHA are cold-water fish such as mackerel, sardines, herring, and salmon. Alfa linoleic acid (ALA) is found in green tissues of plants, soybean oil, flaxseed, and canola. The n-6 fatty acids are found in vegetable oils, except for coconut, cocoa, and palm oil. The main sources of oleic acid are olive, canola, avocado, and oilseeds (peanuts, chestnuts, walnuts, almonds) (SPOSITO et al., 2007).
The degree of saturation of dietary lipids plays an important role in modulating plasma cholesterol concentration, which determines the risk of cardiovascular diseases (FERNANDEZ; WEST, 2005). The isocaloric replacement of saturated fatty acids by PUFA reduces the plasma concentrations of total cholesterol and LDL-C. The PUFA have the disadvantage of inducing increased lipid oxidation and decreasing HDL-C when used in large quantities. The n-3 fatty acids (ALA, EPA, and DHA) promote the reduction of plasma triglycerides by decreasing hepatic synthesis of VLDL. Monounsaturated fatty acids (oleic acid) have the same effect on cholesterol without, however, reducing HDL-C and causing lipid oxidation (SPOSITO et al., 2007).
Several studies have shown that the consumption of EPA and DHA is inversely related to the incidence of cardiovascular diseases. However, this is not observed for the ALA because the ALA from plant sources is converted to EPA at very low rates, less than 5%, and the conversion to DHA is even lower, 0.5% (WANG et al., 2006; PLOURDE; CUNNANE, 2007). Goyens et al (2005) observed that this inefficient conversion of ALA into EPA is probably related to limited incorporation of ALA in the pool of liver phospholipids. The presence of linoleic acid (LA, n = 6) also affects this conversion. The ALA and LA compete for the δ-6/5-dessaturase enzyme in the process of desaturation and elongation. Thus, the incorporation of ALA into plasma and tissue lipids and its conversion into long chain fatty acids of the n-3 fatty acids (EPA and DHA) are influenced by the levels of LA (HU, 2001b)
The triglyceride-lowering effect by the EPA and DHA has been detailed in several studies. The dose-response relationship between EPA and DHA and the reduction of triglycerides is believed to be in the range from 2 to 4 g/day for reducing plasma triglycerides by 20 to 50% (KRIS-ETHERTON; HARRIS, 2002). Although the mechanism of triglyceride reduction by n-3 fatty acids is not completely understood, it is believed that it may be due to the lower hepatic synthesis, which is related to the inhibition of acyl-CoA: 1,2-diacylglycerol O-acyltransferase and/or the induction the peroxisome beta-oxidation in the liver (RUSTAN et al., 1988; JUMP, 2004).
Evidence of the cardioprotective effect of n-3 fatty acids was observed in 2 studies of intervention in secondary prevention (Diet and Reinfarction Trial - DART and Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto miocardico - GISSI). The DART study demonstrated a 29% reduction in total mortality at 2 years in patients recovered from myocardial infarction, advised to consume fat fish 2 times a week (BURR et al., 1989). After two years of study, it was observed that consumption of EPA increased by four times (2.4 g/week) compared to the intake of 0.6 g/week in the control group (BURR et al., 1989). The GISSI prevention study was designed to investigate the effect of fish oil on morbidity and mortality after a heart attack. Patients supplemented with 1 g/day fish oil (850 to 882 mg of EPA + DHA) had a 20% reduction in risk of total mortality and 30% reduction in risk of death from cardiovascular disease, and 47% reduction in risk of sudden death (GISSI, 1999; MARCHIOLI et al., 2002). These cardioprotective benefits have been largely attributed to the anti-arrhythmic effect of EPA and DHA, but are also related to the improvement of other cardiovascular risk factors.
Based on evidences, the Food and Drug Administration (FDA) approved in November 2004 the administration of omega-3 fatty acids to reduce triglyceride levels in hypertriglyceridemic adults (> 500 mg.dL-1) as an adjunct to diet (FDA, 2004). The American Heart Association recommends, for the general population, the consumption of fish twice a week (KRAUSS et al., 2000). For individuals with a history of CVD, the recommendation is 1 g of EPA and DHA per day, and for hypertriglyceridemic individuals, 2 to 4 g/day (-KRIS-ETHERTON; HARRIS, 2002). The Institute of Medicine of the National Academies recommends that 0.6 to 1.2% of total energy come from ALA, and more than 10% of this recommendation should be of DHA and EPA. The inclusion of 1 to 2 teaspoons per day of the flaxseed oil or 1 tablespoon per day of ground flaxseed satisfy the current recommendations of ALA. Additionally, 500 mg per day of EPA and DHA is recommended to reduce the risk of cardiovascular disease, which is equivalent to 2 servings of fish per week, as recommended by the AHA (KATCHER et al., 2009).
Saturated fatty acids
Saturated fatty acids are found predominantly in animal products like butter, lard, and beef fat, but can also be obtained by the hydrogenation process of vegetable oils. Another important source of saturated fatty acids is coconut, cocoa and palm oils. It is estimated that the relative ability of the saturated fatty acids to promote the increase of serum cholesterol is twice greater than lowering the cholesterol promoted by PUFA (JAMA, 1972). Therefore, the dietary recommendations aimed at reducing the consumption of saturated fatty acids arise out of these observations.
