Abstracts
The development of processed foods requires the understanding of the phenomena that dictate the ingredient interactions normally used in food formulations, as well as the effects of the numerous operations involved in the processing of the final product. In ice creams, sugars are responsible for taste, but they also affect the freezing behavior and viscosity of processed mixes. Components such as fats influence mechanical properties, melting resistance, and palatability of final products. The objective was to study the technological effects of different sugars and fats on the structure of ice cream formulations. Fructose syrup was used as a substitute for glucose syrup in blends with sucrose, and palm fat was employed as an alternative to hydrogenated vegetable fat. The analysis of variance showed significant differences in chemical compositions. Hygroscopicity of fructose syrup increased the solids content in the formulations. Melting rate and overrun were higher in products added with this sugar. Palm fat caused changes in melting ranges of formulations, and higher melting rate was observed in the combination of palm fat and fructose syrup.
ice cream; fructose syrup; palm fat; melting behavior
O desenvolvimento de alimentos processados requer a compreensão do fenômeno que dita as interações entre os ingredientes, normalmente usadas em formulações alimentícias, como os efeitos de numerosas operações envolvidas no processamento do produto final. Em sorvetes, açúcares são responsáveis pelo sabor, porém também afetam o comportamento de congelamento e viscosidade das misturas. Componentes como gorduras têm impacto nas propriedades mecânicas, resistência à fusão e palatabilidade dos produtos finais. O objetivo foi estudar os efeitos tecnológicos de diferentes açúcares e gorduras na estrutura das formulações de sorvetes. Xarope de frutose foi usado como substituto de glicose em misturas com sacarose, e gordura de palma foi empregada como alternativa à gordura vegetal hidrogenada. Análises de variância mostraram significante diferença nas composições químicas. A higroscopicidade do xarope de frutose aumentou o conteúdo de sólidos nas formulações. A taxa de fusão e o overrun foram maiores nos produtos com açúcar. A gordura de palma ocasionou trocas nas taxas de fusão das formulações e a maior taxa de fusão foi observada pela combinação entre gordura de palma e xarope de frutose.
sorvete; xarope de frutose; gordura de palma; comportamento de fusão
ORIGINAL
Effect of different sweetener blends and fat types on ice cream properties
Efeito de diferentes misturas de adoçantes e tipos de gordura nas propriedades de sorvetes
Elieste da Silva Junior; Suzana Caetano da Silva Lannes* * A quem a correspondência deve ser enviada
Departamento de Tecnologia Bioquímico-Farmacêutica, Faculdade de Ciências Farmacêuticas, Universidade de São Paulo - USP, Av. Prof. Lineu Prestes, 580, B. 16, Cidade Universitária, CEP 05508-900, São Paulo - SP, Brasil, E-mail: scslan@usp.br
ABSTRACT
The development of processed foods requires the understanding of the phenomena that dictate the ingredient interactions normally used in food formulations, as well as the effects of the numerous operations involved in the processing of the final product. In ice creams, sugars are responsible for taste, but they also affect the freezing behavior and viscosity of processed mixes. Components such as fats influence mechanical properties, melting resistance, and palatability of final products. The objective was to study the technological effects of different sugars and fats on the structure of ice cream formulations. Fructose syrup was used as a substitute for glucose syrup in blends with sucrose, and palm fat was employed as an alternative to hydrogenated vegetable fat. The analysis of variance showed significant differences in chemical compositions. Hygroscopicity of fructose syrup increased the solids content in the formulations. Melting rate and overrun were higher in products added with this sugar. Palm fat caused changes in melting ranges of formulations, and higher melting rate was observed in the combination of palm fat and fructose syrup.
Keywords: ice cream; fructose syrup; palm fat; melting behavior.
RESUMO
O desenvolvimento de alimentos processados requer a compreensão do fenômeno que dita as interações entre os ingredientes, normalmente usadas em formulações alimentícias, como os efeitos de numerosas operações envolvidas no processamento do produto final. Em sorvetes, açúcares são responsáveis pelo sabor, porém também afetam o comportamento de congelamento e viscosidade das misturas. Componentes como gorduras têm impacto nas propriedades mecânicas, resistência à fusão e palatabilidade dos produtos finais. O objetivo foi estudar os efeitos tecnológicos de diferentes açúcares e gorduras na estrutura das formulações de sorvetes. Xarope de frutose foi usado como substituto de glicose em misturas com sacarose, e gordura de palma foi empregada como alternativa à gordura vegetal hidrogenada. Análises de variância mostraram significante diferença nas composições químicas. A higroscopicidade do xarope de frutose aumentou o conteúdo de sólidos nas formulações. A taxa de fusão e o overrun foram maiores nos produtos com açúcar. A gordura de palma ocasionou trocas nas taxas de fusão das formulações e a maior taxa de fusão foi observada pela combinação entre gordura de palma e xarope de frutose.
