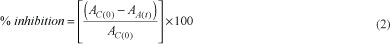Abstracts
Four varieties of an Andean indigenous crop, quinoa (Chenopodium quinoa Willd.), were evaluated as a source of dietary fiber, phenolic compounds and antioxidant activity. The crops were processed by extrusion-cooking and the final products were analyzed to determine the dietary fiber, total polyphenols, radical scavenging activity, and in vitro digestibility of starch and protein. There were no significant differences in the contents of total dietary fiber between varieties of quinoa. In all cases, the contents of total and insoluble dietary fiber decreased during the extrusion process. At the same time, the content of soluble dietary fiber increased. The content of total phenolic compounds and the radical scavenging activity increased during the extrusion process in the case of all 4 varieties. There were significant differences between the varieties and the content of total polyphenols. The in vitro protein digestibility of quinoa varieties was between 76.3 and 80.5% and the in vitro starch digestibility was between 65.1 and 68.7%. Our study demonstrates that quinoa can be considered a good source of dietary fiber, polyphenols and other antioxidant compounds and that extrusion improves the nutritional value.
quinoa; Chenopodium quinoa Willd.; dietary fiber; bioactive compounds; extrusion
Quatro variedades de quinoa (Chenopodium quinoa Willd.), cultura de origem andina, foram avaliados como fonte de fibra dietética, de compostos fenólicos e atividade antioxidante. As quinoas foram processadas por extrusão e os produtos finais foram analisados para determinar a fibra alimentar, o total de polifenóis, atividade de ligar os radicais livres e digestibilidade in vitro do amido e proteínas. Não houve diferença significativa no conteúdo de fibra dietética total entre as variedades de quinoa. Em todos os casos, o teor de fibra alimentar insolúvel e total diminuiu durante o processo de extrusão. Ao mesmo tempo, o teor de fibra alimentar solúvel teve um incremento. O teor de compostos fenólicos totais e a atividade de ligar os radicais livres foram aumentados durante o processo de extrusão, no caso das quatro variedades. Houve diferenças significativas entre o conteúdo total de polifenóis por variedades. A digestibilidade proteica in vitro das variedades de quinoa ficou entre 76,3 e 80,5%, e a digestibilidade in vitro do amido situou-se entre 65,1 e 68,7%. Nosso estudo demonstra que a quinoa pode ser considerada como uma boa fonte de fibra dietética, polifenóis e outros compostos antioxidantes e que o processo de extrusão - cocção pode melhorar o valor nutricional dos grãos.
quinoa; Chenopodium quinoa Willd.; fibra dietética; componentes funcionais; processo de extrusão - cocção
ORIGINAL
Quinoa (Chenopodium quinoa, Willd.) as a source of dietary fiber and other functional components
Quinoa (Chenopodium quinoa, Willd.) como fonte de fibra alimentar e de outros componentes funcionais
Ritva Ann-Mari Repo-Carrasco-Valencia* * A quem a correspondência deve ser enviada ; Lesli Astuhuaman Serna
Universidad Nacional Agraria La Molina (Lima), Lima, Peru, E-mail: ritva@lamolina.edu.pe
ABSTRACT
Four varieties of an Andean indigenous crop, quinoa (Chenopodium quinoa Willd.), were evaluated as a source of dietary fiber, phenolic compounds and antioxidant activity. The crops were processed by extrusion-cooking and the final products were analyzed to determine the dietary fiber, total polyphenols, radical scavenging activity, and in vitro digestibility of starch and protein. There were no significant differences in the contents of total dietary fiber between varieties of quinoa. In all cases, the contents of total and insoluble dietary fiber decreased during the extrusion process. At the same time, the content of soluble dietary fiber increased. The content of total phenolic compounds and the radical scavenging activity increased during the extrusion process in the case of all 4 varieties. There were significant differences between the varieties and the content of total polyphenols. The in vitro protein digestibility of quinoa varieties was between 76.3 and 80.5% and the in vitro starch digestibility was between 65.1 and 68.7%. Our study demonstrates that quinoa can be considered a good source of dietary fiber, polyphenols and other antioxidant compounds and that extrusion improves the nutritional value.
Keywords: quinoa; Chenopodium quinoa Willd.; dietary fiber; bioactive compounds; extrusion.