Note that not all saturated fatty acids alter serum lipids in the same way. It is recognized that the fatty acids of short chains (6:0 to 10:0) and stearic acid (18:0) cause little change in serum cholesterol levels (Keys et al., 1965). This is because the fatty acids of short chains are absorbed directly into the hepatic portal vein, and the stearic acid is rapidly converted to oleic acid (cis-18: 1n-9) (BONAMONE; GRUND, 1988). Saturated fatty acids of intermediate chains, lauric acid (12:0), myristic acid (14:0), and palmitic acid (16:0) consumption promotes increasing concentrations of plasma cholesterol, probably because they influence the reduction in the rate of LDL-C catabolism with little or no effect on its production rates (GRUNDY; DENKA, 1990; MATTHAN et al., 2004).
The American Heart Association recommends the consumption of less than 7% of total dietary energy from SFA (LICHTENSTEIN et al., 2006). The replacement of SFA by unsaturated fat, carbohydrates, or proteins is an effective alternative to reduce total cholesterol and LDL-C (van HORN et al., 2008).
Trans fatty acids
The main sources of trans fatty acids (TFA) are partially hydrogenated fats and products made with these fats, such as bakery products and fried foods. A small proportion of trans fatty acids (TFA) of the diet originates from fat of ruminant animals found mainly in meat and whole milk; vaccenic acid (trans 18:1 n-7) and elaidic acid (trans 18:1 n-9) represent most of the TFAS originated from the partial hydrogenation of vegetable oils.
The consumption of trans fatty acids adversely affects plasma lipids and lipoproteins, increasing LDL-C and lowering HDL-C. The TFA consumption increases the levels of fasting triglycerides as in comparison to the same amounts of MUFA and PUFA consumption (MOZAFFARIAN; CLARKE, 2009). In an observational study, it was found that the consumption of 2.6 to 3.6 g/day of TFA was associated with higher levels of LDL-C, lower levels of HDL-C, and higher LDL-C/HDL-C ratios (SUN et al., 2007). The mechanisms by which TFA promote these changes seems to be due to the increased activity of the protein carrier of cholesteryl esters (CETP), which may contribute to the increase in the production of LDL-C and low production of HDL-C when the TFA is consumed (van TOL et al., 1995).
Ascherio et al. (1999) reviewed some studies that evaluated the effect of consumption of TFA on plasma levels of LDL and HDL and suggested that 2% increase in the intake of trans fatty acids may be responsible to an increase of 0.1 in the LDL-C/HDL-C ratio. It has been observed that the increase of one unit (1.0) in this relationship is associated with an elevation in about 53% of the risk of cardiovascular disease (STAMPFER et al., 1991). Trans isomers may also interfere with the biological functions of LA and ALA, which compete for the δ-6/5-desaturase enzyme. This enzyme is responsible for the desaturation of ALA in the process of converting the same into EPA and DHA (KINSELLA et al., 1981).
According to the IV Brazilian Guidelines on Dyslipidemia and Prevention of Atherosclerosis, there is no consensus on the maximum allowed amount of trans fat in the diet. However, it is recommended that the intake of this fat should be less than 1% of total calories (SPOSITO et al., 2007). The recommendation of ANVISA (2003) is that the consumption of TFAs should not exceed 2 g/day for adults. The Brazilian Society of Cardiology, under the guidelines of the American Heart Association, recommends that the consumption of total fat is between 25 to 35% of the total energy of the diet. The consumption of SFA should be <7%, polyunsaturated fatty acids <10%, monounsaturated fatty acids, and <20% of the total energy of the diet (SPOSITO et al., 2007).
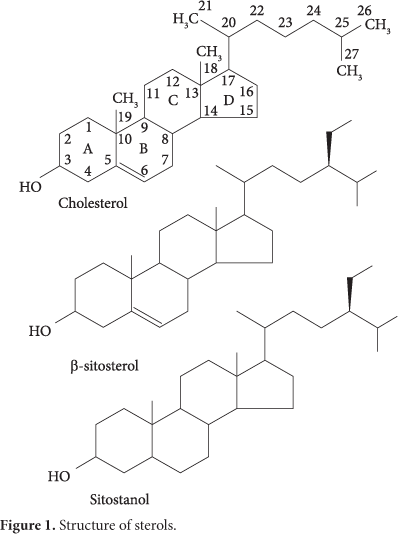
Dietary sterols
Sterols are unsaponifiable lipids containing a perhi-dropenteno-phenanthrene core. Sterols can be of animal or vegetable origin, being cholesterol and phytosterol their major components, respectively (Figure 1).
The difference between cholesterol and phytosterols is that phytosterols have an ethyl group attached to carbon 24. The hydrogenation of the double bond 5-6 β-sitosterol converts it into sitostanol.
Cholesterol
Cholesterol is a sterol found in animal products such as eggs, organ meats, whole milk and its derivatives, sausages, cold cuts, skinless poultry and seafood (shrimp, oysters, shellfish, octopus, lobster), and pig meats.