Palavras-chave: sorvete; xarope de frutose; gordura de palma; comportamento de fusão.
1 Introduction
Besides ability and experience of the researcher, the development of processed foods also requires a proper understanding of the major phenomena that dictate the interactions among different ingredients to achieve the desired characteristics of the final product (SILVA JUNIOR et al., 2010).
Ice cream has been identified as a three-component foam made up of a network of fat globules and ice crystals dispersed in a high viscosity aqueous phase (AIME et al., 2001). According to Adapa et al. (2000a), the composition of ice cream varies depending on the market requirements and processing conditions. Although the quality of the final product depends largely on processing and freezing parameters, the ingredients also play an important role. The physical structure of ice cream affects its melting rate and hardness although the specific relationships have not all been worked out (MUSE; HARTEL, 2004).
Sugars are an economic path to increase the solid content in ice creams. Usually, blends containing sucrose and glucose or fructose are employed as partial substitutes to sucrose (ARBUCKLE, 2000). The sweetener used in formulations dictates the freezing point depression of the mix, which can be correlated to the equilibrium ice phase volume using a freezing point depression curve and the draw temperature (DREWETT; HARTEL, 2007); it can also contribute to the viscosity of the unfrozen phase (MILLER-LIVNEY; HARTEL, 1997).
The development of structure in ice cream is often attributed to the macromolecules present in the ice cream mix - milk fat, protein, and complex carbohydrates. Milk fat interacts with other ingredients to develop the texture, mouthfeel, creaminess, and overall sensations of lubricity. Typically, ice cream contains 10 to 16% of fat, and its type and amount influence the characteristics of the resultant products by affecting their rheological properties (ADAPA et al., 2000b).
As suggested by some authors, the fat content can influence the size of the ice crystals. Fat globules could mechanically impede the ice crystal growth (TRGO; KOXHOLT; KESSLER, 1999). Since each type of fat exhibits a specific polymorphism function of its triacylglycerol composition, the thermal behavior of fats during the processing of ice cream may influence the physicochemical properties of the intermediate and final products (GRANGER et al., 2005).
In this study, the chemical, physical, and mechanical properties of four chocolate ice cream formulations produced using different sugar blends and fat types were evaluated.
2 Materials and methods
2.1 Ice cream formulation and production
Formulas for the mixes are shown in Table 1. Sucrose, whole milk, and chocolate powdered were purchased from the local market. Hydrogenated vegetable fat (Glaze, Cargill), palm fat (370 S, Agropalma), glucose syrup (Excell 1040, 40 DE, Corn Products), fructose syrup (80.9 °Brix, Getec), and emulsifier/stabilizer (Cremodan, SIM-B, Danisco) were used. After weighing, the mixtures were preheated, pasteurized at 82 °C for 25 seconds, cooled at 10 °C, mixed using a Fisaton 713D mixer at 850 rpm, and aged overnight at 4 °C. Aeration and freezing were performed in a scraped surface heat exchanger. The ice cream formulations were packed in plastic containers (V = 2 L) and stored at -18 °C.
2.2 Physicochemical analysis
Determinations of residual moisture, proteins, lipids, and ashes content were performed in triplicate, according to AOAC (ASSOCIATION..., 1995). The Nifext fraction (carbohydrates) was obtained by the calculation of the difference in other analyzed fractions. Measurements of pH were obtained by direct immersion of the electrode in in the samples. Factor 6.38 was used to calculate the ratio nitrogen/protein, according to the TBCAUSP-Table of food composition (UNIVERSIDADE DE SÃO PAULO, 2008). The energetic content was calculated from the caloric coefficient of proteins, lipids, and carbohydrates as 16.7 kJ.g-1, 37.6 kJ.g-1, and 16.7 kJ.g-1, respectively.
2.3 Melting rate
According to the methodology proposed by Lee and White (1991), the ice cream samples (100.0 ± 2.0 g; -12 °C) were placed on a mesh attached to a graduated cylinder and maintained in a controlled temperature chamber at 25 °C, under constant humidity (≈ 50%). The dripped volume was measured for 45 minutes every 5 minutes. The data recorded were use to determine the melting rate (mL/minute).