RESUMO
Quatro variedades de quinoa (Chenopodium quinoa Willd.), cultura de origem andina, foram avaliados como fonte de fibra dietética, de compostos fenólicos e atividade antioxidante. As quinoas foram processadas por extrusão e os produtos finais foram analisados para determinar a fibra alimentar, o total de polifenóis, atividade de ligar os radicais livres e digestibilidade in vitro do amido e proteínas. Não houve diferença significativa no conteúdo de fibra dietética total entre as variedades de quinoa. Em todos os casos, o teor de fibra alimentar insolúvel e total diminuiu durante o processo de extrusão. Ao mesmo tempo, o teor de fibra alimentar solúvel teve um incremento. O teor de compostos fenólicos totais e a atividade de ligar os radicais livres foram aumentados durante o processo de extrusão, no caso das quatro variedades. Houve diferenças significativas entre o conteúdo total de polifenóis por variedades. A digestibilidade proteica in vitro das variedades de quinoa ficou entre 76,3 e 80,5%, e a digestibilidade in vitro do amido situou-se entre 65,1 e 68,7%. Nosso estudo demonstra que a quinoa pode ser considerada como uma boa fonte de fibra dietética, polifenóis e outros compostos antioxidantes e que o processo de extrusão - cocção pode melhorar o valor nutricional dos grãos.
Palavras-chave: quinoa; Chenopodium quinoa Willd.; fibra dietética; componentes funcionais; processo de extrusão - cocção.
1 Introduction
Quinoa (Chenopodium quinoa Willd.) is a crop used by pre-Columbian cultures in South America for centuries. There is a long history of safe use of the grain in South America. Cultivated and collected Chenopodium species have been part of the Tiahuanacotan and Incan cultures. Quinoa has fulfilled various roles in these ancestral cultures, in addition to its role in human and animal nutrition, quinoa had a sacred importance (BONIFACIO, 2003). Archaeological studies have shown that quinoa was already known in 5000 B.C. (TAPIA et al., 1979). For the Incas, quinoa was a very important crop together with corn and potato. Quinoa is currently grown for its grain in the South American countries of Peru, Bolivia, Ecuador, Argentina, Chile and Colombia. The plant is cold and drought tolerant and it can be cultivated in high altitudes in the mountain areas. Quinoa can be grown in a wide range of pH of the soil, including acidic soils, and it can tolerate poor and rough environments. This crop grows perfectly at high altitudes, where it is not possible to grow maize, and it matures in 4 to 7 months, depending on the variety or ecotype (CARMEN, 1984). The genetic variability of quinoa is great, with cultivars of quinoa being adapted to growth from sea level to an altitude of 4,000 m, from 40° S to 2° N latitude, and from the cold highland climate to subtropical conditions. This makes it possible to select, adapt, and breed cultivars for a wide range of environmental conditions, providing basic nutrition in demanding environmental conditions (JACOBSEN, 2003).
Quinoa is usually referred to as a pseudo-cereal since it is not a member of the Gramineae family, but it produces seeds that can be milled in to flour and used as a cereal crop. It is an annual dicotyledonous plant usually standing 0.5-2.0 m high with large panicles of 1.8-2.2 mm long seeds produced at the end of the stem. The seed is usually pale yellow, but it may vary from almost white through pink, orange or red to brown and black. The embryo can hold 60% of the seed weight and it forms a ring around the endosperm that loosens when the seed is cooked (NATIONAL RESEARCH COUNCIL, 1989). Grain yields vary from 1 to 3 t.ha-1, depending on the variety and the level of cultivation technology (CARMEN, 1984).
Most of the varieties of quinoa contain saponins, bitter-tasting triterpenoid glycosides, which are concentrated in the seed coat and must be removed before consumption. The most popular method for removing saponins involves washing the grains with water in the ratio of 1:8 quinoa:water, although there are several other traditional methods (ANTUNEZ DE MAYOLO, 1981). Large-scale commercial methods haven been developed in Peru and Bolivia.
Quinoa is one of the most nutritive grains used as human food and it has been selected by FAO as one of the crops destined to offer food security in this century (FOOD..., 1998). Its protein content is remarkable and the composition of the essential amino acids is excellent. The nutritional value of quinoa protein is comparable to that of milk protein (KOZIOL, 1992; RANHOTRA et al., 1993). The content of lysine, methionine and cysteine in quinoa is higher than in common cereals and legumes, making it complementary to these crops. Quinoa is rich in oil, containing beneficial fatty acids and a high content of tocopherols (REPO-CARRASCO-VALÊNCIA; ESPINOZA; JACOBSEN, 2003). Based on the high quality of the oil, and on the fact that some varieties show oil concentrations of up to 9.5%, quinoa could be considered as a potentially valuable new oil crop (KOZIOL, 1992).