Epidemiological studies and clinical trials have shown that dietary cholesterol is positively associated with the risk of CVD through an increase in total cholesterol and LDL-C; although the main determinant of the increase in LDL-C in humans is the consumption of saturated and trans fatty acids (HOWEL et al., 1997). It is difficult to estimate the absolute effect of dietary cholesterol on plasma lipid concentrations because of the high degree of individual variability of plasma responses (KATAN; BEYENE, 1987). However, a review study of McNamara (2000) shows that the additional consumption of 100 mg per day of cholesterol increases the total cholesterol by approximately 2.4 mg.dL-1 and LDL-C by 2.1 mg.dL-1.
Among the mechanisms by which dietary cholesterol changes the plasma lipid concentrations, it is featured the inhibition of the activity of LDL receptors on the membrane. Thus, VLDL-C and LDL-C are not efficiently removed from plasma and the effect of the increasing rate of the conversion of VLDL into LDL (GRUNDY; DENKA, 1990) is added. The American Heart Association recommends that cholesterol intake stays below 300 mg per day (LICHTENSTEIN et al., 2006), and the National Cholesterol Education Program ATP III recommends the consumption of less than 200 mg per day to maximize the reduction of cholesterol by diet (NCEP, 2002). To achieve this recommendation, the substitution of meat and other animal products for plant foods such as fruits, vegetables, beans, peanuts and seeds is recommended since they do not contain cholesterol (LICHTENSTEIN et al., 2006). This is a dietary strategy indicated for patients at high risk, who are seeking significant reduction in total cholesterol and LDL-C.
Phytosterols
Phytosterols, sterols, and stanols are structurally similar to cholesterol. Stanols are saturated sterols and less abundant in nature than sterols (Figure 1). Phytosterols are present in small amounts in nuts, seeds, and vegetable oils and perform analogous structural functions to cholesterol in animal tissues. More than 40 phytosterols have been identified, but sitosterol, campesterol, and stigmasterol are the most abundant. The main sterol found in foods is the β-sitosterol. While cholesterol is absorbed in the gastrointestinal tract in approximately 50%, stanols and sterols are absorbed to a lesser extent: 10 to 15% of campesterol and campestanol, and 4 to 7% of sitosterol and 1% of sitostanol (HEINEMANN et al., 1993).
Studies have shown that phytosterols reduce LDL-C in a dose-dependent way. When they are consumed in quantities above 2.5 g per day, they present a lowering effect on plasma LDL-C in normocholesterolemic and in hypercholesterolemic adults (POLI et al., 2008). A meta-analysis of 41 trials reported that the intake of 2 g per day of phytosterols reduces by 10% LDL-C and a higher intake confers little additional benefit (KATAN et al., 2003). The mechanism of action by which phytosterols reduce plasma cholesterol is by competing in the absorption of cholesterol in the intestine. After the hydrolysis of phytosterol esters in the small intestine, free sterols replace cholesterol in the micelles and thus reduce the absorption of cholesterol, but the exact mechanism is still unknown. It is believed that the effects of stanols and sterols involve the membrane transport proteins ABCG5 and ABCG8, which selectively pump the phytosterols of enterocytes to the intestinal lumen (BERGE et al., 2000).
A balanced diet with adequate amounts of vegetables provides approximately 200 to 400 mg of phytosterols (SPOSITO et al., 2007). However, the intake of 2 g/day is necessary, according to NCEP (2002), for the average reduction of 10 to 15% of LDL-C (GRUNDY, 2005). Phytosterols do not influence plasma levels of HDL-C and triglycerides (SPOSITO et al., 2007).
2.2 Vegetable protein, phytoestrogens and bioactive peptides
Vegetable protein
The potential of soy protein to reduce cholesterol has been studied extensively in animals and humans. A meta-analysis reviewed 38 controlled clinical studies and suggested that the average intake of 47 g/day of isolated or textured soy protein resulted in a significant reduction of 9.3% of total cholesterol, 12.9% LDL-C and 10.5% triglycerides, with no significant changes in HDL-C, in comparison to the group that received animal protein (ANDERSON et al., 1995). This change in total cholesterol and LDL-C was dependent on the concentration of cholesterol at baseline, and it is greater the hypocholesterolemic effect in individuals with higher cholesterol levels at baseline.
A more recent meta-analysis of 10 studies published between 1995 and 2002, including 959 individuals whose cholesterol levels at baseline were between 209 and 255 mg.dL-1 and with greater uniformity in experimental designs, showed a weak correlation between the change in LDL-C and the consumption of soy isoflavones associated (r = -0.33, P = 0.14). An average of 52 mg of isoflavones associated with 36 g of soy protein daily resulted in a 4% reduction in LDL-C and a 3% increase in HDL-C (WEGGEMANS; TRAUTWEIN, 2003). Thus, it seems that the hypocholesterolemic effect of soy is relatively modest on subjects with mild hypercholesterolemia. A study with women in hypercholesterolemic postmenopausal to assess the effect of soy protein containing varying amounts of isoflavones (56 or 90 mg/day) showed that the ingestion of 40 g of soy protein with isoflavones was able to reduce the TC: HDL-C at 10% for the group receiving soy protein with 56 mg of isoflavones (IPS56) and 8% for the group receiving soy protein with 90 mg of isoflavones (IPS90), while the casein group increased by 3%. With regard to HDL-C, a significant increase of 5.2 and 3.6% for IPS56 and IPS90 groups, respectively, and a reduction of 4.1% in the group receiving casein (BAUM et al., 1998) was observed. Since there was no difference in the lipoprotein concentration of the 2 groups that received soy protein, this can be interpreted in two ways: either the isoflavone is not involved in the effect on humans, or the isoflavone has reached a plateau with respect to the ability to influence the blood lipid profile. The mechanism of action of soy protein in reducing blood cholesterol has not been fully elucidated. The most accepted proposal is that there is a change in the hepatic metabolism with an increase in the clearance of low density lipoprotein (LDL) and lipoproteins, and very low density (VLDL) by hepatocytes, due to increased transcription of LDL receptors (LOVATI et al., 2000; CHO et al., 2008; AZIZ et al., 2008). In addition, some evidence has shown that isoflavones found in soy stimulate the activity of proteins in reducing blood cholesterol although isoflavones alone do not possess this reduction ability (JOINT HEALTH CLAIMS INITIATIVE, 2002; FUKUI et al., 2004).