2.4 Overrun
Overrun was measured after batch freezing by carefully filling a capsule of known volume with the ice cream and weighing. Comparisons of the weight of the original ice cream mix allow calculation of the overrun.
2.5 Statistical analysis
The statistical evaluation was performed using Statistica 6.0/2001 (StatSoft, Tulsa, OK, USA). Tuckey HSD test, at a 5% significance level, was used to define the difference among formulas.
3 Results and discussion
Physicochemical characteristics are presented in Table 2.
Ice creams containing fructose syrup presented solid content higher than that of the products added with glucose syrup. The hygroscopicity attributed to the fructose molecules was pointed as the most probable cause for this behavior. Trgo, Koxholt and Kessler (1999) found that a reduction in the dry matter results in an increase in the freezing temperature. According to the authors, higher water content leads to a longer hardening time since more heat of fusion has to be eliminated. No effect of formulation was observed in the proteins and lipid content. The differences in the caloric contents of the ice creams were due to variations in content of carbohydrates. The pH of the ice creams varied with the different treatments and was systematically higher than that found in the literature (ARBUCKLE, 2000), probably as a consequence of the alkalinity normally measured in chocolate. A significant difference in pH was found among the formulations. Segall and Goff (2002) reported that to control the pH in an emulsion containing proteins is very important because when values are comprised between 4 and 6, flocculation and creaming can occur. However, the same reduction in pH is desired, and thus the proteins get near to an isoelectric point and the repulsion between the groups with the same electric charge increases protein-protein interactions increasing the formation of a second layer around the fat globules increasing the emulsion stability.
3.1 Melting behavior
The effects of composition on the melting properties of ice creams are shown in Figure 1. Melting times were independent of protein content for all ice creams but were affected by sugar type. The formulations added with fructose syrup, generally, presented higher melting rate when compared to products made with glucose syrup. A probable cause for this behavior was the impact of fructose syrup on the freezing point of the mixes. On the other hand, the slower thawing of the ice creams produced with glucose syrup was associated to the size and molar weight of the chains of this monosaccharide, which result in more entanglement among the macromolecules leading to an increase in viscosity. The melting rates (curve slopes) were obtained by linear regression (R2 > 0.98). The initial melting times (intersection to "xx" axis) was similar for all ice creams. The lower and higher melting times were obtained for the formulations based on the hydrogenated vegetable fat/glucose syrup and palm fat/fructose syrup, respectively.
The structural arrangement of the ice creams reflects their thermal properties. The optimal formation of fat structure in ice cream is responsible for the slowness of meltdown after hardening (GOFF, 2002) and increases with the solid fat content. The formulation factors, such as emulsifiers, have been shown to influence the extent of fat destabilization (CHANG; HARTEL, 2002). Moreover, Muse and Hartel (2004) found important relationships between the sugar type applied in formulations and the physical behavior of products. They reported that ice creams made with 20 DE corn syrup generally had the highest levels of destabilized fat, which was attributed to the high viscosity and percentage of ice during freezing, which leads to high shear forces and enhanced fat destabilization. According to the authors, a greater extent of destabilized fat increases the resistance to flow of the serum phase as ice melted, which leads to slower meltdown. On the other hand, ice creams with low levels of destabilized fat (up to 30%) melted quickly and did not retain their shape. Thus, from the results (Figure 1), it was possible to conclude that for a given sweetener blend, products made with palm fat had lower levels of destabilization as suggested by the melting point behavior.
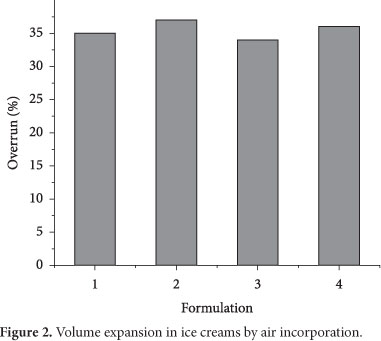
3.2 Overrun
Ice cream and related products are generally aerated and characterized as frozen foams. The gas phase volume varies greatly from a maximum of 50% to a minimum of 10-15% (GOFF, 2002). As seen in Figure 2, the overrun was comprised between 35 and 40%. According to Moretti (1979), the incorporation of air in mixtures that contain cocoa solids, which have higher viscosity, is more difficult. In addition, Chang and Hartel (2002) reinforce that other formulation factors such as fat, emulsifier, and stabilizer contents as well as processing conditions (whipping temperature and freezing power) can influence air cells development. Comparing the ice creams, it seems that the fat type did not influence the air incorporation. However, it was observed that for the same fat type sugar blends impacted upon aeration levels. Mixes containing glucose syrup presented lower overrun. The products added with this carbohydrate generally exhibit higher viscosity, and in agreement to Adapa et al. (2000b), more viscous systems do not favor foaming capacity. Hence, this parameter could have been the primary cause for the decrease in whipping ability.