Actually, quinoa is widely used in many South American countries, especially in Peru, Bolivia, Ecuador, Chile, and Argentina. In Peru, the population of Lima is aware of the nutritive qualities of quinoa and other Andean crops. They consume quinoa once a day on average. In rural areas of southern Peru, the population is accustomed to eating quinoa every day in different preparations (AYALA, 2003).
Quinoa grains do not contain gluten and thus, they cannot be used alone for bread- making. However, they can be mixed with wheat flour in the preparation of bread with high nutritional value (MORITA et al., 2001). The fact that quinoa contains no gluten, makes it an interesting ingredient for the diets of persons who have celiac disease. Quinoa flour is commonly used in infant foods. Flakes, similar to oat flakes, have also been prepared from quinoa. Puffed grains of quinoa are produced commercially in Peru and Bolivia. The plant is sometimes grown as a green vegetable and its leaves are eaten fresh or cooked (NATIONAL RESEARCH COUNCIL, 1989). The saponins obtained as a by-product in the processing of quinoa can be utilized by the cosmetics and pharmaceutical industries (TAPIA et al., 1979).
Cereals are commonly known as good sources of dietary fiber, phenolic compounds, and natural antioxidants. Some studies on dietary fiber, phenolic compounds, and antioxidant activity of quinoa have been published (RUALES; NAIR, 1994; GORINSTEIN et al., 2007; NSIMBA; KIKUZAKI; KONISHI, 2008). These investigations have shown that quinoa is a very good source of antioxidants and it can be a substitute for common cereals. However, we did not find any reports on the content of phenolic compounds and antioxidant activity of processed quinoa. Thus, the aim of this study was to analyze the content of dietary fiber, phenolic compounds, and antioxidant activity in 4 varieties of raw and extruded quinoa.
2 Materials and methods
2.1 Raw material
The following 4 varieties of quinoa (Chenopodium quinoa, Willd.) were used in this study: ´La Molina 89´, of the leguminous and cereal program of National Agrarian University La Molina, Lima, Peru; ´Kcancolla´, 'Blanca de Juli´ and ´Sajama´ from the Agronomical Experimentation Center of Altiplano University, Puno, Peru.
2.2 Preparation of raw material
Quinoa was washed for 20 minutes with tap water with the aim to eliminate bitter taste and toxic saponins. Washed grains were dried at 45 °C for 12 hours. Dried seeds were packed in polyethylene bags and stored at 4 °C until they used in analysis and processing.
2.3 Extrusion
Washed and dried quinoa seeds were moistened to 12% humidity for the extrusion process. The extrusion process was carried out using a low-cost extruder simulating local processing conditions (Jarcon del Peru, Huancayo, Peru). The single-screw extruder was operated with the following parameters: 389.4 rpm, 10-13 seconds resident time, 200 °C work temperature, 2 orifices on the die. No external heat was transferred to the barrel on the screw during extrusion. The aim was to produce results applicable to local conditions where this technology is widely used.
2.4 Extraction of polyphenols and radical scavenging analysis
All samples were ground through a Foss cyclotec mill before extraction. Five grams of milled quinoa or extrudate were mixed with 25 mL methanol and homogenized using the Ultra-Turrax homogenizer. The homogenates were allowed to stand for 12-24 hours under refrigeration (4 °C) and then centrifuged for 15 minutes. The supernatant was recovered and stored until analysis.
2.5 Analysis
Proximate analysis. Water content, proteins (N × 6.25), fat, crude fiber and ash were determined according to AOAC (ASSOCIATION..., 1995). The carbohydrates (CHO) were calculated by difference, using the Equation 1:
Dietary fiber. The total, soluble and insoluble dietary fiber were determined by an enzymatic-gravimetric method according to the Approved Method 32-21 (AMERICAN..., 1995) using the TDF-100 kit from Sigma Chemical Company (St. Louis, MO, U.S.A.). Radical scavenging activity. Radical scavenging activity was determined according to Re et al. (1999) based on the decrease of absorbance at 734 nm produced by reduction of ABTS (2,2´-azinobis-(3-ethylbenzothiazoline-6- sulfonic acid)) by an antioxidant. Trolox was used as the reference compound for calibration curve. The percentage inhibition of absorbance at 734 nm was calculated using the formula according to Katalinic et al. (2006), Equation 2:
where AC(0) is the absorbance of the control at t = 0 minute and AA(t) is the absorbance of the antioxidant at t = 16 minutes.
Total polyphenols. The content of total polyphenols was analyzed according to the method of Swain and Hillis (1959). The phenolic compounds were extracted with methanol and the extract was allowed to react with the Folin- Ciocalteau phenol reagent. The absorbance was measured at 725 nm. Gallic acid equivalents (GAE) were determined from a standard concentration curve.
Protein in vitro digestibility. Digestibility of proteins was determined by an in vitro method according to Hsu et al. (1977). The multi-enzymatic method is based on the decrease of pH during 10 minutes. The percentage of digestibility was calculated using the Equation 3:
where: X = pH of the protein suspension after 10 minutes of digestion and Y = percentage of protein hydrolysis.
Starch in vitro digestibility. The digestibility of starch was determined by an in vitro method according to Holm et al. (1985). Starch (500 mg) was mixed with phosphate buffer (pH 6.9) and incubated with α-amylase at 37 °C for 1 hour. The sugars released were determined by spectrophotometry.
Degree of gelatinization was based on the method of Wooton; Weeden and Munk (1971).
2.6 Statistical analysis
Each analysis was done at least in duplicate and expressed as means and standard deviation (SD). The data were analyzed by analysis of variance and Tukey´s test (significance of differences p < 0.05) was used to find significant differences between the samples and treatments.
3 Results and Discussion
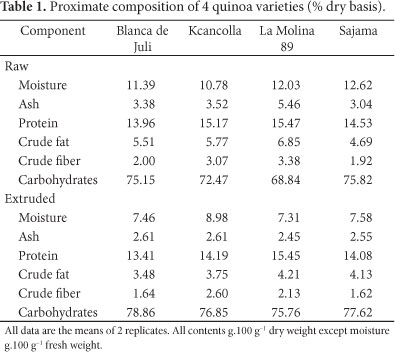
The results of analysis of the proximate composition of 4 quinoa varieties and their extrudates are presented in Table 1. Then, protein content of the grain of the 4 varieties was between 14.0 and 15.5%. This coincides with values by Guzman-Maldonado and Paredes-Lopez (1998). According to these authors, the protein content of quinoa is between 11.0 and 15.0%. There were differences between the 4 varieties of quinoa in protein content, La Molina 89 having the highest and Blanca de Juli the lowest protein contents. Extrusion affected the protein content, decreasing it. La Molina 89 had the highest crude fat content. The contents of moisture, ash, and crude fiber were reduced during the extrusion process in all varieties.
In Table 2, the contents of total (TDF), insoluble (IDF), and soluble dietary fiber (SDF) are presented for raw and extruded quinoa varieties. There were no significant differences in the contents of TDF, IDF, and SDF between the varieties. In all cases, the contents of total and insoluble dietary fiber decreased during the extrusion process, however, this decrease was significant only in the case of the Sajama variety. At the same time, the content of soluble dietary fiber increased during the extrusion process. The increase of content of soluble dietary fiber was statistically significant in the case of Blanca de Juli, Kcancolla, and La Molina 89 varieties. Gualberto et al. (1997) also found a decrease in the content of insoluble dietary fiber and an increase in the content of soluble fiber during extrusion-cooking. This could be due to shear stress caused by high screw speed and also to high temperature. The exposure to shear stress and high temperature causes chemical bond breakage creating smaller particles, which are soluble. There is a transformation of some insoluble fiber components into soluble fiber during extrusion. Rinaldi; NG and Bennink (2000) studied the effect of extrusion on dietary fiber of wheat extrudates enriched with wet okara and his results coincide with ours. Extrusion of the formulations resulted in decreased insoluble fiber and increased soluble fiber contents of the products. Extrusion-cooking of white heat flour has also been found to cause a redistribution of insoluble to soluble dietary fiber (BJORCK; NYMAN; ASP, 1984).
The extrusion-cooking process was investigated by Lue, Hsieh and Huff (1991) with the expectancy that mechanic rupture of the glycosidic bonds would lead to an increase of soluble fiber. In some cases, an increase of insoluble fiber was observed (UNLU; FALLER, 1998). Esposito et al. (2005) studied the effect of extrusion on dietary fiber of durum wheat. The data showed that the extrusion-cooking process did not have an effect on the amount of soluble dietary fiber, independently from the fiber typology of the different samples. This difference in fiber solubilization during processing could be explained by the variability in the raw material composition, but also by different experimental conditions, for example, screw share forces and pressure in extrusion. The high mechanical stress during extrusion may cause breakdown of polysaccharide glycosidic bonds releasing oligosaccharides and, therefore, end up with an increase of soluble dietary fiber (ESPOSITO et al., 2005).
Ruales and Nair (1994) determined the contents of dietary fiber in raw and processed quinoa samples. They found 13.4% of total dietary fiber for raw quinoa. This value is comparable to our values for Blanca de Juli and Sajama varieties. The content of total dietary fiber was decreased only in cooked quinoa, while in autoclaved and drum-dried samples it remained the same. Some soluble fiber was lost during cooking, and in autoclaved samples, it was lost probably due to depolymerization of fiber components.
Content of the total phenolic compounds and the radical scavenging activity increased during the extrusion process in the case of all 4 varieties (Table 3.). There were significant differences between the varieties and the contents of total polyphenols. The contents of total polyphenols in the 4 quinoa varieties ranged from 1.43 to 1.97 mg.GAE.g-1. Pasko et al. (2009) defined the content of total polyphenols in quinoa to be 3.75 mg.GAE.g-1 by using a 2-step extraction process, first with methanol and then with acetone. As we used methanol only, some polyphenols may not have been included in the extract.
Figure 1 presents the radical scavenging activities of raw and extruded quinoa. These increased during the extrusion process. The finding is in agreement with the report of Dewanto, Wu and Hai Liu (2002) who discovered that the antioxidant activity and the content of total phenolics of sweet corn increased during thermal processing. Increase of the total antioxidant activity in processed grains could be explained by the increase of soluble phenolic compounds released by thermal processing. In cereals, the phenolic acids are in free, esterified and insoluble bound forms. Dewanto, Wu and Hai Liu (2002) found that heat treatment increased the free and conjugated ferulic acid contents in sweet corn due to the release of bound ferulic acid across both the heating time and heating temperature parameters.
Xu and Chang (2008) studied the effect of thermal processing on total phenolics and specific phenolic compounds in yellow and black soybeans. They found that the content of phenolics increased in yellow varieties and decreased in black varieties during the heat treatment. In yellow soybean varieties, thermal processing caused more free gallic acid to be released leading to higher total phenolic content and antioxidant activity compared to raw beans.
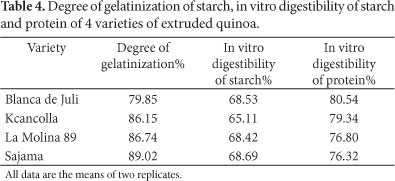
The values of degree of gelatinization (DG), as well as the in vitro digestibility of starch and protein are presented in Table 4. DG of the 4 quinoa varieties was between 79.9 and 89.0%. These values are lower than those found by Ruales and Nair (1994) for drum-dried (96%) and cooked (97%) quinoa samples, but higher than for the autoclaved (27%) samples. Dogan and Karwe (2003) investigated the physicochemical properties of quinoa extrudates. They found that the starch was only partially gelatinized with a maximum of 84.4%, depending on the extrusion conditions (feed moisture, screw speed and die temperature). During extrusion-cooking, both temperature and shear are responsible for starch gelatinization.
Ruales and Nair (1994) reported the in vitro digestibility of starch in raw, autoclaved, cooked, and drum-dried quinoa. Their values for raw, autoclaved and cooked quinoa were lower than ours (22, 32 and 45%, respectively), but the value for drum-dried quinoa was higher (73%). As the starch granules of quinoa are surrounded by a protein matrix, they are not very easily hydrolysable by α-amylase. The degree of starch hydrolysis could be improved by treating quinoa flour with proteolytic enzymes prior to hydrolysis with α-amylase.
The in vitro digestibility of protein of the 4 extruded quinoa varieties was between 76.3 and 80.5%. Zia-Ur-Rehman and Shah (2001) studied the protein in vitro digestibility of black grams after soaking and cooking. They obtained values of digestibility as in our study, between 75 and 84% for cooked black grams. Dahlin and Lorenz (1993) studied protein in vitro digestibility of extruded cereal grains, including quinoa. The effect of extrusion on in vitro protein digestibility was similar in all cereals investigated. The optimum extrusion process conditions for cereals used in this study were 15% feed moisture, 100/150 °C product temperatures and screw speed of 100 rpm.
4 Conclusions
Altogether, this study demonstrates that quinoa can be considered a very nutritive cereal when compared to commonly consumed cereals such as wheat, barley, and corn. It has a relatively high content of good-quality protein and it can be considered a good source of dietary fiber and other bioactive compounds such as phenolics. It has a long history of safe use in South America, especially in low-income areas. Therefore, it may present a new viable crop option for low-income areas and also provide a new ingredient for specific foods for particular target populations with potential health benefits. Thus, further studies should be conducted to characterize the phenolic compound composition and the antioxidant content and activity, especially of colored varieties of quinoa.
Acknowledgements
We thank Dr. Seppo Salminen and Dr. Heikki Kallio, Department of Biochemistry and Food Chemistry, University of Turku, Finland, for their critical reading and helpful comments on the manuscript. Financing from CONCYTEC (Concejo Nacional de Ciencia, Tecnologia e Innovación Tecnológica, Peru) is gratefully acknowledged.
Recebido para publicação em 2/6/2009
Aceito para publicação em 25/9/2009 (004229)
- AMERICAN ASSOCIATION OF CEREAL CHEMISTS - AACC. Approved Methods of American Association of Cereal Chemists. 9th ed. St. Paul: AACC, 1995.
- ANTUNEZ DE MAYOLO, S. E. La Nutrición en el Antiguo Perú. Lima: Banco Central de Reserva de Perú, Oficina Numismática, 1981.
- ASSOCIATION OF OFFICIAL ANALYTICAL CHEMISTS - AOAC. Official Methods of Analysis. 16th ed. Washington: AOAC, 1995.
- AYALA, G. Consumption of quinoa in Peru. Food Reviews International, v. 19, p. 221-227, 2003.
- BJORCK, I.; NYMAN, M.; ASP, N. G. Extrusion-cooking and dietary fiber: Effects on dietary fiber content and on degradation in the rat intestinal tract. Cereal Chemistry, v. 61, p. 174-179, 1984.
- BONIFACIO, A. Chenopodium Sp.: Genetic resources, ethnobotany, and geographic distribution. Food Reviews International, v. 19, p. 1-7, 2003.
- CARMEN, M. Acclimatization of quinoa (Chenpodium quinoa, Willd) and canihua (Chenopodium pallidicaule, Aellen) to Finland. Annales Agriculturae Fenniae, v. 23, p. 135-144. 1984.
- DAHLIN, K.; LORENZ, K. Protein digestibility of extruded cereal grains. Food Chemistry, v. 48, p. 13-18, 1993.
- DEWANTO, V.; WU, X.; HAI LIU, R. Processed Sweet Corn Has Higher Antioxidant Activity. Journal of Agricultural and Food Chemistry, v. 50, p. 4959-4964, 2002.
- DOGAN, H.; KARWE, M. Physicochemical properties of quinoa extrudates. Food Science and Technology International, v. 2, p. 101-114, 2003.
- ESPOSITO, F. et al. Antioxidant activity and dietary fibre in durum wheat bran by-products. Food Research International, v. 38, p. 1167-1173, 2005.
- FOOD AND AGRICULTURE ORGANIZATION - FAO. Under-utilized Andean Food Crops Rome: FAO, 1998.
- GORINSTEIN, S. et al. The total polyphenols and the antioxidant potentials of some selected cereals and pseudocereals. European Food Research and Technology, v. 225, p. 321-328, 2007.
- GUALBERTO, D. et al. Effect of extrusion processing on the soluble and insoluble fiber and phytic acid contents of cereal brans. Plant Foods for Human Nutrition, v. 51, p. 187-198, 1997.
- GUZMAN-MALDONADO, S.; PAREDES- LOPEZ, O. Functional Products of Plant Indigenous to Latin America Amaranth and Quinoa, Common Beans and Botanicals. In: MAZZA, G. (Ed.) Functional Foods Biochemical and Processing Aspects Pennsylvania: Technomic Publishing Company, 1998. p. 293-328.
- HOLM, J. et al. Starch availability in vitro and in vivo after flaking, steam-cooking and popping of wheat. Journal of Cereal Science, v. 3, p. 193-206, 1985.
- HSU, H. et al. A multienzyme technique for estimating protein digestibility. Journal of Food Science, v. 42, p. 1269-1273, 1977.
- JACOBSEN, S. E. The worldwide potential for quinoa (Chenopodium quinoa Willd.). Food Reviews International, v. 19, p.167-177, 2003.
- KATALINIC, V. et al. Screening of 70 medicinal plant extracts for antioxidant capacity and total phenols. Food Chemistry, v. 94, p. 550-557, 2006.
- KOZIOL, M. Chemical composition and nutritional evaluation of quinoa (Chenopodium quinoa Willd.). Journal of Food Composition and Analysis, v. 5, p. 35-68, 1992.
- LUE, S.; HSIEH, F.; HUFF, H. Extrusion cooking of corn meal and sugar beet fiber: effects on expansion properties, starch gelatinization and dietary fiber content. Cereal Chemistry, v. 68, p. 227-234, 1991.
- MORITA, N. et al. Quinoa flour as a new foodstuff for improving dough and bread. Journal of Applied Glycoscience, v. 48, p. 263-270, 2001.
- NATIONAL RESEARCH COUNCIL. Lost Crops of the Incas: Little-Known Plants of the Andes with Promise for Worldwide Cultivation Washington: National Academy Press, 1989.
- NSIMBA, Y. R.; KIKUZAKI, H.; KONISHI, Y. Antioxidant activity of various extracts and fractions of Chenopodium quinoa and Amaranthus spp. Seeds. Food Chemistry, v. 106, p.760-766, 2008.
- PASKO, P. et al. Anthocyanins, total polyphenols and antioxidant activity in amaranth and quinoa seeds and sprouts during their growth. Food Chemistry, v. 115, n. 3, p. 994-998, 2009.
- RANHOTRA, G. et al. Composition and protein nutritional quality of quinoa. Cereal Chemistry, v. 70, p. 303-305, 1993.
- RE, R. et al. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biology and Medicine, v. 26, p. 1231-1237, 1999.
- REPO-CARRASCO-VALÊNCIA, R. A.-M.; ESPINOZA, C.; JACOBSEN, S.-E. Nutritional value and use of the Andean crops quinoa (Chenopodium quinoa) and kañiwa (Chenopodium pallidicaule). Food Reviews International, v. 19, p. 179-189, 2003.
- RINALDI, V. E. A. ; NG, P. K. W.; BENNINK, M. R. Effects of extrusion on dietary fiber and isoflavone contents of wheat extrudates enriched with wet okara. Cereal Chemistry, v. 77, p. 237-240, 2000.
- RUALES, J.; NAIR, B. Properties of starch and dietary fibre in raw and processed quinoa (Chenopodium quinoa, Willd) seeds. Plant Foods for Human Nutrition, v. 45, p. 223-246, 1994.
- SWAIN, T.; HILLIS, E. The phenolic constituents of Prunus Domestica Journal of the Science of Food and Agriculture, v. 10, p. 63-68, 1959.
- TAPIA, M. et al. Quinua y Kaniwa. Cultivos Andinos. Centro Internacional de Investigación para el Desarrollo (CIID). Instituto Interamericano de Ciencias Agrícolas (IICA), 1979.
- UNLU, E.; FALLER, F. Formation of resistant starch by a twinscrew extruder. Cereal Chemistry, v. 75, p. 346-350, 1998.
- WOOTON, M.; WEEDEN, D.; MUNK, N. A rapid method for the estimation of starch gelatinization in processed foods. Food Technology in Australia., v. 23, p. 612-615, 1971.
- XU, B.; CHANG, S. Total phenolics, phenolic acids, isoflavones, and anthocyanins and antioxidant properties of yellow and black soybeans as affected by thermal processing. Journal of Agricultural and Food Chemistry, v. 56, p. 7165-7175, 2008.
- ZIA-UR-REHMAN; SHAH, W. Tannin contents and protein digestibility of black grams (Vigna mungo) after soaking and cooking. Plant Foods for Human Nutrition, v. 56, p. 265-273, 2001.
Publication Dates
-
Publication in this collection
27 May 2011 -
Date of issue
Mar 2011
History
-
Received
02 June 2009 -
Accepted
25 Sept 2009