So far, only soy protein health claim has been approved, both nationally and internationally. ANVISA and Joint Health Claims Initiative (2002) endorsed the claim that the inclusion of at least 25 g of soy protein in the diet can help reduce blood cholesterol. The two bodies, respectively, also recommended that soy consumption should be associated with a balanced diet and a healthy lifestyle, and a diet low in saturated fatty acids. The main sources of soy in the diet are soybean oil, soy bean curd (tofu), soy sauce, soy flour, soy milk, and soy protein concentrate.
Other legume seed proteins are being studied in order to evaluate their hypocholesterolemic effect. The consumption of protein isolated from cowpea in hypocholesterolemized hamsters with 20% casein + 13% saturated fat + excess cholesterol could reduce plasma total cholesterol by 20% and non-HDL cholesterol by 22%, in comparison to animals fed casein as the protein source (FROTA et al., 2008).
Another grain that had been recently studied is amaranth, a pseudoceral, whose protein isolate (IPA) proved quite effective in reducing cholesterol in hypercholesterolemized hamsters. The ingestion of 20% amaranth protein or 20% casein + 10% amaranth protein resulted in a reduction of total cholesterol of 48 and 27%, respectively (MENDONÇA et al., 2009).
These results illustrated how plant proteins often show a protective effect against hypercholesterolemia. However, this effect should not be considered alone. The ideal situation is to associate the intake of vegetable protein to the diet, and/or feeding a low intake of saturated and trans fat and, to the highest possible extent, with limited sources of animal protein, which are associated with saturated fat and cholesterol.
Isoflavones
Isoflavones are a class of phytoestrogens, a group of phytochemical from plant with non-steroidal estrogen-like activity. The chemical structure of 17 β-estradiol and equol (a metabolite of the phytoestrogen) are very similar (Figure 2).
The presence of the phenol ring and the distance between the hydroxyl group, which are identical in estradiol and isoflavones, are considered prerequisites for estrogen action (SETCHELL, 1998). It is this chemical and structural similarity of endogenous estrogen that leads to the hypothesis that isoflavones may be responsible for the hypocholesterolemic effect of soy (SETCHELL, 1998). Isoflavones are widespread in legumes and are present in greater amounts in soy. Isoflavones are associated with the protein, and it is depleted when the protein isolation is obtained by alcohol extraction (SETCHELL, 1998; WANG; MURPHY, 1994).
Genistein, daidzein and glycitein are the major soy isoflavones. Genistein and daidzein are conjugated to sugar as glycosides. Isoflavone glycosides cannot be absorbed, unless they are hydrolyzed by intestinal microbiota or by in vitro fermentation (MINIELLO et al., 2003).
In a review of the effects of soy on health, the 4th International Symposium on the Role of Soy in Preventing and Treating Chronic Diseases, Messina,Gardne and Barnes (2002) concluded that the consumption of up to 10g of soy protein rich in isoflavones per day may be associated with health benefits. In contrast with studies using soy protein, research using purified isoflavone supplements has consistently reported no effect on plasma lipoprotein LDL-C (NESTEL et al., 1999; DEWELL; HOLLENBECK; BRUCE, 2002). It is suggested that the potential beneficial effect of soy may be due to the presence of the metabolite equol produced by intestinal microbiota from daidzein, the main isoflavone in soy (SETCHELL; BROWN; LYDEKING-OLSEN, 2002). It has been shown that equol has a higher affinity for estrogen receptors than daidzein. It seems that about 50 to 70% of the general population can produce equol, possibly due to a difference in species of the microbiota of the large intestine (SETCHELL; BROWN; LYDEKING-OLSEN, 2002). However, the publications so far do not support the effect of isolated soy isoflavones on the lipid profile (FUKUI et al., 2004, HALL et al., 2006). Hall et al. (2006) studying the effect of isolated isoflavones on lipid biomarkers of risk for cardiovascular disease found no significant beneficial effect on the concentration of plasma lipids.
Bioactive peptides
Biologically active peptides or functional peptides are peptides derived from food, which also present physiological effects to the body (YAMAMOTO et al., 2003). They are inactive in the original protein, but once released, they function as regulatory components with activity similar to hormones (YAMAMOTO et al., 2003). They can be released from the original protein during gastrointestinal digestion or during food processing. The active peptides generally contain from 2 to 20 amino acid residues and are partially or totally resistant to hydrolysis. They can be absorbed, and thus exert a systemic effect.
Since isolated soy protein appears to be the bioactive component in soy responsible for the beneficial effect on lipid metabolism, a new line of research was developed recently, in which the soy peptides are studied. Several studies have analyzed the effect of polypeptide subunit of 7S globulin or β-conglycinin. Lovati et al. (2000) have identified a polypeptide sequence of the 7S globulin protein, present in soybean (subunit α', corresponding to residues 127-150), which are capable of increasing the regulation of LDL receptors in Hep G2 cells in "in vitro" studies. Moreover, they suggest that this mechanism may explain why the effect of soy protein on LDL-C is substantially lower in normocholesterolemic individuals and higher in individuals with higher level of cholesterol, assuming that the down-regulation of LDL receptors is proportional to the degree of hypercholesterolemia (LOVATI et al., 2000).
Duranti et al. (2004) first demonstrated that biologically active peptides, derived from the α' subunit of soybean 7 S globulin, are able to modulate cholesterol homeostasis by up-regulation of LDL receptors produced "in vivo". They detected a marked increase in β-VLDL receptor in hypercholesterolemic rats treated with the α' subunit.
A recent study developed with liver cells (Hep G2) treated with small peptides from soy 7S subunit showed changes in lipid metabolism with a 30% reduction in the accumulation of apoB-100 (MOCHIZUKI et al., 2009).
2.3 Fiber
The fibers are complex undigested carbohydrates classified according to their solubility into soluble and insoluble (SLAVIN, 1987). Soluble fibers are represented by pectin, guar gum, β-glucan and psyllium. Observational studies have shown a negative association between the consumption of soluble fiber and CVD, and clinical trials show the lowering effect of soluble fiber on LDL-C (PEREIRA et al., 2004; THEUWISSEN; MENSINK, 2008). The primary mechanism of the soluble fiber action for reducing LDL-C is via the absorption of cholesterol and bile acids (LIA et al., 1995; MARLETT et al., 1994).
Soluble fibers are found in fruits (apple, pear, and guava), oats, barley, legumes (beans, chickpeas, lentils, and peas), and in oat bran food that is rich in soluble fiber (THEUWISSEN; MENSINK, 2008). Clinical studies show that the use of extract of soluble fiber (β-glucan from barley) in patients with moderate dyslipidemia causes a reduction of LDL-C from 9 to 15% (BEHALL; SCHOLFIELD; HALLFRISCH, 2004; KEENAN et al., 2007).
A meta-analysis evaluated the effectiveness of various soluble fibers and the conclusion was that oats, psyllium, pectin, and guar gum had a similar effect in reducing the total cholesterol and LDL-C (BROWN et al., 1999). This meta-analysis reveals that each gram of oat reduced the total cholesterol and LDL-C by 1.42 and 1.23 mg.dL-1, respectively, each gram of psyllium by 1.10 and 1.11 mg.dL-1, respectively, each gram of pectin by 2.69 and 1.96 mg.dL-1, respectively, and each gram of guar gum by 1.13 and 1.20 mg.dL-1, respectively.
The recommended intake of total fiber for adults is 20 to 30 g/day; 5 to 10 g of these should be soluble fiber, as an additional measure to reduce cholesterol and therefore, to reduce the cardiovascular risk (NCEP, 2001; SPOSITO et al., 2007).
3 Final remarks
In order to have the desirable blood cholesterol levels and lipoprotein profiles, changes in lifestyle are recommended, which include primarily the establishment of a healthy diet. This diet should limit the intake of foods with high amounts of saturated fat, trans fat, and cholesterol, replacing them with grains and unsaturated fats from vegetable, fish, legumes, and seeds. Food products may be improved by aggregating legume, amaranth, quinoa, and other whole grains or their proteins. Fiber, especially the soluble one, is another supplement that will provide a healthier food product than the original formulation. The choice of appropriate foods for a healthy diet is based on scientific evidence for each food component. However, it is important to emphasize the maintenance of balanced dietary patterns, i.e. the suitable combination of macro and micronutrients, which cannot be neglected.
Recebido para publicação em 4/1/2010
Aceito para publicação em 30/3/2010 (003941)
- ANDERSON, J. W.; JOHNSTONE, B. M.; COOK, N. Meta-analysis of the effects of soy protein intake on serum lipids. New England Journal of Medicine, v. 333, n. 5, p. 276-282, 1995.
- ASCHERIO, A. et al. Trans fatty acids and coronary heart disease. New England Journal of Medicine, v. 340, n. 25, p. 1994-1998, 1999.
- AZIZ, A. et al. Impact of dietary protein on lipid metabolism in hamsters is source-dependent and associated with changes in hepatic gene expression. British Journal of Nutrition, v. 100, n. 3, p. 503-511, 2008.
- BAUM, J. A. et al. Long-term intake of soy protein improves blood lipid profiles and increases mononuclear cell low-density-lipoprotein receptor messenger RNA in hypercholesterolemic, postmenopausal women. American Journal of Clinical Nutrition, v. 68, n. 3, p. 545-551, 1998.
- BEHALL, K. M.; SCHOLFIELD, D. J.; HALLFRISCH, J. Lipids significantly reduced by diets containing barley in moderately hypercholesterolemic men. Journal of the American College of Nutrition, v. 23, n. 1, p. 55-62, 2004.
- BERGE, K. E. et al. Accumulation of dietary cholesterol in sitosterolemia caused by mutations in adjacent ABC transporters. Science, v. 290, n. 5497, p. 1771-1775, 2000.
- BONAMONE, A.; GRUNDY, S. M. Effect of dietary stearic acid on plasma cholesterol and lipoprotein levels. New England Journal of Medicine, v. 318, n. 19, p. 1244-1248, 1988.
- BRASIL. Agência Nacional de Vigilância Sanitária - ANVISA. Alegações de propriedade funcional aprovada Brasília, DF, 2003. Disponível em: <http://portal.anvisa.gov.br/wps/portal/anvisa/home/alimentos?tax=Alimentos>
- BRASIL. Agência Nacional de Vigilância Sanitária - ANVISA Gordura Trans Brasília, DF, 2009. Disponível em: <http://www.anvisa.gov.br/alimentos/gordura_trans.pdf>. Acesso em: 20 jul. 2009.
- BROWN, L. et al. Cholesterol-lowering effects of dietary fiber: a meta-analysis. American Journal of Clinical Nutrition, v. 69, n. 1, p. 30-42, 1999.
- BURR, M. L. et al. Effects of changes in fat, fish, and fibre intakes on death and myocardial reinfarction: diet and reinfarction trial (DART). Lancet, v. 334, n. 8666, p. 757-761, 1989.
- CANNON, C. P. et al. Meta-analysis of cardiovascular outcomes trials comparing intensive versus moderate statin therapy. Journal of the American College of Cardiology, v. 48, n. 3, p. 438-445, 2006.
- CHO, S. J.; JUILLERAT, M. A.; LEE, C. H. Identification of LDL-receptor transcription stimulating peptides from soybean hydrolysate in human hepatocytes. Journal of Agricultural and Food Chemistry, v. 56, n. 12, p. 4372-4376, 2008.
- DEWELL, A.; HOLLENBECK, C. B.; BRUCE, B. The effects of soy-derived phytoestrogens on serum lipids and lipoproteins in moderately hypercholesterolemic postmenopausal women. Journal of Clinical Endocrinology and Metabolism, v. 87, n. 1, p. 118-121, 2002.
- DIET and coronary heart disease: a council statement. Journal of American Medical Association, v. 222, n. 13, p. 1647, 1972.
- DURANTI, M. et al. The alpha' subunit from soybean 7S globulin lowers plasma lipids and upregulates liver beta- VLDL receptors in rats fed a hypercholesterolemic diet. Journal of nutrition, v. 134, n. 6, p. 1334-1339, 2004.
- FERNANDEZ, M. L.; WEST, K. L. Mechanisms by which Dietary Fatty Acids Modulate Plasma Lipids. Journal of Nutrition, v. 135, n. 9, p. 2075-2078, 2005.
- FOOD DRUG ADMINISTRATION - FDA. FDA Announces Qualified Health Claims for Omega-3 Fatty Acids New Hampshire, 2004. Disponível em: <http://www.fda.gov/newsEvents/Newsroom/PressAnnouncements/2004/ucm83>
- FROTA, K. M. G. et al. Cholesterol-lowering properties of whole cowpea seed and its protein isolate in hamsters. Journal of Food Science, v. 73, n. 9, p. H235-H240, 2008.
- FUKUI, K. et al. Ethanol washing does not attenuate the hypocholesterolemic potential of soy protein. Nutrition, v. 20, n. 11-12, p. 984-990, 2004.
- GISSI - PREVENZIONE INVESTIGATORS. Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin e after myocardial infarction: results of the GISSI-Prevenzione trial. Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto miocardico. Lancet, v. 354, n. 9177, p. 447-455, 1999.
- GOYENS, P. L. et al. Compartmental modeling to quantify alphalinolenic acid conversion after longer term intake of multiple tracer boluses. Journal of Lipid Research, v. 46, n. 7, p. 1474-1483, 2005.
- GRUNDY, S. M. Stanol esters as a component of maximal dietary therapy in the National Cholesterol Education Program Adult Treatment Panel III report. American Journal of Cardiology, v. 96, n. 1A, p. 47D-50D, 2005.
- GRUNDY, S. M.; DENKE, M. A. Dietary influences on serum lipids and lipoproteins. Journal of Lipid Research, v. 31, n. 7, p. 1149-1172, 1990.
- HALL, W. L. et al. Soy-isoflavone-enriched foods and markers of lipid and glucose metabolism in postmenopausal women: interactions with genotype and equol production. American Journal of Clinical Nutrition, v. 83, n. 3, p. 592- 600, 2006.
- HEINEMANN, T.; AXTMANN, G.; von BERGMANN, K. Comparison of intestinal absorption of cholesterol with different plant sterols in man. European Journal of Clinical Investigation, v. 23, n. 12, p. 827-831, 1993.
- HOWELL, W. H. et al. Plasma lipid and lipoproteína responses to dietary fat and cholesterol: a meta-analysis. American Journal of Clinical Nutrition, v. 65, n. 6, p. 1747-1764, 1997.
- HU, F. B. The Balance Between n-6 and n-3 Fatty Acids and the Risk of Coronary Heart Disease. Nutrition, v. 17, n. 9, p. 741-742, 2001b.
- HU, F. B.; MANSON, J. E.; WILLETT, W. C. Types of dietary fat and risk of coronary heart disease: a critical review. Journal of the American College of Nutrition, v. 20, n. 1, p. 5-19, 2001a.
- JUMP, D. B. Fatty acid regulation of gene transcription. Critical Reviews in Clinical Laboratory Sciences, v. 41, n. 1, p. 41-78, 2004.
- KASIM-KARAKAS, S. E. et al. Changes in plasma lipoproteins during low-fat, high carbohydrate diets: effects of energy intake. American Journal of Clinical Nutrition, v. 71, n. 6, p. 1439-1447, 2000.
- KATAN, M. B. et al. Efficacy and safety of plant stanols and sterols in the management of blood cholesterol levels. Mayo Clinic Proceedings, v. 78, n. 8, p. 965-78, 2003.
- KATAN, M. B.; BEYNEN, A. C. Characteristics of human hypo- and hyper responders to dietary cholesterol. American Journal of Epidemiology, v. 125, n. 3, p. 387-399, 1987.
- KATCHER, H. I. et al. Lifestyle Approaches and Dietary Strategies to Lower LDL-Cholesterol and Triglycerides and Raise HDL-Cholesterol. Endocrinology and Metabolism Clinics of North America, v. 38, n. 1, p. 45-78, 2009.
- KEENAN, J. M. et al. The effects of concentrated barley b-glucan on blood lipids in a population of hypercholesterolaemic men and women. British Journal of Nutrition, v. 97, n. 6, p. 1162-1168, 2007.
- KEYS, A.; ANDERSON, J. T.; GRANDE, F. Serum cholesterol response to changes in the diet. IV. Particular saturated fatty acids in the diet. Metabolism, v. 14, n. 7, p. 776-787, 1965.
- KINSELLA, J. E. et al. Metabolism of trans fatty acid enphasys on the effects of trans, trans-octadecadienoate on lipid composition, essential fatty acid and prostaglandins: an overview. American Journal of Clinical Nutrition, v. 34, n. 10, p. 2307-2318, 1981.
- KRAUSS, R. M.; ECKEL, R. H.; HOWARD, B. AHA Dietary Guidelines: revision 2000: a statement for healthcare professionals from the Nutrition Committee of the American Heart Association. Circulation, v. 102, n. 18, p. 2284-2299, 2000.
- KRIS-ETHERTON, P. M.; HARRIS, W. S.; APPEL, L. J. Nutrition Committee. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation, v. 106, n. 21, p. 2747-2757, 2002.
- LIA, A. et al. Oat beta-glucan increases bile acid excretion and a fiber-rich barley fraction increases cholesterol excretion in ileostomy subjects. American Journal of Clinical Nutrition, v. 62, n. 6, p. 1245-1251, 1995.
- LICHTENSTEIN, A. H. et al. Diet and lifestyle recommendations revision 2006: a scientific statement from the American Heart Association Nutrition Committee. Circulation, v. 114, n. 1, p. 82-96, 2006.
- LOVATI, M. R. et al. Soy protein peptides regulate cholesterol homeostasis in Hep G2 cells. Journal of Nutrition, v. 130, n. 10, p. 2543-2549, 2000.
- MARCHIOLI, R. et al. Early protection against sudden death by n-3 polyunsaturated fatty acids after myocardial infarction: time-course análisis of the results of the Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto Miocardico (GISSI) - Prevenzione. Circulation, v. 105, n. 16, p. 1897-1903, 2002.
- MARLETT, J. A. et al. Mechanism of serum colesterol reduction by oat bran. Hepatology, v. 20, n. 6, p. 1450-1457, 1994.
- MATTHAN, N. R. et al. Dietary hydrogenated fat increases high density lipoprotein apoA-I catabolism and decreases low-density lipoprotein apoB-100 catabolism in hypercholesterolemic women. Arteriosclerosis, Thrombosis and Vascular Biology, v. 24, n. 6, p. 1092-1097, 2004.
- MCNAMARA, D. J. The impact of egg limitations on coronary heart disease risk: do the numbers add up? Journal of the American College of Nutrition, v. 19, n. 5, p. 540S-548S, 2000.
- MENDONÇA, S. et al. Amaranth protein presents cholesterol-lowering effect. Food Chemistry, v. 116, n. 3, p. 738-742, 2009.
- MESSINA, M.; GARDNER, C.; BARNES, S. Gaining insight into the health effects of soy but a long way still to go: commentary on the fourth international symposium on the role of soy in preventing and treating chronic disease. Journal of Nutrition, v. 132, n. 3, p. 547-551, 2002.
- MINIELLO, V. L. et al. Soy-based formulas and phyto-oestrogens: a safety profile. Acta Paediatrica, v. 91, n. 9, p. 93-100, 2003. (Supplement 441).
- MOCHIZUKI, Y. et al. Changes in Lipid Metabolism by Soy β-Conglycinin-Derived Peptides in HepG2 Cells. Journal of Agricultural and Food Chemistry, v. 57, n. 4, p. 1473-1480, 2009.
- MOZAFFARIAN, D.; CLARKE, R. Quantitative effects on cardiovascular risk factors and coronary heart disease risk of replacing partially hydrogenated vegetable oils with other fats and oils. European Journal of Clinical Nutrition, v. 63, p. S22-S33, 2009.
- NATIONAL CHOLESTEROL EDUCATION PROGRAM - NCEP. Executive Summary of the Third Report of the Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment panel III). Journal of American Medical Association, v. 285, n. 19, p. 2486-2497, 2001.
- NATIONAL CHOLESTEROL EDUCATION PROGRAM - NCEP. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Circulation, v. 106, n. 25, p. 3143-3421, 2002.
- NESTEL, P. J. et al. Isoflavones from red clover improve systemic arterial compliance but not plasma lipids in menopausal women. Journal of Clinical Endocrinology and Metabolism, v. 84, n. 3, p. 895-898, 1999.
- PEREIRA, M. A. et al. Dietary fiber and risk of coronary heart disease: a pooled analysis of cohort studies. Archives of Internal Medicine, v. 164, n. 4, p. 370-376, 2004.
- PLOURDE, M.; CUNNANE, S. C. Extremely limited synthesis of long chain polyunsaturates in adults: implications for their dietary essentiality and use as supplements. Applied Physiology, Nutrition and Metabolism, v. 32, n. 4, p. 619-634, 2007.
- POLI, A. et al. Non-pharmacological control of plasma cholesterol levels. Nutrition, Metabolism and Cardiovascular Disease, v, 18, n. 2, p. S1-S16, 2008.
- RUSTAN, A. C. et al. Eicosapentaenoic acid reduces hepatic synthesis and secretion of tri acylglycerol by decreasing the activity of acyl-coenzyme A:1,2-diacylglycerol acyl transferase. Journal of Lipid Research, v. 29, n. 11, p. 1417-1426, 1988.
- SCHAEFER, E. J. Lipoproteins, nutrition, and heart disease. American Journal of Clinical Nutrition, v. 75, n. 2, p.191-212, 2002.
- SETCHELL, K. D. R. Phytoestrogens: the biochemistry, physiology, and implications for human health of soy isoflavones. American Journal of Clinical Nutrition, v. 68, n. 6, p. 1333S-1346S, 1998.
- SETCHELL, K. D. R.; BROWN, N. M.; LYDEKING-OLSEN, E. The clinical importance of the metabolite equol - a clue to the effectiveness of soy and its isoflavones. Journal of Nutrition, v. 132, n. 12, p. 3577-3584, 2002.
- SLAVIN, J. L. Dietary fiber: classification, chemical analyses, and food sources. Journal of the American Dietetic Association, v. 87, n. 9, p. 1164-1171, 1987.
- SOYA PROTEIN ASSOCIATION - SPA. Does the inclusion of 25g soya protein per day as part of a diet low in saturated fat help to reduce blood cholesterol?: A Health Claim Submission by the Soya Protein Association to the JHCI For a Generic Soya Protein Health Claim In The UK London, 2002. Disponível em: <http://www.jhci.org.uk/approv/FINALJHCI%20Soya%20Submission.pdf>. Acesso em: 10 maio 2009.
- SPOSITO, A. C. et al. IV Diretriz Brasileira Sobre Dislipidemias e Prevenção da Aterosclerose Departamento de Aterosclerose da Sociedade Brasileira de Cardiologia. Arquivos Brasileiros de Cardiologia, v. 88, n. 1, p. 2-19, 2007.
- STAMPFER, M. J. et al. A prospective-study of cholesterol, apolipoproteins, and the risk of myocardial-infarction. New England Journal of Medicine, v. 325, n. 6, p. 373-381, 1991.
- SUN, Q. et al. A prospective study of trans fatty acids in erythrocytes and risk of coronary heart disease. Circulation, v. 115, n. 14, p. 1858-1865, 2007.
- THEUWISSEN, E.; MENSINK, R. P. Water-soluble dietary fibers and cardiovascular disease. Physiology and Behaviour, v. 94, n. 2, p. 285-292, 2008.
- VAN HORN, L. et al. The Evidence for Dietary prevention and treatment of cardiovascular disease. Journal of the American Dietetic Association, v. 108, n. 2, p. 287-331, 2008.
- VAN TOL, A. et al. Dietary trans fatty acids increase serum cholesterylester transfer protein activity in man. Atherosclerosis, v. 115, n. 1, p. 129-134, 1995.
- WANG, C. et al. N-3 Fatty acids from fish or fish-oil supplements, but not a-linolenic acid, benefit cardiovascular disease outcomes in primary- and secondary-prevention studies: a systematic review. American Journal of Clinical Nutrition, v. 84, n. 1, p. 5-17, 2006.
- WANG, H.; MURPHY, P. A. Isoflavone content in commercial soybean foods. Journal of Agricultural and Food Chemistry, v. 42, n. 8, p. 1666-1673, 1994.
- WEGGEMANS, R. M.; TRAUTWEIN, E. A. Relation between soy-associated isoflavones and LDL and HDL cholesterol concentrations in humans: a meta-analysis. European Journal of Clinical Nutrition, v. 57, n. 8, p. 940-946, 2003.
- YAMAMOTO, N.; EJIRI, M.; MIZUNO, S. Biogenic peptides and their potential use. Current Pharmaceutical Design, v. 9, n. 9, p. 1345-1355, 2003.
Publication Dates
-
Publication in this collection
05 July 2010 -
Date of issue
May 2010
History
-
Received
04 Jan 2010 -
Accepted
30 Mar 2010