4 Conclusions
In addition to the expected effects on melting behavior and solid content, the sugar blends used in this study affected the air incorporation. It is known that greater air content increases the melting resistance. However, despite the lower overrun, ice creams made with fructose syrup melted more slowly. Thus, the levels of air added into the products did not allow safe conclusions about the influence of this parameter in the physical behavior of the assessed ice creams in this study.
The replacement of hydrogenated vegetable fat for palm fat caused changes in the melting ranges of the formulations. Higher melting rate was observed in the combination of palm fat and fructose syrup.
Acknowledgements
The authors are grateful for the financial support provided by CAPES- Brazilian research supporting foundation.
Recebido para publicação em 23/1/2010
Aceito para publicação em 08/1/2011 (004181)
- ADAPA, S. et al. Mechanisms of ice crystallization and recrystallization in ice cream: a review. Food Reviews International, v. 16, p. 259-271, 2000a.
- ADAPA, S. et al. Rheological properties of ice cream mixes and frozen ice creams containing fat and fat replacers. Journal of Dairy Science, v. 83, p. 2224-2229, 2000b.
- AIME, D. B. et al. Textural analysis of fat reduced vanilla ice cream products. Food Research International, v.34, p. 237-246, 2001.
- ARBUCKLE, W. S. Ice cream. 4th ed. Aspen: Chapman & Hall, 2000.
- ASSOCIATION OF OFFICIAL ANALYTICAL CHEMISTS - AOAC. Official Methods of Analysis. 16th ed. Washington: AOAC, 1995.
- CHANG, Y.; HARTEL, R. W. Development of air cells in a batch ice cream freezer. Journal of Food Engineering, v. 55, p. 71-78, 2002.
- DREWETT, E. M. HARTEL, R. W. Ice crystallization in a scraped surface freezer. Journal of Food Engineering, v. 78, p. 1060-1066, 2007.
- GOFF, H. D. Formation and stabilisation of structure in ice cream and related products. Current Opinion in Colloid and Interface Science, v. 7, p. 432-437, 2002.
- GRANGER, C. et al. Influence of formulation on the thermal behavior of ice cream mix and ice cream. Journal of American Oil Chemists Society, v. 82, p. 427-431, 2005.
- LEE, F. Y.; WHITE, C. H. Effect of ultrafiltration retentates and whey protein concentrates on ice cream quality during storage. Journal of Dairy Science, v. 74, p. 1170-1180, 1991.
- MILLER-LIVNEY, T.; HARTEL, R. W. Ice recrystallization in ice cram: interactions between sweeteners and stabilizers. Journal of Dairy Science, v. 80, p. 447-456, 1997.
- MORETTI, R. H. Elaboração de sorvetes 2. ed. Campinas: Fundação Tropical de Pesquisa e Tecnologia, 1979. 118 p.
- MUSE, M. R.; HARTEL, R. W. Ice cream structural elements that affect melting rate and hardness. Journal of Dairy Science, v. 87, p. 1-10, 2004.
- SEGALL, K. I.; GOOF, H. D. A modified ice cream processing routine that promotes fat destabilization in the absence of added emulsifier. International Dairy Journal, v. 12, p. 1013-1018, 2002.
- SILVA JUNIOR, E. et al. Transient process in ice creams evaluated by laser speckles. Food Research International, v. 43, p. 1470-1475, 2010.
- TRGO, C.; KOSHOLT, M.; KESSLER, G. Effect of freezing point and texture regulation parameters on the initial ice crystal growth in ice cream. Journal of Dairy Science, v. 82, p. 460-465, 1999.
- UNIVERSIDADE DE SÃO PAULO . Faculdade de Ciências Farmacêuticas. Tabela Brasileira de Composição de Alimentos 2008. Disponível em: <http://www.fcf.usp.br/tabela/>. Acesso em: 19 fev. 2009.
Publication Dates
-
Publication in this collection
27 May 2011 -
Date of issue
Mar 2011
History
-
Received
23 Jan 2010 -
Accepted
08 Jan 2011