Abstract
This work aimed to study the use of fruit salads as carriers for Lactobacillus rhamnosus HN001. We evaluated the viability of this probiotic in fruit salads and the phsyico-chemical, microbiological and sensory properties of this food. Scanning electron microscopy (SEM) was used to verify microorganism adhesion on the fruit tissues. The viability of L. rhamnosus in fruit salads was 8.49 log CFU.g-1 after 120 hours. SEM images showed that fruit tissue provided protection for probiotic. Adhesion sites were observed in higher quantity in banana, apple and guava. The addition of L. rhamnosus did not alter texture of fruits (p > 0.05). Fruit salads containing probiotic had different values of pH and acidity compared to the control (p < 0.05). Ascorbic acid content decreased over time; however, total carotenoids did not significantly decrease (p > 0.05). Fruit salads containing L. rhamnosus showed counts of psychotrophic microorganisms of at least 2.0 log CFU.g-1 lower than control salad after 120 h of refrigerated storage. The fruit salad was well accepted by consumers. Therefore, this product can be used as a carrier for probiotic and an alternative to consuming functional foods.
Keywords:
acceptability; fruit mix; microscopy; probiotic carrier
1 Introduction
Studies have shown the human health benefits of functional foods with probiotic microorganisms. Several definitions of probiotics have been published, however, the internationally accepted definition states probiotics are microorganisms that when administered in adequate amounts confer health benefits to the host (Food and Agriculture Organization & World Health Organization, 2001Food and Agriculture Organization – FAO, & World Health Organization – WHO. (2001). Evaluation of health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria (Report of a Joint FAO/WHO Expert Consultation, Córdoba, Argentina). Rome: FAO/WHO.). Rivera-Espinoza & Gallardo-Navarro (2010)Rivera-Espinoza, Y., & Gallardo-Navarro, Y. (2010). Non-dairy probiotic products. Food Microbiology, 27(1), 1-11. http://dx.doi.org/10.1016/j.fm.2008.06.008. PMid:19913684.
http://dx.doi.org/10.1016/j.fm.2008.06.0...
reported that fermented milk products as yogurt (Batista et al., 2015Batista, A. L. D., Silva, R., Cappato, L. P., Almada, C. N., Garcia, R. K. A., Silva, M. C., Raices, R. S. L., Arellano, D. B., Sant’Ana, A. S., Conte, C. A., Jr., Freitas, M. Q., & Cruz, A. G. (2015). Quality parameters of probiotic yogurt added to glucose oxidase compared to commercial products through microbiological,physical-chemical and metabolic activity analyses. Food Research International, 77, 627-635. http://dx.doi.org/10.1016/j.foodres.2015.08.017.
http://dx.doi.org/10.1016/j.foodres.2015...
; Cruz et al., 2013Cruz, A. G., Castro, W. F., Faria, J. A. F., Bolini, H. M. A., Celeghini, R. M. S., Raices, R. S. L., Oliveira, C. A. F., Freitas, M. Q., Conte, C. A., Jr., & Mársico, E. T. (2013). Stability of probiotic yogurt added with glucose oxidase in plastic materials with different permeability oxygen rates during the refrigerated storage. Food Research International, 51(2), 723-728. http://dx.doi.org/10.1016/j.foodres.2013.01.028.
http://dx.doi.org/10.1016/j.foodres.2013...
), petit suisse cheese (Pereira et al., 2016Pereira, E. P. R., Faria, J. A. F., Cavalcanti, R. N., Garcia, R. K. A., Silva, R., Esmerino, E. A., Cappato, L. P., Arellano, D. B., Raices, R. S. L., Silva, M. C., Padilha, M. C., Meireles, M. A., Bolini, H. M. A., & Cruz, A. G. (2016). Oxidative stress in probiotic Petit Suisse: is the jabuticaba skin extract a potential option? Food Research International, 81, 149-156. http://dx.doi.org/10.1016/j.foodres.2015.12.034.
http://dx.doi.org/10.1016/j.foodres.2015...
), minas frescal cheese (Dantas et al., 2016Dantas, A. B., Jesus, V. F., Silva, R., Almada, C. N., Esmerino, E. A., Cappato, L. P., Silva, M. C., Raices, R. S. L., Cavalcanti, R. N., Carvalho, C. C., Sant’Ana, A. S., Bolini, H. M. A., Freitas, M. Q., & Cruz, A. G. (2016). Manufacture of probiotic Minas Frescal cheese with Lactobacillus casei Zhang. Journal of Dairy Science, 99(1), 18-30. http://dx.doi.org/10.3168/jds.2015-9880. PMid:26519974.
http://dx.doi.org/10.3168/jds.2015-9880...
), and dairy beverages (Castro et al., 2013aCastro, W. F., Cruz, A. G., Bisinotto, M. S., Guerreiro, L. M. R., Faria, J. A. F., Bolini, H. M. A., Cunha, R. L., & Deliza, R. (2013a). Development of probiotic dairy beverages: rheological properties and application of mathematical models in sensory evaluation. Journal of Dairy Science, 96(1), 16-25. http://dx.doi.org/10.3168/jds.2012-5590. PMid:23102956.
http://dx.doi.org/10.3168/jds.2012-5590...
, bCastro, W. F., Cruz, A. G., Rodrigues, D., Ghiselli, G., Oliveira, C. A. F., Faria, J. A. F., & Godoy, H. T. (2013b). Short communication: effects of different whey concentrations on physicochemical characteristics and viable counts of starter bacteria in dairy beverage supplemented with probiotics. Journal of Dairy Science, 96(1), 96-100. http://dx.doi.org/10.3168/jds.2012-5576. PMid:23182354.
http://dx.doi.org/10.3168/jds.2012-5576...
) are good carrier matrices for probiotic microorganisms. However, raw food products have recently been intensively investigated as potential substrates for the production of probiotic non-dairy foods (Martins et al., 2013Martins, E. M. F., Ramos, A. M., Vanzela, E. S. L., Stringheta, P. C., Pinto, C. L. O., & Martins, J. M. (2013). Products of vegetable origin: a new alternative for the consumption of probiotic bacteria. Food Research International, 51(2), 764-770. http://dx.doi.org/10.1016/j.foodres.2013.01.047.
http://dx.doi.org/10.1016/j.foodres.2013...
; Peres et al., 2012Peres, C. M., Peres, C., Hernández-Mendoza, A., & Malcata, F. X. (2012). Review on fermented plant materials as carriers and sources of potentially probiotic lactic acid bacteria – with an emphasis on table olives. Trends in Food Science & Technology, 26(1), 31-42. http://dx.doi.org/10.1016/j.tifs.2012.01.006.
http://dx.doi.org/10.1016/j.tifs.2012.01...
; Soccol et al., 2010Soccol, C. R., Vandenberghe, L. P. S., Spier, M. R., Medeiros, A. B. P., Yamaguishi, C. T., Lindner, J. D., Pandey, A., & Soccol, V. T. (2010). The potential of probiotics. Food Technology and Biotechnology, 48(4), 413-434.; Yu et al., 2012Yu, Z. H., Zhang, X., Li, S. Y., Li, C. Y., Li, D., & Yang, Z. N. (2012). In vitro evaluation of probiotic properties of strains isolated from Chinese Sauerkraut. Lactobacillus plantarumAfrican Journal of Biotechnology, 11(21), 4868-4875.).
Products of plant origin, such as fruit, may be considered ideal substrates for probiotic since they contain nutrients such as vitamins, minerals, carbohydrates, fibers and antioxidant compounds (Nicolescu & Buruleanu, 2010Nicolescu, C. L., & Buruleanu, L. C. (2010). Correlation of some substrate parameters in growing on Vegetable and fruit cocktail juices. Lactobacillus acidophilusBulletin of the University of Agricultural Sciences and Veterinary Medicine, 67(2), 352-359.; Russo et al., 2014Russo, P., Chiara, M. L. V., Vernile, A., Amodio, M. L., Arena, M. P., Capozzi, V., Massa, S., & Spano, G. (2014). Fresh-cut pineapple as a new carrier of probiotic lactic acid bacteria. BioMed Research International, 2014, 1-9. http://dx.doi.org/10.1155/2014/309183.
http://dx.doi.org/10.1155/2014/309183...
; Soccol et al., 2010Soccol, C. R., Vandenberghe, L. P. S., Spier, M. R., Medeiros, A. B. P., Yamaguishi, C. T., Lindner, J. D., Pandey, A., & Soccol, V. T. (2010). The potential of probiotics. Food Technology and Biotechnology, 48(4), 413-434.; Yoon et al., 2004Yoon, K. Y., Woodams, E. E., & Hang, Y. D. (2004). Probiotication of tomato juice by lactic acid bacteria. Journal of Microbiology (Seoul, Korea), 42(4), 315-318. PMid:15650688.). Also, these foods contain no allergenic substances, which are present in dairy products and could restrict their consumption (Martins et al., 2015Martins, E. M. F., Ramos, A. M., Martins, M. L., & Rodrigues, M. Z. (2015). Research and development of probiotic products from vegetable bases: a new alternative for consuming functional food. In V. R. Rai & J. A. Bai. Beneficial microbes in fermented and functional foods (pp. 207-223). Boca Raton: CRC Press.).
Minimally processed products, such as fruits and vegetables, have great marketing potential due to consumer demand for fresh and healthy food (Oliveira et al., 2011Oliveira, M. A., Souza, V. M., Bergamini, A. M. M., & Martinis, E. C. P. (2011). Microbiological quality of ready-to-eat minimally processed vegetables consumed in Brazil. Food Control, 22(8), 1400-1403. http://dx.doi.org/10.1016/j.foodcont.2011.02.020.
http://dx.doi.org/10.1016/j.foodcont.201...
) and the feasibility of using fresh-cut fruits to vehicle probiotic microorganisms is arising scientific interest (Russo et al., 2014Russo, P., Chiara, M. L. V., Vernile, A., Amodio, M. L., Arena, M. P., Capozzi, V., Massa, S., & Spano, G. (2014). Fresh-cut pineapple as a new carrier of probiotic lactic acid bacteria. BioMed Research International, 2014, 1-9. http://dx.doi.org/10.1155/2014/309183.
http://dx.doi.org/10.1155/2014/309183...
). According to Sheehan et al. (2007)Sheehan, V. M., Ross, P., & Fitzgerald, G. F. (2007). Assessing the acid tolerance and the technological robustness of probiotic cultures for fortification in fruit juices. Innovative Food Science & Emerging Technologies, 8(2), 279-284. http://dx.doi.org/10.1016/j.ifset.2007.01.007.
http://dx.doi.org/10.1016/j.ifset.2007.0...
, probiotic microorganisms found in fruit products provide a promising area for exploration, especially due of their ability to tolerate acidic environments.
Food is considered a major factor regulating the colonization of probiotic in the gastrointestinal tract. Thus, food can influence probiotic growth, survival, viability and functionality, which determines their effectiveness (Ranadheera et al., 2010Ranadheera, R. D. C. S., Baines, S. K., & Adams, M. C. (2010). Importance of food in probiotic efficacy. Food Research International, 43(1), 1-7. http://dx.doi.org/10.1016/j.foodres.2009.09.009.
http://dx.doi.org/10.1016/j.foodres.2009...
). Additionally, probiotics should be able to be carried in food matrices which are easily accepted by consumer.
Fruit juices are already being used as carriers of probiotic bacteria (Ankolekar et al., 2012Ankolekar, C., Pinto, M., Greene, D., & Shetty, K. (2012). In vitro bioassay based screening of antihyperglycemia and antihypertensive activities of . Lactobacillus acidophilus fermented pear juiceInnovative Food Science & Emerging Technologies, 13, 221-230. http://dx.doi.org/10.1016/j.ifset.2011.10.008.
http://dx.doi.org/10.1016/j.ifset.2011.1...
; Antunes et al., 2013Antunes, A. E. C., Liserre, A. M., Coelho, A. L. A., Menezes, C. R., Moreno, I., Yotsuyanagi, K., & Azambuja, N. C. (2013). Acerola nectar with added microencapsulated probiotic. LWT - Food Science and Technology, 54, 125-131.; Fonteles et al., 2011Fonteles, T. V., Costa, M. G. M., Jesus, A. L. T., & Rodrigues, S. (2011). Optimization of the fermentation of cataloupe juice by . Lactobacillus casei NRRL B-442Food and Bioprocess Technology, 5(7), 2819-2826. http://dx.doi.org/10.1007/s11947-011-0600-0.
http://dx.doi.org/10.1007/s11947-011-060...
; Pereira et al., 2011Pereira, A. L. F., Maciel, T. C., & Rodrigues, S. (2011). Probiotic beverage from cashew apple juice fermented with Lactobacillus casei.Food Research International, 44(5), 1276-1283. http://dx.doi.org/10.1016/j.foodres.2010.11.035.
http://dx.doi.org/10.1016/j.foodres.2010...
). However, few studies with minimally processed fruit have been developed. Thus, the development of fruit salads containing probiotic microorganisms is a viable alternative for minimally processed foods, as well as non-dairy probiotic products, considering the acceptability and practicality offered to consumers who can buy a variety of fruits ready to eat containing also the probiotic cultures of high functionality. Therefore, this study aimed to evaluate the potential use of fruit salads as carriers of L. rhamnosus HN001.
2 Materials and methods
2.1 Minimal processing of fruit and salad preparation
In this work, usually consumed fruits as salad in Brazil such as pineapple, banana, guava, apple, papaya and mango were used at commercial maturity. Fruits were bought at the local market of Rio Pomba (Brazil). Fruits were washed in clean water to eliminate impurities and dirt, and were immersed for 20 min. at 5 °C in water with sodium dichloroisocyanurate (Sumaveg® Diversey Lever) at a concentration of 200 mg L-1 of residual active chlorine to inactivate the microorganisms. The fruits were peeled and manually cut with stainless steel knives into cubes of approximately 1x1 cm2. Fruit salads were prepared containing all fruits in equal proportion. All experiments were performed in three replicates.
2.2 Probiotic microorganism inoculation and antibrowning agent addition
Fruit salads were immersed in solution containing approximately 1010 CFU·mL-1 of the probiotic microorganism L. rhamnosus HN001 (DuPont/Danisco, Brazil). The probiotic culture was grown twice in MRS broth (De Man, Rogosa and Sharpe), incubated at 37 °C for 18 h, and again grown in MRS broth for 16 h. Afterwards, it was centrifuged at 5 °C for 15 minutes at 7000 g. The supernatant of the culture medium was discarded and the probiotic cell pellet was aseptically resuspended in a buffer solution of citric acid: sodium citrate at a 1:1 ratio and pH 3.8. The pellet was then resuspended in the same buffer solution, pH 3.8, at a ratio of 1:10; i.e., for every gram of cells, 10 ml of buffer solution was added to obtain at least 1010 cells·mL-1. Thus, to obtain fruit salads containing probiotic cultures, 1 ml of the previously prepared probiotic cell solution was added for each gram of fruit salad. This suspension was kept in contact with the fruit salads for 15 min. Fruit salads were drained for 3 min and immersed in an antibrowning solution containing 1% ascorbic acid (w/v) for 3 min. The ratio of this solution to fruit salad was 3:1. The control was a minimally processed fruit salad without the addition of L. rhamnosus HN001 and without 1% ascorbic acid.
2.3 Enumeration of L. rhamnosus HN001 in minimally processed fruit salad
Samples of 25 g from each treatment of fruit salads were homogenized in 225 mL of peptone saline solution (0.85% NaCl and 0.1% peptone) followed by serial dilutions. The pour plate method was used to enumerate the probiotic microorganisms, with 1 mL of each dilution placed on a Petri dish with a small amount of Rogosa SL agar (HIMEDIA, India). The Petri dishes were incubated in anaerobic jars at 37 °C for 72 h.
2.4 Evaluation of L. rhamnosus HN001 present in fruit salads by Scanning Electron Microscopy
Fruit salad treated with L. rhamnosus HN001 were analyzed by Scanning Electron Microscopy (SEM) according to Silveira (1989)Silveira, M. (1989). Preparo de amostras biológicas para microscopia eletrônica de varredura. In W. Souza, A. Haddad, A. Sesso, M. Silveira, O. M. Barth, R. D. Machado & T. Souto-Padrón (Eds.), Manual sobre técnicas básicas em microscopia eletrônica (pp. 71-79). Rio de Janeiro: Sociedade Brasileira de Microscopia Eletrônica.. In this analysis, each fruit component of salad was used to verify microbial adhesion, distribution and morphology of probiotic culture to plant tissue. These were analyzed at 0 h and after 120 h of storage at 8 °C. The experiment was performed in three repetitions and some images were presented.
Initially, fruits were sliced into 0.5 x 0.5 cm2 sections, 1-2 mm thick. To fix plant tissue cells, fragments of each fruit were transferred to a 5% (v/v) glutaraldehyde solution in 0.1 M phosphate buffer at 1:1 ratio. The final concentration of both reagents was 2.5% glutaraldehyde and 0.05 M phosphate buffer. Fruit fragments were kept in this solution for 18 h at 7 °C, then washed for 1 min in sodium phosphate buffer (0.05 mol L-1, pH 7.2). Then, they were dehydrated with acetone at 30 °GL, 50 ºGL, 70 ºGL and 90 ºGL for 10 min. Afterwards, the fragments were treated three times with acetone at 100 °GL for 10 min. Fruit fragments were transferred to the critical point dryer (model CPD020, Balzers, Liechstenstein) for total dehydration and samples were metalized using the Sputter Coater equipment (model FDU 010, Bal-Tec, Balzers, Liechtenstein) for observation in the SEM (LEO 1430 VP Zeiss, Cambridge, England) and image capture.
2.5 Phsyico-chemical evaluation
Acidity, pH, soluble solids (°Brix) and ascorbic acid determination
Acidity and pH were determined after 0, 24, 72 and 120 h of storage at 8 °C, according to AOAC (Association Official Methods of Analysis, 1995Association Official Methods of Analysis – AOAC. (1995). Official methods of analysis of the Association of Official Analytical Chemist. Washington: AOAC.). Samples of 1.667 g from each fruit were combined to achieve a total sampling mass of 10.00 g, for both the control and fruit salads treated with L. rhamnosus HN001. The soluble solids of the fruit salads were determined by refractometry (Association Official Methods of Analysis, 1995Association Official Methods of Analysis – AOAC. (1995). Official methods of analysis of the Association of Official Analytical Chemist. Washington: AOAC.), using an Abbe refractometer (model 100 RTA) at 0, 24, 72 and 120 h of storage at 8 °C.
Ascorbic acid content in control and fruit salads treated with L. rhamnosus was determined after 0, 24, 72 and 120 h of storage at 8 °C using the method of Tillmans according to Zenebon & Pascuet (2004)Zenebon, O., & Pascuet, N. S. (2004). Métodos físico-químicos para análise de alimentos. São Paulo: Instituto Adolfo Lutz..
Determination of total carotenoids
Total carotenoid content was analyzed for the control and for fruit salads treated with L. rhamnosus. Total carotenoids were extracted from samples according to Rodriguez-Amaya (2001)Rodriguez-Amaya, D. B. (2001). A guide to carotenoid analysis in foods (pp. 41-45). Washington: ISLI Press.. Acetone was used as the extractor solvent and total carotenoids were quantified in the spectrophotometer (450 nm). Analyses were done at 0h and after 120 h of storage at 8 °C. Results were expressed as mg of total carotenoids/g of salad, according to equation: Total carotenoids = V x A x 10 / P x E1cm1%. V is the volume of sample extract; A is the absorbance; P is the sample weight and E1cm1% is the molar extinction coefficient (ε = 2592 at 450 nm).
Determination of fruit firmness
The firmness of fruits from the control and fruit salad treated with L. rhamnosus was determined by compression test using a texture analyzer (CT3, Brookfield, USA) set with a load cell of 25 kg. Analyses were done after 0, 24, 72 and 120 h of processing. Results were expressed in Newtons (N). Three samples from each fruit were selected from each treatment of fruit salad analyzed by placing them individually on a flat surface being compressed by a 3.5 cm diameter probe (SMSP/35). The distance between the sample and the probe was 60 mm and the test speed was 5 mm s-1.
2.6 Microbiological analysis
Total and fecal coliforms were determined using samples from the control and from the fruit salad treated with probiotic culture. Analyses were done using the most probable number (MPN) method according Kornacki & Johnson (2001)Kornacki, J. L., & Johnson, J. L. (2001). Enterobacteriaceae, coliforms, and Escherichia coli as quality and safety indicators. In F. P. Downes & K. Ito (Eds.), Compendium of methods for the microbiological examination of foods (pp. 69-82). Washington: APHA.. To quantify Salmonella sp., samples of each treatment of fruit salad (25 g) were homogenized in 225 mL of buffered peptone water, according to Andrews et al. (2001)Andrews, W. H., Flower, R. S., Silliker, J., & Bailey, J. S. (2001). Salmonella. In F. P. Downes & K. Ito (Eds.), Compendium of methods for the microbilological examination of foods (pp. 357-380). Washington: APHA.. Psychrotrophic bacteria were enumerated according to Cousin et al. (2001)Cousin, M. A., Jay, J. M., & Vasavada, P. C. (2001). Psychrotrophic microrganisms. In F. P. Downes, & K. Ito (Eds.), Compendium of methods for the microbilological examination of foods (pp. 159-166). Washington: APHA. with Plate Count Agar (PCA). Petri dishes were incubated for 10 days at 7 °C and this microbiota was counted by selecting plates containing 25-250 colonies. The results were expressed in CFU (colony forming units) per gram of fruit salad. All microbiological analyses were performed in duplicate for the control treatment and for the fruit salads containing L. rhamnosus, at 0 h and after 120 h of storage at 8 °C.
2.7 Sensory evaluation of fruit salads
Sensory analysis was performed by following the technical standards of biosafety and ethics, under the approval of the ethics committee in research with human beings, of the Federal University of Viçosa (process number 084/2012). This analysis was carried out in individual sensory booths by 50 untrained tasters, 25 men and 25 women aged from 18 to 30 years old, that were students of Federal University of Viçosa, Brazil. Samples were codified with random 3-digit numbers. The acceptance test was performed on the control treatment and the fruit salads treated with L. rhamnosus at 0 h and after 120 h of storage at 8 °C. Each consumer received a form containing a nine-point hedonic scale, ranging from “like extremely” (score 9) to “dislike extremely” (score 1) (Morais et al., 2014Morais, E. C., Morais, A. R., Cruz, A. G., & Bolini, H. M. A. (2014). Development of chocolate dairy dessert with addition of prebiotics and replacement of sucrose with different high-intensity sweeteners. Journal of Dairy Science, 97(5), 2600-2609. http://dx.doi.org/10.3168/jds.2013-7603. PMid:24612793.
http://dx.doi.org/10.3168/jds.2013-7603...
; Santos et al., 2015Santos, B. A., Campagnol, P. C. B., Cruz, A. G., Morgano, M. A., Wagner, R., & Pollonio, M. A. R. (2015). Is there a potential consumer market for low-sodium fermented sausages? Journal of Food Science, 80(5), 1093-S1099. http://dx.doi.org/10.1111/1750-3841.12847. PMid:25808547.
http://dx.doi.org/10.1111/1750-3841.1284...
). Each untrained consumer evaluated the samples regarding color, flavor and overall impression. The results of the acceptance test regarding color, flavor and overall impression of fruit salads were analyzed with a 2x2 completely randomized factorial design. The independent variables analyzed were two treatments (control and fruit salad treated with L. rhamnosus) and two storage times (0 and 120 h).
2.8 Statistical analyzes
To study the viability of L. rhamnosus as well as the texture, pH, acidity and soluble solids of fruit salads, we used a completely randomized statistical design with three replications. Results were subjected to analysis of variance (ANOVA) followed by the Tukey test. To determine ascorbic acid content, we did a completely randomized 2x4 full factorial design with three replications. The independent variables studied were two treatments (control and fruit salads treated with L. rhamnosus) and four storage times (0, 48, 72 and 120 h). Results were subjected to ANOVA, followed by analysis of regression of variable time. The regression model was selected according to the coefficient of determination (r2). To determine total carotenoid content, we did a completely randomized 2x2 full factorial design with four replications. The independent variables studied were two treatments (control and fruit salads treated with L. rhamnosus) and two storage times (0 and 120 h). Only the F-test analysis of ANOVA was used. All statistical procedures were performed considering 5% level of probability using the R software (R Core Team, 2012R Core Team2012R: A language and environment for statistical computingViennaR Foundation for Statistical Computing).
3 Results and discussion
3.1 L. rhamnosus HN001 viability in minimally processed fruit salad during storage
L. rhamnosus HN001 count, in fruit salad with this probiotic, after processing (time 0) was 8.5 log CFU.g-1 and after 120 hours of storage at 8 °C was 8.49 CFU.g-1. Time did not affect (p > 0.05) the viability of L. rhamnosus and there was no correlation between time and the probiotic microorganism (p > 0.05). The viability of L. rhamnosus HN001 was maintained during the storage time in fruit salad.
According to Champagne & Gardner (2008)Champagne, C. P., & Gardner, N. J. (2008). Effect of storage in a fruit drink on subsequent survival of probiotic lactobacillito gastro-intestinal stresses. Food Research International, 41(5), 539-543. http://dx.doi.org/10.1016/j.foodres.2008.03.003.
http://dx.doi.org/10.1016/j.foodres.2008...
Lactobacillus strains viability in presence of 0.3% bile salts or pancreatic enzymes was not affected by refrigerated storage. Then, due to the good viability in fruit salad during the 120 hours of storage, it is expected that, for this period the functional properties of L. rhamnosus HN001 should be kept in the salad. However, this is dependent on the food matrix, probiotic strain, conditions and time of storage, since Fernandes et al. (2013)Fernandes, M. S., Cruz, A. G., Arroyo, D. M. D., Faria, J. A. F., Cristianini, M., & Sant’Ana, A. S. (2013). On the behavior of and . Listeria innocuaLactobacillus acidophilus co-inoculated in a dairy dessert and the potential impacts on food safety and product’s functionalityFood Control, 34(2), 331-335. http://dx.doi.org/10.1016/j.foodcont.2013.04.040.
http://dx.doi.org/10.1016/j.foodcont.201...
found loss of L. acidophilus viability in milk dessert during shelf life. In addition, the authors highlighted that probiotic cultures do not replace good manufacturing practices, since they found no antagonistic effect of Lactobacillus acidophilus on Listeria innocua. Also, in vivo studies should be performed to confirm the funtionaly of the probiotic in fruit salad (Lollo et al., 2015Lollo, P. C. B., Morato, P. N., Moura, C. S., Almada, C. N., Felicio, T. L., Esmerino, E. A., Barros, M. E., Amaya-Farfan, J., Sant’Ana, A. S., Raices, R. R. S., Silva, M. C., & Cruz, A. G. (2015). Hypertension parameters are attenuated by the continuous consumption of probiotic Minas cheese. Food Research International, 76, 611-617. http://dx.doi.org/10.1016/j.foodres.2015.07.015.
http://dx.doi.org/10.1016/j.foodres.2015...
).
The minimum concentration of probiotic microorganisms needed to provide a beneficial effect for the host organism remains unclear in the literature. Some researchers suggest concentrations higher than 106 CFU.g-1 (Dave & Shah, 1997Dave, R. I., & Shah, N. P. (1997). Effectiveness of ascorbic acid as an oxygen scavenger in improving viability of probiotic bacteria in yoghurts made with commercial starter cultures. International Dairy Journal, 7(6-7), 435-443. http://dx.doi.org/10.1016/S0958-6946(97)00026-5.
http://dx.doi.org/10.1016/S0958-6946(97)...
) while others suggest concentrations of at least 107-108 CFU.g-1 (Lourens-Hattingh & Viljeon, 2001Lourens-Hattingh, A., & Viljeon, C. B. (2001). Yogurt as probiotic carrier food. International Dairy Journal, 11(1-2), 1-17. http://dx.doi.org/10.1016/S0958-6946(01)00036-X.
http://dx.doi.org/10.1016/S0958-6946(01)...
). So, prepared fruit salads can be considered probiotic since they contain over 108 CFU of L. rhamnosus per gram. In addition, this product can be consumed by children, the elderly, vegetarians and individuals who are lactose intolerant or on cholesterol-restricted diets. In this way, the probiotic fruit salad developed here constitute a promising functional probiotic product that can be consumed by everybody.
A similar result was observed by Rößle et al. (2010)Rößle, C., Auty, M. A. E., Brunton, N., Gormley, R. T., & Butler, F. (2010). Evaluation of fresh-cut apple slices enriched with probiotic bacteria. Innovative Food Science & Emerging Technologies, 11(1), 203-209. http://dx.doi.org/10.1016/j.ifset.2009.08.016.
http://dx.doi.org/10.1016/j.ifset.2009.0...
, who used L. rhamnosus GG in minimally processed apples and found that after 10 days of storage the product contained 8.0 Log CFU.g-1 of this bacterium.
3.2 Scanning Electron Microscopy (SEM)
SEM images showed that a high number of L. rhamnosus HN001 adhered to the surface of the fruits present in minimally processed fruit salad (Figure 1). Bacterial cells were predominantly well distributed in the fruit tissue, and some fruits presented the formation of bacterial agglomeration. No changes due to stress, such as the low pH of fruit tissue, were observed in cell morphology, and bacterial cells were mainly rod-shaped.
L. rhamnosus HN001 on the surface of pineapple (A), banana (B), guava (C), apple (D), papaya (E), and manga (F) after processing of fruit salads (0 h) or after 120 h of storage. White arrows indicate cells adhered to the fruit tissue.
L. rhamnosus HN001 showed good adhesion capacity in fruits, being this greater in banana, apple and guava. The adherence of probiotic bacteria to the tissue of these fruits can be attributed to tissue format and microstructure. This protects microorganisms, enabling their survival. Moreover, intrinsic characteristics of the microarchitecture of the fruit surface protect probiotic bacteria from the acidic environment of the stomach, due to the presence of ridges and natural compounds of prebiotics, such as oligosaccharides. Typical operations of processing such as peeling and cutting can promote microbial adhesion to the fruits tissue, increasing the surface contact and the release of cellular content rich in nutrients which are ideal substrates for probiotic culture growth (Oliveira et al., 2011Oliveira, M. A., Souza, V. M., Bergamini, A. M. M., & Martinis, E. C. P. (2011). Microbiological quality of ready-to-eat minimally processed vegetables consumed in Brazil. Food Control, 22(8), 1400-1403. http://dx.doi.org/10.1016/j.foodcont.2011.02.020.
http://dx.doi.org/10.1016/j.foodcont.201...
; Russo et al., 2014Russo, P., Chiara, M. L. V., Vernile, A., Amodio, M. L., Arena, M. P., Capozzi, V., Massa, S., & Spano, G. (2014). Fresh-cut pineapple as a new carrier of probiotic lactic acid bacteria. BioMed Research International, 2014, 1-9. http://dx.doi.org/10.1155/2014/309183.
http://dx.doi.org/10.1155/2014/309183...
).
According Mitsou et al. (2011)Mitsou, E. K., Kougia, E., Nomikos, T. Z., Yannakoulia, M., Mountzouris, K. C., & Kyriacou, A. (2011). Effect of banana consumption on faecal microbiota: a randomised, controlled trial. Anaerobe, 17(6), 384-387. http://dx.doi.org/10.1016/j.anaerobe.2011.03.018. PMid:21524710.
http://dx.doi.org/10.1016/j.anaerobe.201...
, banana has considerable amounts of prebiotic fructooligosaccharides, which can contribute to the viability and persistence of L. rhamnosus HN001 in fruit salad. Thus, we verified that banana has potential to serve as a probiotic carrier. Apples are rich in carbohydrates, pectin, fiber and minerals. Also, apple is a good source of nutrients for probiotic cultures (Mahawar et al., 2012Mahawar, M., Singh, A., & Jalgaonkar, K. (2012). Utility of apple pomace as a substrate for various products: A review. Food and Bioproducts Processing, 90(4), 597-605. http://dx.doi.org/10.1016/j.fbp.2012.04.007.
http://dx.doi.org/10.1016/j.fbp.2012.04....
). In the present study, we found that L. rhamnosus HN001 adhered to fruit tissue in high numbers (Figures 1D and 1E).
The adhesion of L. rhamnosus HN001 to apple tissue is related to the intercellular spaces, also known as pores, which are in the parenchymal tissue of the fruit. These pores may play an important role in microorganism penetration and adherence since they occupy 20-25% of the total volume of the fruit. They are large enough to ensure that microbes can enter the fruit. Mature parenchymal cells of apple may have sizes ranging from 50 to 500 µm in diameter. Moreover, the size of bacterial cells ranges from 0.2 to 2.0 µm in diameter and 2.0 to 8.0 µm in length (Tortora et al., 2006Tortora, G. J., Funke, B. R., & Case, C. L. (2006). Microbiology an introduction. San Francisco: Benjamin Cummings.), which ensures the internalization of probiotic cultures in cellular compartments of fruit, such as apples.
In addition, guava also showed potential for use as a carrier matrix for L. rhamnosus HN001 (Figure 1C), but pineapple presented a low number of microorganisms attached to its tissue (Figure 1A). This is probably due to pineapple’s intrinsic characteristics, such as pH and acidity, which may limit the growth of probiotic bacteria.
It is interesting to note that the results obtained in SEM were consistent with the plate count, which demonstrated the viability of L. hamnosus HN001 in fruit salad.
Incorporating probiotic bacteria in processed fruits is a challenge. However, it is also highly advantageous, because these kinds of foods are rich in nutrients and are consumed and well accepted by most individuals (Saad et al., 2011Saad, S. M. I., Komatsu, T. R., Granato, D., Branco, G. F., & Buriti, F. C. A. (2011). Probióticos e prebióticos em alimentos: aspectos tecnológicos, legislação e segurança no uso. In S. M. I. Saad, A. G. Cruz & J. A. F. Faria (Eds.), Probióticos e prebióticos em alimentos: fundamentos e aplicações tecnológicas (pp. 23-49). São Paulo: Editora Varela.). Few studies demonstrate the effect of fruits as food matrices on the survival and activity of probiotic microorganisms. In this way, recent studies indicate a neutral and even a positive effect of fruits on probiotic microorganisms and on the interaction of the probiotic culture-host (Espírito-Santo et al., 2011Espírito-Santo, A. P., Perego, P., Converti, A., & Oliveira, M. O. (2011). Influence of food matrices on probiotic viability – A review focusing on the fruity bases. Trends in Food Science & Technology, 22(7), 377-385. http://dx.doi.org/10.1016/j.tifs.2011.04.008.
http://dx.doi.org/10.1016/j.tifs.2011.04...
; Martins et al., 2013Martins, E. M. F., Ramos, A. M., Vanzela, E. S. L., Stringheta, P. C., Pinto, C. L. O., & Martins, J. M. (2013). Products of vegetable origin: a new alternative for the consumption of probiotic bacteria. Food Research International, 51(2), 764-770. http://dx.doi.org/10.1016/j.foodres.2013.01.047.
http://dx.doi.org/10.1016/j.foodres.2013...
).
3.3 Phsyico-chemical characteristics of fruit salad
The fruit salad treated with L. rhamnosus HN001 showed pH and acidity values different from the control treatment (p<0.05) (Table 1). The reduction of pH and increase in acidity in the product with probiotic bacteria is probably due to the food product being immersed in a buffered solution of citric acid: sodium citrate, at pH 3.8. This was not done for the control treatment. Time did not influence pH, acidity or soluble solids content of fruit salads for either treatment (p>0.05). In this work, the pH and acidity values did not exert a negative effect on the survival of the probiotic culture.
Mean values of pH, acidity and soluble solids (°Brix) of minimally processed fruit salads and control treatments containing L. rhamnosus HN001 at different storage times.
Soluble solids content was not affected by the probiotic bacteria buffer solution, presenting no difference (p>0.05) among treatments. Similar results were also reported by Rößle et al. (2010)Rößle, C., Auty, M. A. E., Brunton, N., Gormley, R. T., & Butler, F. (2010). Evaluation of fresh-cut apple slices enriched with probiotic bacteria. Innovative Food Science & Emerging Technologies, 11(1), 203-209. http://dx.doi.org/10.1016/j.ifset.2009.08.016.
http://dx.doi.org/10.1016/j.ifset.2009.0...
, who observed no changes regarding this parameter in minimally processed apples inoculated with L. rhamnosus GG and stored at 2 to 4 °C for 10 days.
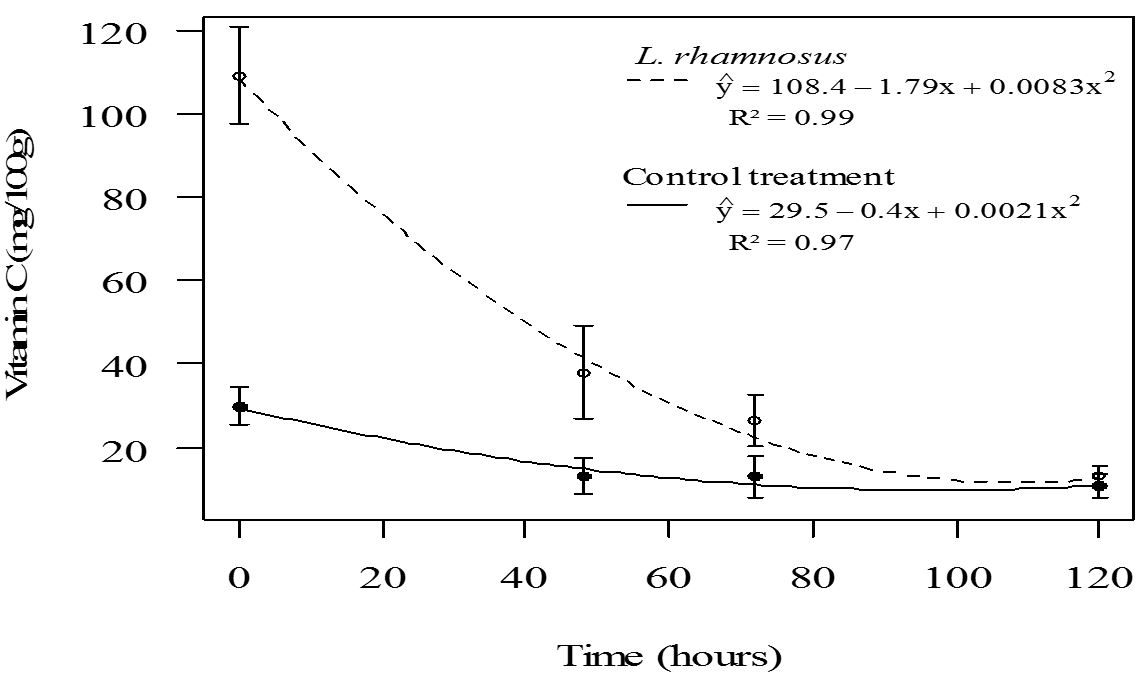
Also, it was verified that fruit salad with L. rhamnosus HN001 showed a higher content of ascorbic acid (p<0.05) compared to the control treatment, up to 72 h of storage (Figure 2). The high ascorbic acid content in fruit salads containing this probiotic is because the fruits were immersed in 1% ascorbic acid to minimize enzymatic browning, which promoted product enrichment compared to the control. Moreover, a marked reduction of ascorbic acid was observed over time in the fruit salad containing 1% ascorbic acid. This compound was free in the product and not naturally present, as observed in the control fruit salad, where a lower reduction was noted (Figure 2).
Regression of ascorbic acid content of control fruit salad and fruit salad treated with L. rhamnosus HN001, as a function of storage time.
The Daily Intake (DI) of vitamin C varies according to age and health. According to the RDC. n.° 269 of the Brazilian National Health Surveillance Agency (Anvisa), the recommended intake of vitamin C is 45 mg/day (Brasil, 2005Brasil. Agência Nacional de Vigilância Sanitária. (2005, September 23). Regulamento técnico sobre a Ingestão Diária Recomendada (IDR) de proteína, vitaminas e minerais (Resolução RDC n. 269, de 22 de setembro de 2005). Diário Oficial [da] República Federativa do Brasil.). Thus, considering the Brazilian legislation and the obtained results, consumption of 200 g of fruit salad of the control treatment or 100 g of fruit salad treated with probiotic culture, immediately after processing, will achieve the Anvisa recommended DI of vitamin C.
Moreover, storage time and the interaction of treatment and storage time had a significant effect (p < 0.05) on the ascorbic acid content of fruit salads. Thus, a regression equation for this variable was adjusted as a function of storage time for each treatment (Figure 2). The decrease of ascorbic acid content in fruit salads of both treatments as a function of time of storage (Figure 2) is consistent with Beaulieu (2011)Beaulieu, J. C. (2011). Factors affecting sensory quality of fresh-cut produce. In O. Martín-Belloso & R. Soliva-Fortuny (Eds.), Advances in fresh-cut fruits and vegetables processing (pp. 115-143). London: CRC Press., who reported that the vitamin C content is generally reduced after processing and tends to decrease with storage time. This is due to mechanical damage caused by minimal processing in plant tissues causing cellular disorganization, which promotes the oxidation of ascorbic acid to dehydroascorbic acid by the direct action of the enzyme ascorbate oxidase or by the action of oxidizing enzymes, such as peroxidase.
Total carotenoid content was not significantly different (p > 0.05) over the storage time among the control treatment and fruit salad treated with L. rhamnosus HN001. In this way, both treatments showed 0.01 mg/g of total carotenoids after 120 h of storage at 8 °C.
Rodriguez-Amaya et al. (2008)Rodriguez-Amaya, D. B., Kimura, M., & Amaya-Farfan, J. (2008). Fontes brasileiras de carotenóides: tabela brasileira de composição de carotenóides em alimentos. Brasília: MMA/SBF. reported that foods containing more than 0.02 mg/g of carotenoid are rich sources of this pigment. According to De-Ancos et al. (2011)De-Ancos, B., Sánchez-Moreno, C., Plaza, L., & Cano, M. P. (2011). Nutritional and health aspects of fresh-cut vegetables. In O. Martín-Belloso, & R. Soliva-Fortuny (Eds.), Advances in fresh-cut fruits and vegetables processing (pp. 145-184). London: CRC Press., there is no recommended DI for carotenoids; however, some studies suggest a DI of 5 to 6 mg/day. Thus, consumption of 100 g of prepared fruit salad offers the consumer 1 mg of total carotenoids and we consider this fruit salad as a good source of these beneficial compounds.
The addition of L. rhamnosus HN001 did not produce significant changes (p > 0.05) in fruit salad texture (Table 2). However, unlike what was observed in this study, Rößle et al. (2010)Rößle, C., Auty, M. A. E., Brunton, N., Gormley, R. T., & Butler, F. (2010). Evaluation of fresh-cut apple slices enriched with probiotic bacteria. Innovative Food Science & Emerging Technologies, 11(1), 203-209. http://dx.doi.org/10.1016/j.ifset.2009.08.016.
http://dx.doi.org/10.1016/j.ifset.2009.0...
found that apples from the control treatment and those treated with L. rhamnosus GG lost their firmness after the second day of storage.
Mean values of firmness (N) of fruits in control prepared fruit salads and containing L. rhamnosus.
The reduction of fruit firmness after processing and during storage was expected, due to damages in the fruit tissues, which promoted the release of water and exudate. The texture of mango and papaya contained in fruit salad treated with L. rhamnosus HN001 was influenced by storage time (p < 0.05). However, the texture of these fruits was not affected by the treatments (Table 2), or by the interaction of time and treatment.
Climacteric fruit, such as papaya and mango, have a short shelf life. Papaya is a fruit of high perishability. In this way, Sañudo-Barajas et al. (2009)Sañudo-Barajas, J. A., Labavitch, J., Greve, C., Osuna-Enciso, T., Muy-Rangel, D., & Siller-Cepeda, J. (2009). Cell wall disassembly during papaya softening: role of ethylenein changes in composition, pectin-derived oligomers (PDOs) production and wall hydrolases. Postharvest Biology and Technology, 51(2), 158-167. http://dx.doi.org/10.1016/j.postharvbio.2008.07.018.
http://dx.doi.org/10.1016/j.postharvbio....
studied the cellular metabolism and enzymes involved in the postharvest softening of papaya and found that at harvest time such fruits showed firmness equal to 144 N, diminishing to 17 N on the sixth day of storage. Tapia et al. (2008)Tapia, M. S., Rojas-Grau, M. A., Carmona, A., Rodriguez, F. J., Soliva-Fortuny, R., & Martin-Belloso, O. (2008). Use of alginate and gellan-based coatings for improving barrier, texture and nutritional properties of fresh-cut papaya. Food Hydrocolloids, 22(8), 1493-1503. http://dx.doi.org/10.1016/j.foodhyd.2007.10.004.
http://dx.doi.org/10.1016/j.foodhyd.2007...
found that the texture of minimally processed papaya was 2 N after eight days of storage at 4 °C. These authors found that papaya softening was caused mainly by hydrolysis of pectic acid in the cell walls, which promoted the loss of firmness, as observed in this study.
3.4 Microbiological characteristics of fruit salad
The quality and safety of minimally processed food products depend on the adoption of good agricultural practices and good manufacturing practices during all processing steps, especially the hygienic conditions of the food handlers and the storage temperature. Results of microbiological analysis after processing of control fruit salad and fruit salad with L. rhamnosus HN001 showed count of psychrotrophic microorganisms of 2,6 Log CFU/g and 2,5 Log CFU/g, respectively. However, the number of these microorganisms increased over time, with this number being higher in control fruit salad (5,1 Log CFU/g) compared to the fruit salad treated with L. rhamnosus HN001 (2,9 Log CFU/g).
Thus, the fruit salad with probiotic bacteria showed counts of psychrotrophic microorganisms of at least 2.0 log CFU.g-1 lower than control fruit salad after 120 h of storage at 8 °C. It could be due to the buffer solution used to add the probiotic to the fruit salad, and not due to the action of probiotic bacterium (biopreservation), once the buffer solution contained citric acid: sodium citrate at a 1:1 ratio, and pH 3.8.
However, previous studies have indicated that probiotic culture produces acids that promote the reduction of pH, creating conditions unfavorable to microbial growth. Pithva et al. (2011)Pithva, S., Ambalam, P., Dave, J. M., & Vyas, B. R. M. (2011). Antimicrobial peptides of probiotic Lactobacillus strains. In A. Méndez-Vilas (Ed.), Science against microbial pathogens: communicating current research and technological advances (pp. 987-991). Badajoz: Formatex. found that strains of L. rhamnosus produce antimicrobial peptides that inhibit Escherichia coli, Enterobacter aerogenes, Salmonella typhi, Shigella sp., Proteus vulgaris, Pseudomonas aeruginosa, Bacillus cereus and Staphylococcus aureus, among others. Alegre et al. (2011)Alegre, I., Viñas, I., Usall, J., Anguera, M., & Abadias, M. (2011). Microbiological and physicochemical quality of fresh-cut apple enriched with the probiotic strain Lactobacillus rhamnosus GG. Food Microbiology, 28(1), 59-66. http://dx.doi.org/10.1016/j.fm.2010.08.006. PMid:21056776.
http://dx.doi.org/10.1016/j.fm.2010.08.0...
evaluated the addition of L. rhamnosus GG in minimally processed apple and its effect on the growth of pathogens such as L. monocytogenes and Salmonella of different strains and found that L. monocytogenes decreased by 1 Log cycle in apples inoculated with this probiotic.
In this study, both treatments showed <3,0 MPN/g of total and fecal coliforms, and absence of Salmonella sp., which met the microbiological standards established by Brazilian legislation (Brasil, 2001Brasil. Agência Nacional de Vigilância Sanitária. (2001, January 10). Regulamento técnico sobre padrões microbiológicos para alimentos (Resolução RDC n.12, de 02 de janeiro de 2001). Diário Oficial [da] República Federativa do Brasil.). Thus, prepared fruit salad with L. rhamnosus HN001 is safe for consumption for up to 120 h after processing.
3.5 Sensory evaluation of fruit salads
According to Granato et al. (2010)Granato, D., Branco, G. F., Nazzaro, F., Cruz, A. G., & Faria, J. A. F. (2010). Functional food and non dairy probiotic food development: trends, concepts, and products. Comprehensive Reviews in Food Science and Food Safety, 9(3), 292-302. http://dx.doi.org/10.1111/j.1541-4337.2010.00110.x.
http://dx.doi.org/10.1111/j.1541-4337.20...
, the food industry takes into consideration many variables to develop non-dairy probiotic foods, among them sensory evaluation. Acceptance notes for both treatments (control and fruit salad with L. rhamnosus HN001) were above 7.0, “like moderately” on a nine-point hedonic scale. This result was observed for all the attributes evaluated (Table 3), indicating that control fruit salad and fruit salad with L. rhamnosus HN001 had a good acceptability and no significant differences (p > 0.05).
Mean values of scores for color, flavor and overall impression of fruit salads, control and treated with L. rhamnosus HN001, immediately after processing (0 h) and after 120 h of storage at 8 °C.
Probiotic bacteria did not promote (p > 0.05) changes in the acceptance of fruit salads regarding acceptance of color, flavor and overall impression after processing (time 0 h). However, storage time had a significant effect (p < 0.05) on attributes evaluated. Similar to our results, Rößle et al. (2010)Rößle, C., Auty, M. A. E., Brunton, N., Gormley, R. T., & Butler, F. (2010). Evaluation of fresh-cut apple slices enriched with probiotic bacteria. Innovative Food Science & Emerging Technologies, 11(1), 203-209. http://dx.doi.org/10.1016/j.ifset.2009.08.016.
http://dx.doi.org/10.1016/j.ifset.2009.0...
evaluated the acceptability of minimally processed apples enriched with probiotic culture and also found that the product was well accepted by consumers.
4 Conclusions
Minimally processed fruit salads can be considered a promising carrier for probiotic bacteria, since the count of L. rhamnosus in this food was similar to that found in fermented dairy products. SEM analysis showed that there was excellent adhesion and distribution of probiotic on banana, apple and guava, probably due to the internal structure of fruit tissue. The fruit salads were well accepted by consumers, indicating that they are a marketable product. Moreover, probiotic fruit salad presents all the benefits provided by probiotic functional food, with the advantage that it can be consumed by everybody. However, selection of a plant matrix with potential as a probiotic carrier is important. Therefore, more studies and clinical trials are needed in order to evaluate the adhesion and permanence of probiotic bacteria in the intestine when it is consumed with fruit salad.
Acknowledgements
The authors thank Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for financial support.
-
Practical Application: Fruit salads can be used as a carrier for probiotic bacteria.
References
- Alegre, I., Viñas, I., Usall, J., Anguera, M., & Abadias, M. (2011). Microbiological and physicochemical quality of fresh-cut apple enriched with the probiotic strain Lactobacillus rhamnosus GG. Food Microbiology, 28(1), 59-66. http://dx.doi.org/10.1016/j.fm.2010.08.006 PMid:21056776.
» http://dx.doi.org/10.1016/j.fm.2010.08.006 - Andrews, W. H., Flower, R. S., Silliker, J., & Bailey, J. S. (2001). Salmonella. In F. P. Downes & K. Ito (Eds.), Compendium of methods for the microbilological examination of foods (pp. 357-380). Washington: APHA.
- Ankolekar, C., Pinto, M., Greene, D., & Shetty, K. (2012). In vitro bioassay based screening of antihyperglycemia and antihypertensive activities of . Lactobacillus acidophilus fermented pear juiceInnovative Food Science & Emerging Technologies, 13, 221-230. http://dx.doi.org/10.1016/j.ifset.2011.10.008
» http://dx.doi.org/10.1016/j.ifset.2011.10.008 - Antunes, A. E. C., Liserre, A. M., Coelho, A. L. A., Menezes, C. R., Moreno, I., Yotsuyanagi, K., & Azambuja, N. C. (2013). Acerola nectar with added microencapsulated probiotic. LWT - Food Science and Technology, 54, 125-131.
- Association Official Methods of Analysis – AOAC. (1995). Official methods of analysis of the Association of Official Analytical Chemist. Washington: AOAC.
- Batista, A. L. D., Silva, R., Cappato, L. P., Almada, C. N., Garcia, R. K. A., Silva, M. C., Raices, R. S. L., Arellano, D. B., Sant’Ana, A. S., Conte, C. A., Jr., Freitas, M. Q., & Cruz, A. G. (2015). Quality parameters of probiotic yogurt added to glucose oxidase compared to commercial products through microbiological,physical-chemical and metabolic activity analyses. Food Research International, 77, 627-635. http://dx.doi.org/10.1016/j.foodres.2015.08.017
» http://dx.doi.org/10.1016/j.foodres.2015.08.017 - Beaulieu, J. C. (2011). Factors affecting sensory quality of fresh-cut produce. In O. Martín-Belloso & R. Soliva-Fortuny (Eds.), Advances in fresh-cut fruits and vegetables processing (pp. 115-143). London: CRC Press.
- Brasil. Agência Nacional de Vigilância Sanitária. (2001, January 10). Regulamento técnico sobre padrões microbiológicos para alimentos (Resolução RDC n.12, de 02 de janeiro de 2001). Diário Oficial [da] República Federativa do Brasil.
- Brasil. Agência Nacional de Vigilância Sanitária. (2005, September 23). Regulamento técnico sobre a Ingestão Diária Recomendada (IDR) de proteína, vitaminas e minerais (Resolução RDC n. 269, de 22 de setembro de 2005). Diário Oficial [da] República Federativa do Brasil.
- Castro, W. F., Cruz, A. G., Bisinotto, M. S., Guerreiro, L. M. R., Faria, J. A. F., Bolini, H. M. A., Cunha, R. L., & Deliza, R. (2013a). Development of probiotic dairy beverages: rheological properties and application of mathematical models in sensory evaluation. Journal of Dairy Science, 96(1), 16-25. http://dx.doi.org/10.3168/jds.2012-5590 PMid:23102956.
» http://dx.doi.org/10.3168/jds.2012-5590 - Castro, W. F., Cruz, A. G., Rodrigues, D., Ghiselli, G., Oliveira, C. A. F., Faria, J. A. F., & Godoy, H. T. (2013b). Short communication: effects of different whey concentrations on physicochemical characteristics and viable counts of starter bacteria in dairy beverage supplemented with probiotics. Journal of Dairy Science, 96(1), 96-100. http://dx.doi.org/10.3168/jds.2012-5576 PMid:23182354.
» http://dx.doi.org/10.3168/jds.2012-5576 - Champagne, C. P., & Gardner, N. J. (2008). Effect of storage in a fruit drink on subsequent survival of probiotic lactobacillito gastro-intestinal stresses. Food Research International, 41(5), 539-543. http://dx.doi.org/10.1016/j.foodres.2008.03.003
» http://dx.doi.org/10.1016/j.foodres.2008.03.003 - Cousin, M. A., Jay, J. M., & Vasavada, P. C. (2001). Psychrotrophic microrganisms. In F. P. Downes, & K. Ito (Eds.), Compendium of methods for the microbilological examination of foods (pp. 159-166). Washington: APHA.
- Cruz, A. G., Castro, W. F., Faria, J. A. F., Bolini, H. M. A., Celeghini, R. M. S., Raices, R. S. L., Oliveira, C. A. F., Freitas, M. Q., Conte, C. A., Jr., & Mársico, E. T. (2013). Stability of probiotic yogurt added with glucose oxidase in plastic materials with different permeability oxygen rates during the refrigerated storage. Food Research International, 51(2), 723-728. http://dx.doi.org/10.1016/j.foodres.2013.01.028
» http://dx.doi.org/10.1016/j.foodres.2013.01.028 - Dantas, A. B., Jesus, V. F., Silva, R., Almada, C. N., Esmerino, E. A., Cappato, L. P., Silva, M. C., Raices, R. S. L., Cavalcanti, R. N., Carvalho, C. C., Sant’Ana, A. S., Bolini, H. M. A., Freitas, M. Q., & Cruz, A. G. (2016). Manufacture of probiotic Minas Frescal cheese with Lactobacillus casei Zhang. Journal of Dairy Science, 99(1), 18-30. http://dx.doi.org/10.3168/jds.2015-9880 PMid:26519974.
» http://dx.doi.org/10.3168/jds.2015-9880 - Dave, R. I., & Shah, N. P. (1997). Effectiveness of ascorbic acid as an oxygen scavenger in improving viability of probiotic bacteria in yoghurts made with commercial starter cultures. International Dairy Journal, 7(6-7), 435-443. http://dx.doi.org/10.1016/S0958-6946(97)00026-5
» http://dx.doi.org/10.1016/S0958-6946(97)00026-5 - De-Ancos, B., Sánchez-Moreno, C., Plaza, L., & Cano, M. P. (2011). Nutritional and health aspects of fresh-cut vegetables. In O. Martín-Belloso, & R. Soliva-Fortuny (Eds.), Advances in fresh-cut fruits and vegetables processing (pp. 145-184). London: CRC Press.
- Espírito-Santo, A. P., Perego, P., Converti, A., & Oliveira, M. O. (2011). Influence of food matrices on probiotic viability – A review focusing on the fruity bases. Trends in Food Science & Technology, 22(7), 377-385. http://dx.doi.org/10.1016/j.tifs.2011.04.008
» http://dx.doi.org/10.1016/j.tifs.2011.04.008 - Fernandes, M. S., Cruz, A. G., Arroyo, D. M. D., Faria, J. A. F., Cristianini, M., & Sant’Ana, A. S. (2013). On the behavior of and . Listeria innocuaLactobacillus acidophilus co-inoculated in a dairy dessert and the potential impacts on food safety and product’s functionalityFood Control, 34(2), 331-335. http://dx.doi.org/10.1016/j.foodcont.2013.04.040
» http://dx.doi.org/10.1016/j.foodcont.2013.04.040 - Fonteles, T. V., Costa, M. G. M., Jesus, A. L. T., & Rodrigues, S. (2011). Optimization of the fermentation of cataloupe juice by . Lactobacillus casei NRRL B-442Food and Bioprocess Technology, 5(7), 2819-2826. http://dx.doi.org/10.1007/s11947-011-0600-0
» http://dx.doi.org/10.1007/s11947-011-0600-0 - Food and Agriculture Organization – FAO, & World Health Organization – WHO. (2001). Evaluation of health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria (Report of a Joint FAO/WHO Expert Consultation, Córdoba, Argentina). Rome: FAO/WHO.
- Granato, D., Branco, G. F., Nazzaro, F., Cruz, A. G., & Faria, J. A. F. (2010). Functional food and non dairy probiotic food development: trends, concepts, and products. Comprehensive Reviews in Food Science and Food Safety, 9(3), 292-302. http://dx.doi.org/10.1111/j.1541-4337.2010.00110.x
» http://dx.doi.org/10.1111/j.1541-4337.2010.00110.x - Kornacki, J. L., & Johnson, J. L. (2001). Enterobacteriaceae, coliforms, and Escherichia coli as quality and safety indicators. In F. P. Downes & K. Ito (Eds.), Compendium of methods for the microbiological examination of foods (pp. 69-82). Washington: APHA.
- Lollo, P. C. B., Morato, P. N., Moura, C. S., Almada, C. N., Felicio, T. L., Esmerino, E. A., Barros, M. E., Amaya-Farfan, J., Sant’Ana, A. S., Raices, R. R. S., Silva, M. C., & Cruz, A. G. (2015). Hypertension parameters are attenuated by the continuous consumption of probiotic Minas cheese. Food Research International, 76, 611-617. http://dx.doi.org/10.1016/j.foodres.2015.07.015
» http://dx.doi.org/10.1016/j.foodres.2015.07.015 - Lourens-Hattingh, A., & Viljeon, C. B. (2001). Yogurt as probiotic carrier food. International Dairy Journal, 11(1-2), 1-17. http://dx.doi.org/10.1016/S0958-6946(01)00036-X
» http://dx.doi.org/10.1016/S0958-6946(01)00036-X - Mahawar, M., