Abstracts
Fruits are important sources of nutrients in human diet, and Barbados Cherry (Malpighia glabra L.) is of particular interest due to its high content of antioxidants. Diets rich in fruits and vegetables protect individuals against diseases and cancer, but excessive intake of vitamins may act as pro-oxidant and generate changes in DNA. To evaluate the effect of different in natura (BAN) and frozen (BAF) Barbados Cherry pulp concentrations and synthetic vitamin C in liquid form (VC) on the chromosome level and the cell cycle division, root meristeme cells of Allium cepa L. and bone marrow cells of Wistar rats Rattus norvegicus, were used as test system. In Allium cepa L., BAN, at the highest concentration (0.4 mg.mL-1) and BAF, at the lowest concentration (0.2 mg.mL-1), inhibited cell division, and there was recovery of cell division after the recovery period in water only for BAN. In the Wistar rats, all treatments with Barbados Cherry, either acute or subchronic, were not cytotoxic or mutagenic; only the highest concentration of VC increased significantly the rate of chromosomal abnormalities. The data obtained are important to reinforce the use of Barbados Cherry fruit in the diet.
antioxidants; cytotoxicity; mutagenesis
As frutas são importantes fontes de nutrientes na dieta humana e a Acerola (Malpighia glabra L.) é de particular interesse devido ao seu alto teor de antioxidantes. Dietas ricas em frutas e legumes protegem os indivíduos contra doenças e câncer, mas a ingestão excessiva de vitaminas pode atuar como pró-oxidante e gerar alterações no DNA. Para avaliar o efeito de diferentes concentrações da polpa in natura da Acerola (BAN) e congelada (BAF), e da vitamina C sintética na forma líquida (VC), em nível cromossômico e sobre o ciclo de divisão celular, foram utilizadas células meristemáticas de raiz de Allium cepa L. e células da medula óssea de ratos Wistar, Rattus norvegicus, como sistema teste. Em Allium cepa L., BAN, na maior concentração (0,4 mg.mL-1) e BAF, na menor concentração (0,2 mg.mL-1), houve inibição da divisão celular, e apenas para BAN houve recuperação da divisão celular após o período de recuperação em água. Em ratos Wistar, todos os tratamentos com Acerola, agudo ou subcrônico, não foram citotóxicos e mutagênicos, apenas a maior concentração de VC aumentou significativamente o percentual de anormalidades cromossômicas. Os dados obtidos são importantes porque reforçam o uso das frutas de Acerola na dieta.
antioxidantes; citotoxicidade; mutagênese
Investigation of cytotoxic and mutagenic effects of Malpighia glabra L. (barbados cherry) fruit pulp and vitamin C on plant and animal test systems
Investigação do efeito citotóxico e mutagênico da polpa da fruta Malpighia glabra L. (acerola) e da vitamina C em sistema teste vegetal e animal
Elisângela DüsmanI,* * Author corresponding ; Márcia Flores da Silva FerreiraII; Alessandra Paim BertiI; Rosinete Gonçalves MariucciI; Mário Sérgio MantovaniIII; Veronica Elisa Pimenta VicentiniI
IDepartment of Cell Biology and Genetics, Biological Sciences Center, State University of Maringá UEM, Av. Colombo 5790, Bloco H67, 11, Jardim Universitário, CEP 87020-900, Maringá, PR, Brazil, e-mail: lisdusman@hotmail.com
IIAgricultural Science Center, Federal University of Espirito Santo UFES, CP 16, CEP 29500-000, Vitória, ES, Brazil
IIIDepartment of General Biology, Biological Sciences Center, State University of Londrina UEL, CP 6001, CEP 86061-990, Londrina, PR, Brazil
ABSTRACT
Fruits are important sources of nutrients in human diet, and Barbados Cherry (Malpighia glabra L.) is of particular interest due to its high content of antioxidants. Diets rich in fruits and vegetables protect individuals against diseases and cancer, but excessive intake of vitamins may act as pro-oxidant and generate changes in DNA. To evaluate the effect of different in natura (BAN) and frozen (BAF) Barbados Cherry pulp concentrations and synthetic vitamin C in liquid form (VC) on the chromosome level and the cell cycle division, root meristeme cells of Allium cepa L. and bone marrow cells of Wistar rats Rattus norvegicus, were used as test system. In Allium cepa L., BAN, at the highest concentration (0.4 mg.mL1) and BAF, at the lowest concentration (0.2 mg.mL1), inhibited cell division, and there was recovery of cell division after the recovery period in water only for BAN. In the Wistar rats, all treatments with Barbados Cherry, either acute or subchronic, were not cytotoxic or mutagenic; only the highest concentration of VC increased significantly the rate of chromosomal abnormalities. The data obtained are important to reinforce the use of Barbados Cherry fruit in the diet.
Keywords: antioxidants; cytotoxicity; mutagenesis.
RESUMO
As frutas são importantes fontes de nutrientes na dieta humana e a Acerola (Malpighia glabra L.) é de particular interesse devido ao seu alto teor de antioxidantes. Dietas ricas em frutas e legumes protegem os indivíduos contra doenças e câncer, mas a ingestão excessiva de vitaminas pode atuar como pró-oxidante e gerar alterações no DNA. Para avaliar o efeito de diferentes concentrações da polpa in natura da Acerola (BAN) e congelada (BAF), e da vitamina C sintética na forma líquida (VC), em nível cromossômico e sobre o ciclo de divisão celular, foram utilizadas células meristemáticas de raiz de Allium cepa L. e células da medula óssea de ratos Wistar, Rattus norvegicus, como sistema teste. Em Allium cepa L., BAN, na maior concentração (0,4 mg.mL1) e BAF, na menor concentração (0,2 mg.mL1), houve inibição da divisão celular, e apenas para BAN houve recuperação da divisão celular após o período de recuperação em água. Em ratos Wistar, todos os tratamentos com Acerola, agudo ou subcrônico, não foram citotóxicos e mutagênicos, apenas a maior concentração de VC aumentou significativamente o percentual de anormalidades cromossômicas. Os dados obtidos são importantes porque reforçam o uso das frutas de Acerola na dieta.
Palavras-chave: antioxidantes; citotoxicidade; mutagênese.
1 Introduction
In the last decades, many studies have examined the relationship between vegetables, fruit and health. The presence of compounds such as polyphenols, alkaloids, flavonoids, and other secondary metabolites in medicinal plants has provided a scientific validation for its popular use and served as an indicator in the selection of antimutagenic/anticarcinogenic activity of plants (KAUR et al., 2005).
The Barbados Cherry fruit (Malpighia glabra L.), for example, which belongs to the plant family Malpighiaceae, native of the Peninsula of Mexico, Central America, has the following major constituents in 100 g of fruit: vitamin C (1.500 mg), vitamin A (77-215 mg) carotenoids (1.44 mg), vitamin B1 (0.02 mg), vitamin B2 (0.07 mg), anthocyanins (16 mg), proteins (0.21 to 0.80 g), fat (0.23 to 0.80 g), carbohydrates (3.6 to 7.8 g), and minerals especially iron (0.24 mg), calcium (11.7 mg), and phosphorus (17.1 mg) (ROSSO; MERCADANTE, 2005; KUSKOSKI et al., 2006; MEZADRI et al., 2006; BADEJO et al., 2007; MARQUES; FERREIRA; FREIRE, 2007). Due to its constitution, this fruit has many medicinal properties: it is a vitamin source and acts as an antioxidant and antianemic, appetite stimulant, healer, anti-inflammatory, and mineralizer. Therefore, it is prescribed as a nutrient tonic in colds, in pulmonary tuberculosis, diabetes, liver diseases, rheumatism, fevers in general, varicose ulcers, hard to heal wounds, bone fractures, bruises, bleeding gums, and dysentery (MAIA et al., 2007).
Vitamin C can be obtained commercially in the form of effervescent or non- effervescent tablets, liquid, or multi-vitamin tablets, which are largely consumed by the population as supplement. However, only 50% of synthetic vitamin C is absorbed by the body, while Barbados Cherry fruit is almost entirely absorbed by the human body (NASSIF; CÍCERO, 2006).
Studies have shown that diets rich in fruits and vegetables protect individuals against a variety of heart and respiratory diseases and cancer. The antimutagenic and anticarcinogenic effects of these vegetables have been attributed to their antioxidant nutrients such as vitamins C and E and β-carotene, which when present, significantly reduce or prevent the oxidation of the substrate (TELESIL; MACHADO, 2008).
On the other hand, excessive intake of vitamins may act as pro-oxidant and generate changes in DNA (HALLIWELL, 2001). The results of mutagenic studies of vitamins, particularly those that are part of Barbados Cherry fruit constitution, are quite contradictory since some studies show their mutagenic activities (ALY; DONYA, 2002; OHSAWA et al., 2003; BHAT et al., 2006), while others deny it (DE FLORA; BAGNASCO; VAINIO, 1999; NEFIC, 2001; AZEVEDO et al., 2007).
Thus, the aim of this study was to evaluate the cytotoxicity and mutagenicity of in natura and frozen Barbados Cherry pulp and synthetic vitamin C in liquid form using meristematic root cells of Allium cepa L. as the plant test system and bone marrow cells of Wistar rats treated in vivo as the animal test system.
2 Material and methods
2.1 Treatment solution
Frozen Barbados Cherry (Malpighia glabra L.) pulp (BAF) (Brasfrut) and synthetic vitamin C in liquid form (VC) (Redoxon/Roche) were obtained from a commercial source. The treatment solutions were prepared by diluting them in water in the following concentrations: BAF = 0.2 and 0.4 mg.mL1 and VC = 0.3 and 0.6 mg.mL1. The fresh samples of Barbados Cherry in natura (BAN) were collected on the day of analysis from the medicinal garden Irene Silva in the State University of Maringá (UEM), and the solutions were prepared in a blender by mixing the pulp with water in the following concentrations: BAN = 0.1; 0.2; 0.4; and 5.0 mg.mL1.
2.2 Allium cepa L.
The experiment was conducted using Feulgen reaction and Schiffs reagent for staining, according to the methodology originally introduced by Levan in 1949 (in FISKESJÖ, 1985).
The onion bulbs were placed in bottles with water at room temperature and aerated in the dark for rooting. Before each treatment, three roots were collected and fixed (3 methanol: 1 acetic acid) to serve as its own control bulb (Co). Next, the roots of those bulbs were placed in treatment solutions in the following concentrations: 0.2 and 0.4 mg BAF.mL1 water, 0.1, 0.2, and 0.4 mg BAN.mL1 water and 0.3 and 0.6 mg VC.mL1 water for 24 hours. After the treatment period, three roots were withdrawn from each onion and fixed (Tr). The remaining roots were washed, and the bulbs were again placed in water for 24 hours to recover from any damage; the remaining roots were then removed and fixed (Re). The negative control onions remained in filtered water throughout the sampling time (CO).
The analysis of the slides was performed as blind tests using light microscopy with a 40× objective. To determine the mitotic index (MI-%), five bulbs were used as the control group in each treatment group. 1,000 cells per bulb were analyzed totalling 5,000 cells for the control, treatment, and recovery groups. Statistical analysis was performed using the chi-square test (α = 0.05).
2.3 Wistar rats
Three male and three female rats, Rattus norvegicus of 35 days of age and approximately 100 g body weight (bw) were used for each control group. They were obtained from the Central Vivarium of UEM. During the experimentation period, these animals were kept in the Mutagenesis Sector of the Vivarium, Department of Cell Biology and Genetics/UEM under controlled temperature conditions of ± 25ºC, humidity ± 50% with a photoperiod of 12 hours light/dark with water and food ad Libitum, in accordance with rules established by the Ethics Committee on Experimentation with Laboratory Animals/UEM (Case number: PRO 027/2009).
The concentrations measured in the acute treatment were: 0.2 and 0.4 mg BAF.mL1 water, 0.2, 0.4, and 5.0 mg BAN.mL1 water, 0.3 and 0.6 mg VC.mL1 water, administered as a single dose (1 mL.100 g1 bw) via oral gavage for 24 hours. Two acute control treatments were performed: the negative, 1 mL water.100 g1 bw via oral gavage, and the positive, 1.5 mg cyclophosphamide/1 mL water.100 g1 bw injected intraperitoneally for 24 hours.
The evaluated concentration in the subchronic treatment was 5.0 mg BAN.mL1 water via oral gavage once a day for 5 days. A negative control was performed with 1 mL of water.100 g1 bw via oral gavage once a day for 5 days.
The Ford and Hamerton (1956) methodology was used with some modifications. The cells were disrupted in mitotic metaphase with the intraperitoneal administration of 0.5 mL.100 g1 bw of colchicine (0.16%) half an hour before the sacrifice.
The analysis of the slides was performed under light microscope by analyzing 100 metaphases per animal totaling 600 per control and treatment groups. Chromosomal abnormalities such as gaps, breaks, and others were evaluated.
Cytotoxicity was evaluated by the parameter of mitotic index (MI), which was calculated for 5,000 cells per sex totaling 10,000 cells per group. The mitotic index was calculated as a percentage ratio of the number of dividing cells to the total number of cells in these fields. The statistical calculation was performed by the chi-square test (α = 0.05).
3 Results
3.1 Meristematic cells of Allium cepa L.
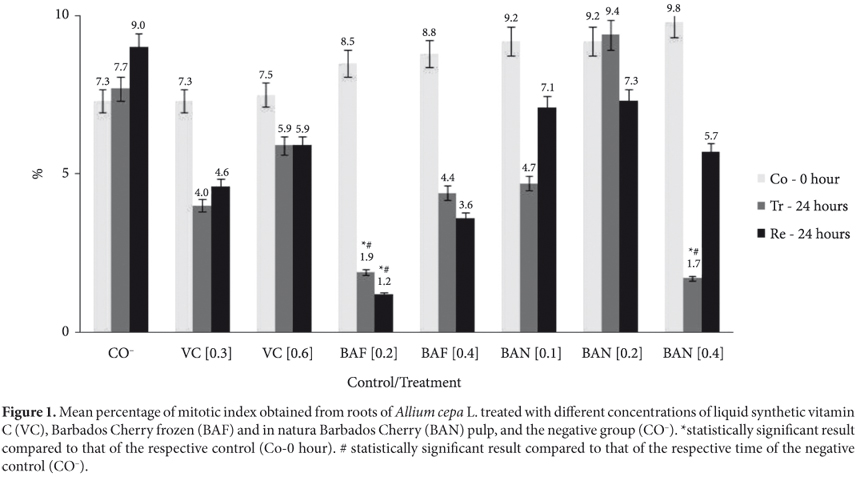
The comparisons of mitotic indices show that there were no statistically significant levels of cell division in the treatments with VC (0.3 and 0.6 mg.mL1), BAF (0.4 mg.mL1), and BAN (0.1 and 0.2 mg.mL1) for 24 hours in terms of the data obtained for the respective controls at 0h (Co), at 24 hours of recovery in water (Re), and when compared with the respective negative control (CO); comparison made with filtered water at the respective sampling times (Figure 1).
However, the mitotic index of the treatments (Tr) using Barbados Cherry frozen pulp (BAF) at lowest concentration (0.2 mg.mL1) and Barbados Cherry in natura (BAN), at the highest concentration (0.4 mg.mL1) were statistically different from those of their respective controls (Co-0 hour) (χ2 = 5.12 and p < 0.025 (BAF) and 6.69 and p < 0.010 (BAN)) and their respective times of negative control (CO) (χ2 = 4.36 and p < 0.050 (BAF) and 4.67 and p < 0.050 (BAN)).
However, for the frozen Barbados Cherry pulp, the inhibition of cell division was irreversible since it dropped even more at recovery time (Re) and was statistically different from its control (Co-0 hour) (χ2 = 6.27 and p < 0.025) and the recovery time of the negative control (CO) (χ2 = 6.76 and p < 0.010). For in natura Barbados Cherry (0.4 mg.mL1), the mitotic indexes of recovery were statistically identical to those of the controls demonstrating the reversibility of inhibition.
3.2 Bone marrow cells of Wistar rats
According to statistical analysis by chi-square, there was no difference in the comparisons between the mitotic index of negative and positive controls, between them, and their acute and subchronic treatments with in natura (BAN) and frozen (BAF) Barbados Cherry pulp, with synthetic vitamin C in liquid form (VC), and between these treatments (Figure 2).
For the average percentages of chromosomal aberrations in each treatment and control group, only the highest concentration of vitamin C (0.6 mg.mL1) showed a statistically significant difference when compared with the negative acute control (χ2 = 6.53 and p < 0.025). The other treatments with BAF (0.2 and 0.4 mg.mL1), BAN (0.2, 0.4 and 5.0 mg.mL1), and VC (0.3 mg.mL1) were statistically similar to the negative control.
Similarly, the subchronic treatment with BAN concentration of 5.0 mg.mL1 was not statistically different from its negative control with respect to the percentage of chromosomal abnormalities; the comparisons of this concentration (5.0 mg.mL1) in acute and subchronic treatments did not differ statistically either.
4 Discussion
The exposure of living organisms, especially humans, to certain substances in the environment can lead to the development of mutations. Since mutations are frequently associated with the development of cancers, knowledge of cytotoxic and mutagenic potential of different compounds is of fundamental importance (RIBEIRO; MARQUES, 2003). Accordingly, this study contributes by increasing the knowledge regarding the cytotoxic and mutagenic potential of fruit and vitamins.
The results obtained for the mitotic index of Barbados Cherry, in natura and frozen pulp, and synthetic vitamin C in liquid form, in Allium cepa, show the cytotoxic effect of lower concentrations (0.2 mg.mL1) of frozen pulp of Barbados Cherry (BAF) (1.9%) that was statistically different from its negative control (8.5%), and from the 24 hour-negative control treatment (7.7%). Furthermore, there was no recovery (1.2%) of the treated group cell division rate after 24 hours. This result may be due to the storage process of Barbados Cherry fruit pulp, which has already been reported by Lima et al. (2002). These authors reported that Barbados Cherry pulp stored under freezing reduces, over time, the levels of anthocyanins and flavonoids by about 4.3 and 13.44% respectively. Reducing the levels of flavonoids may result in the oxidation of ascorbic acid and degradation of anthocyanins in the frozen pulp of Barbados Cherry fruit, which may have resulted in cytotoxicity in the present study with Allium cepa in the frozen pulp of Barbados Cherry becoming more evident at this concentration.
Furthermore, some authors observed that the Barbados Cherry juice stored at low temperature shows a gradual reduction of the vitamin C, beta-carotene, and anthocyanins content over time (GOMES et al., 2001; LIMA et al., 2003; ARAÚJO et al., 2007). The decrease in the levels of antioxidants, especially vitamin C and anthocyanins, may also be due to the processing steps (formulation/homogenization and thermal treatment) of the Barbados Cherry fruit pulp, as shown by Maia et al. (2007). All of these components are essential for the antioxidant activity and benefits of Barbados Cherry fruit for human health. Hence, their decrease may have contributed to the reduction in the mitotic index induced by the frozen Barbados Cherry fruit pulp.
The highest concentration (0.4 mg.mL1) of Barbados Cherry in natura also caused cell division inhibition of (1.7%), and it was statistically different from its negative control (9.8%) and their respective time of the negative control experiment (7.7%), but the cells divided during the recovery period again (5.7%). It is important to mention that the fruits used in this study were collected at a full maturity stage, and according to some studies, vitamin C can decrease during the ripening of fruits (NOGUEIRA et al., 2002; BRUNINI et al., 2004). According to Vendramini and Trugo (2000), the content of ascorbic acid unripe fruit is much lower than in red fruit with a loss of 50% by biochemical oxidation. In addition, Oliveira et al. (1999) showed that the soluble solids and vitamins may vary depending on the amount of rain during harvest, weather, variety, handling, soil, and others.
However, these two groups, frozen pulp (0.2 and 0.4 mg.mL1) and fruit in natura (0.2, 0.4 and 5.0 mg.mL1) showed no cytotoxic nor mutagenic effect in the bone marrow test with mammals Wistar rats in the acute treatment since their percentage of mitotic index (BAF: [0.2] = 2.1 and [0.4] = 0.7; BAN: [0.2] = 1.9, [0.4] = 1.5 and [5.0] = 2.0) and chromosomal abnormalities (BAF : [0.2] = 1.0 and [0.4] = 0.8; BAN: [0.2] = 0.7, [0.4] = 0.3 and [5.0] = 0.8) were statistically similar to those of the acute negative control (MI = 1.7 and CA = 0.3). These results corroborate those found by Nunes et al. (2011), who also showed that 0.5, 1 and 2 mg.mL1 of unripe and ripe Barbados Cherry fruit showed no genotoxicity in rat cells, according to the comet assay.
The negative results obtained for the mitotic index were similar to those obtained by Nefic (2001), who observed that vitamin C, the antioxidant component of Barbados Cherry, did not change the mitotic index value in the human peripheral lymphocytes treated. The non- mutagenicity of in vivo, frozen pulp, and in natura Barbados Cherry may be due to its high antioxidant potential due to the combination of the components with this property, such as vitamins A, B1, B2, C, carotenes, and flavonoids, as shown by Konapacka, Widel and Rezeszowska-Wolny (1998). They may have provided a better protection against the damage caused by the free radicals generated by the rat metabolism preventing mutations that occur in chromosomal aberrations.
Costa et al. (2001) confirmed that the administration of 150 mL of Barbados Cherry juice (average content of ascorbic acid 565 mg.100 mL1) for 35 days to 72 patients with low blood level of vitamin C increased vitamin A and hemoglobin levels in these individuals. Aranha et al. (2004) also observed that supplementation with natural vitamin juice Barbados Cherry (500 mg vitamin C) for 30 days was more efficient than that with vitamin C tablets to normalize the serum levels in elderly patients with vitamin C deficiency (tablet with 500 mg of vitamin C).
The two concentrations of synthetic vitamin C in liquid form evaluated in this study showed no cytotoxic effects (MI = 1.8%), and the lowest dose of vitamin C (0.3 mg.mL1) was not mutagenic (0.7%). However, the higher dose of vitamin C (0.6 mg.mL1) caused a statistically significant increase in the percentage of chromosomal abnormalities (1.7%), especially chromatid breaks (6 of 10 found breaks), compared to that of the control negative (0.3%) for bone marrow cells from Wistar rats.
These data corroborate the findings of Bhat et al. (2006), who, using human peripheral lymphocytes and the comet assay, showed that ascorbic acid (100-200 mM) is capable of causing oxidative damage to the DNA of normal cells. Cai, Koropatnick and Cherian (2001) also found that high concentrations of chromium (50 µM) and vitamin C (2 mM) may react with oxygen radicals and cause DNA damage. Similarly, Khan and Sinha (2008) showed that 10 mg of vitamin C increased the recessive lethal mutation frequency of X-chromosome in Drosophila melanogaster by more than 3 times compared to the control frequency level. Campos-da-Paz et al. (2008), using comet assay, showed that a higher concentration of vitamin C (100 mg.mL1) induced DNA damage on human submandibular gland cells and on oral epithelium cells.
Therefore, although many studies have demonstrated the antimutagenic activity of ascorbic acid (CAI; KOROPATNICK; CHERIAN, 2001; ALY; DONYA, 2003; ORTMANN et al., 2004; JAGETIA; RAJANIKANT; RAO, 2007; MOZDARANI; GHORAEIAN, 2008; WAMBI et al., 2008; ASSAYED; KHALAF; SALEM, 2010), in some cases, it can act as a co-mutagenic leading to the generation of oxygen radicals that induce sister chromatid exchanges and other cytogenetic damage (KONAPACKA; WIDEL; REZESZOWSKA-WOLNY, 1998; KAYA et al., 2002).
The cytotoxic activity of vitamin C is attributed to the formation of oxygen peroxides (H2O2) on surface or inside the cells, which result from the oxidation, through Fenton reaction, of this acid in the presence of oxygen and metal ion (HALLIWELL, 2001). Thus, vitamin C may affect cellular macromolecules, have cytotoxic and genetic effects, and can cause cellular apoptosis (ALY; DONYA, 2002; OHSAWA et al., 2003).
Since synthetic vitamin C in liquid form and the frozen pulp of Barbados Cherry also undergo manufacturing process, storage, and processing, the chemical oxidation and/or thermal degradation of ascorbic acid may have occurred as a result of its bleaching, cooking, pasteurisation, sterilization, or dehydration (MAIA et al., 2007).
The comparison between the percentage of chromosomal aberrations and mitotic index of the acute and subchronic treatments at 5 mg.mL1 of in natura Barbados Cherry in the present study did not show statistically different results. Similar results were found by El-Nahas, Mattar and Mohamed (1993), who found that vitamins C and E (100 and 300 mg.kg1.day1), in a six month-treatment, showed no cytotoxic or mutagenic activity in albino rats. Cameron and Campbell (1974) also reported good clinical response on palliative treatment in 55 advanced cancer patients with the continuous administration of high doses of ascorbic acid.
These data indicate that even receiving high daily doses of Barbados Cherry fruit juice in natura and, therefore, higher doses of vitamin C, this natural vitamin did not undergo oxidation, nor did it form oxygen peroxide which could lead to cytotoxic or mutagenic activity of this group.
This results are different from those found by Franke et al. (2005), who investigated simple (24 hours) and double (24 and 48 hours) treatments with doses of 1 and 30 mg vitamin C.kg1 of mice and confirmed the genotoxic effects of these two doses and found that the highest dose caused greater damage to DNA.
Therefore, the results of the present emphasize the need of greater knowledge about the cytotoxic and mutagenic potential of fruits and vitamins, especially those that are part of human diet.
As evidenced in the present study, the literature data of studies testing the mutagenicity of Barbados Cherry fruit in natura and frozen pulp in Wistar rats show health benefits of fruit and vegetable consumption and demonstrate no cytotoxic or mutagenic potential. Note that the literature emphasizes that the consumption of vegetables is more beneficial than the consumption of concentrated synthetic vitamins, as shown in this study testing synthetic vitamin C in liquid form, which showed mutagenic activity.
In addition, since fruits are natural foods, they have other beneficial compounds such as flavonoids, anthocyanins, and carotenoids, they are easily available in the market and are not expensive, and they can be widely consumed by the population. The negative results of the mutagenic activity of Barbados Cherry fruit stimulate its use in the prevention of diseases and improvement of population health.
5 Conclusion
The results of this study showed that the frozen and in natura pulp of Barbados Cherry inhibited cell division in Allium cepa L. and that all treatments with Barbados Cherry, acute or subchronic, did not cause cytotoxic or mutagenic effects in Wistar rats; however, the largest concentration of vitamin C increased significantly the percentage of chromosomal abnormalities.
The data obtained are important because they encourage the consumption of juices with numerous medicinal properties and no mutagenic potential instead of synthetic compounds.
Acknowledgements
The authors gratefully acknowledge the Coordination for the Improvement of Higher Level Personnel CAPES and the Laboratory of Cytogenetics and Mutagenesis staff of the State University of Maringá UEM.
Received 04/10/2012
Accepted 28/01/2012 (005491)
- ALY, F. A. E.; DONYA, S. M. In vivo antimutagenic effect of vitamins C and E against rifampicin-induced chromosome aberrations in mouse bone-marrow cells. Mutation Research, v. 518, p. 1-7, 2002.
- ARANHA, F. Q. et al. Normalização dos níveis séricos de ácido ascórbico por suplementação com suco de Barbados Cherry (Malpighia glabra L.) ou farmacológica em idosos institucionalizados. Revista de Nutrição, v. 17, p. 309-317, 2004.
- ARAÚJO, P. G. L. et al. Β-caroteno, ácido ascórbico e antocianinas totais em polpa de frutos de aceroleira conservada por congelamento durante 12 meses. Ciência e Tecnologia de Alimentos, v. 27, p. 304-307, 2007.
- ASSAYED, M. E.; KHALAF, A. A.; SALEM, H. A. Protective effects of garlic extract and vitamin C against in vivo cypermethrin-induced cytogenetic damage in rat bone-marrow. Mutation Research, v. 702, p. 1-7, 2010.
- AZEVEDO, L. et al. Diferential response related to genotoxicity between eggplant (Solanum melangena) skin aqueous extract and its main purified anthocyanin (delphinidin) in vivo. Food and Chemical Toxicology, v. 45, p. 852-858, 2007. PMid:17194516. http://dx.doi.org/10.1016/j.fct.2006.11.004
- BADEJO, A. A. et al. Cloning and expression of GDP-d-mannose pyrophosphorylase gene and ascorbic acid content of Barbados Cherry (Malpighia glabra L.) fruit at ripening stages. Plant Physiology and Biochemistry, v. 45, p. 665-672, 2007. PMid:17764967. http://dx.doi.org/10.1016/j.plaphy.2007.07.003
- BHAT, S. H. et al. Ascorbic acid mobilizes endogenous copper in human peripheral lymphocytes leading to oxidative DNA breakage A putative mechanism for anticancer properties. The International Journal of Biochemistry & Cell Biology, v. 38, p. 2074-2081, 2006. http://dx.doi.org/10.1016/j.biocel.2006.05.017
- BRUNINI, M. A. et al. Caracterização física e química de Barbados Cherrys provenientes de diferentes regiões de cultivo. Revista Brasileira de Fruticultura, v. 26, p. 486-489, 2004. http://dx.doi.org/10.1590/S0100-29452004000300027
- CAI, L.; KOROPATNICK, J.; CHERIAN, M. G. Roles of vitamin C in radiation-induced DNA damage in presence and absence of copper. Chemico-Biological Interactions, v. 137, p. 75-88, 2001. http://dx.doi.org/10.1016/S0009-2797(01)00210-1
- CAMERON, E.; CAMPBELL, A. The orthomolecular treatment of cancer II. Clinical trial of high-dose ascorbic acid supplements in advanced human cancer. Chemico-Biological Interactions, v. 9, p. 285-315, 1974. http://dx.doi.org/10.1016/0009-2797(74)90019-2
- CAMPOS-DA-PAZ, M. et al. Interaction of bracken-fern extract with vitamin C in human submandibular gland and oral epithelium cell lines. Mutation Research, v. 652, p. 158-163, 2008.
- COSTA, M. J. C. et al. Efeito da suplementação com Barbados Cherry nos níveis sangüíneos de vitamina C e de hemoglobina em crianças pré-escolares. Revista de Nutrição, v. 14, p. 13-20, 2001. http://dx.doi.org/10.1590/S1415-52732001000100003
- DE FLORA, S.; BAGNASCO, M.; VAINIO, H. Modulation of genotoxic and related effects by carotenoids and vitamin A in experimental models mechanistic issues. Mutagenesis, v. 14, p. 153-172, 1999. PMid:10229917. http://dx.doi.org/10.1093/mutage/14.2.153
- EL-NAHAS, S. M.; MATTAR, F. E.; MOHAMED, A. A. Radioprotective effect of vitamins C and E. Mutation Research, v. 301, p. 143-147, 1993. http://dx.doi.org/10.1016/0165-7992(93)90037-V
- FISKESJÖ, G. The Allium test as a standard in environmental monitoring. Hereditas, v. 102, p. 99-112, 1956.
- FORD, C. E.; HAMERTON, J. L. A colchicine, hypotonic citrate, squash sequence for mammalian chromosome. Stain Technology, v. 31, p. 247-251, 1956.
- FRANKE, S. I. R. et al. Possible repair action of vitamin C on DNA damage induced by methyl methanesulfonate, cyclophosphamide, FeSO4 and CuSO4 in mouse blood cells in vivo. Mutation Research, v. 583, p. 75-84, 2005.
- GOMES, J. E. et al. Comportamento de propriedades físicas, químicas e reológicas do suco de Barbados Cherry armazenado a baixa temperatura. Revista Brasileira de Engenharia Agrícola e Ambiental, v. 5, p. 296-300, 2001. http://dx.doi.org/10.1590/S1415-43662001000200020
- HALLIWELL, B. Vitamin C and genomic stability. Mutation Research, v. 475, p. 29-35, 2001. http://dx.doi.org/10.1016/S0027-5107(01)00072-0
- JAGETIA, G. C.; RAJANIKANT, G.K.; RAO, K. V. N. M. Ascorbic acid increases healing of excision wounds of mice whole body exposed to different doses of γ-radiation. Burns, v. 33, p. 484-494, 2007. PMid:17223272. http://dx.doi.org/10.1016/j.burns.2006.08.025
- KAYA, B. et al. Genotoxicity is modulated by ascorbic acid studies using the wing spot test in DrosophiLa. Mutation Research, v. 520, p. 93-101, 2002. PMid:12297148.
- KAUR, S. et al. The in vitro cytotoxic and apoptotic activity of Triphala-an Indian Herbal drug. Jounal of Ethopharmacology, v. 97, p. 15-20, 2005. PMid:15652269. http://dx.doi.org/10.1016/j.jep.2004.09.050
- KHAN, P. K.; SINHA, S. P. Antimutagenic Profile of Antioxidant Vitamins in Drosophila Mulation Test. Biomedical and Environmental Sciences, v. 21, p. 163-166, 2008. http://dx.doi.org/10.1016/S0895-3988(08)60023-9
- KONAPACKA, M.; WIDEL, M.; REZESZOWSKA-WOLNY, J. Modifying effect of vitamins C, E and beta-carotene against gamma-ray-induced DNA damage in mouse cells. MUTATION RESEARCH, v. 417, p. 85-94, 1998. PMid:9733928.
- KUSKOSKI, E. et al. Frutos tropicais silvestres e polpas de frutas congeladas atividade antioxidante, polifenóis e antocianinas. Ciência Rural, v. 36, p. 1283-1287, 2006. http://dx.doi.org/10.1590/S0103-84782006000400037
- LIMA, V. L. A. G. et al. Polpa congelada de Barbados Cherry efeito da temperatura sobre os teores de antocianinas e flavonóides totais. Revista Brasileira de Fruticultura, v. 24, p. 669-670, 2002. http://dx.doi.org/10.1590/S0100-29452002000300024
- LIMA, V. L. A. G. et al. Avaliação do teor de antocianinas em polpa de Barbados Cherry congelada proveniente de frutos de 12 diferentes aceroleiras (Malpighia emarginata D.C.). Ciência e Tecnologia de Alimentos, v. 23, p. 101-103, 2003. http://dx.doi.org/10.1590/S0101-20612003000100021
- MAIA, G. A. et al. Efeito do processamento sobre componentes do suco de Barbados Cherry. Ciência e Tecnologia de Alimentos, v. 27, p. 130-134, 2007. http://dx.doi.org/10.1590/S0101-20612007000100023
- MARQUES, L. G.; FERREIRA, M. C.; FREIRE, J. T. Freeze-drying of Barbados Cherry (Malpighia glabra L.). Chemical Engineering Processing, v. 46, p. 451-457, 2007. http://dx.doi.org/10.1016/j.cep.2006.04.011
- MEZADRI, T. et al. The Barbados Cherry fruit composition, productive characteristics and economic importance. Archivos Latinoamericanos Nutricion, v. 56, p. 101-109, 2006. PMid:17024954.
- MOZDARANI, H.; GHORAEIAN, P. Modulation of gamma-ray-induced apoptosis in human peripheral blood leukocytes by famotidine and vitamin C. Mutation Research, v. 649, p. 71-78, 2008. PMid:17851119.
- NASSIF, D. S. P.; CÍCERO, S. M. Avaliação de sementes de Barbados Cherry por meio de Raios-X. Revista Brasileira de Fruticultura, v. 28, p. 542-545, 2006. http://dx.doi.org/10.1590/S0100-29452006000300047
- NEFIC, H. Anticlastogenic effect of Vitamin C on cisplatin induced chromosome aberrations in human lymphocyte cultures. Mutation Research, v. 498, p. 89-98, 2001. PMid:11673074.
- NOGUEIRA, R. J. M. C. et al. Efeito do estágio de maturação dos frutos nas características físico-químicas da Barbados Cherry. Pesquisa Agropecuária Brasileira, v. 37, p. 463-470, 2002. http://dx.doi.org/10.1590/S0100-204X2002000400006
- NUNES, R.S. et al. Antigenotoxicity and Antioxidant Activity of Acerola Fruit (Malpighia glabra L.) at Two Stages of Ripeness. Plant Foods for Human Nutrition, v. 66, p. 129-135, 2011. PMid:21503669. http://dx.doi.org/10.1007/s11130-011-0223-7
- OHSAWA, K. et al. Detection of in vivo genotoxicity of endogenously formed N-nitroso compounds and supression by ascorbic acid, teas and fruit juices. Mutation Research, v. 539, p. 65-76, 2003. PMid:12948815.
- OLIVEIRA, M. E. B. et al. Avaliação de parâmetros de qualidade físico-químicos de polpas congeladas de Barbados Cherry, cajá e caju. Ciência e Tecnologia de Alimentos, v. 19, p. 1-18, 1999.
- ORTMANN, E. K. et al. Effect of antioxidant vitamins on radiation-induced apoptosis in cells of a human lymphoblastic cell line. Radiation Research v. 161, p. 48-55, 2004. PMID: 14680397.
- RIBEIRO, L. R.; MARQUES, E. K. A importância da mutagênese ambiental na carcinogênese humana. In: RIBEIRO, L. R. et al. Mutagênese Ambiental Canoas: Editora da ULBRA, 2003. p. 21-27.
- ROSSO, V. V.; MERCADANTE, A. Z. Carotenoid composition of two Brasilian genotypes of Barbados Cherry (Malpighia glabra L.) from two harvests. Food Research International, v. 38, p. 1073-1077, 2005. http://dx.doi.org/10.1016/j.foodres.2005.02.023
- TELESIL, M.; MACHADO, F. A. A influência do exercício físico e dos sistemas antioxidantes na formação de radicais livres no organismo humano. Sabios Revista de Saúde e Biologia, v. 3, p. 40-49, 2008.
- VENDRAMINI, A. L.; TRUGO, L. C. Chemical composition of Barbados Cherry fruit (Malpighia punicifoLia L.) at three stages of maturity. Food Chemistry, v. 71, p. 185-198, 2000. http://dx.doi.org/10.1016/S0308-8146(00)00152-7
- WAMBI, C. et al. Dietary antioxidants protect hematopoietic cells and improve animal survival after total-body irradiation. Radiation Research, v. 169, p. 384-396, 2008.
Publication Dates
-
Publication in this collection
10 May 2012 -
Date of issue
June 2012
History
-
Received
04 Oct 2012 -
Accepted
28 Jan 2012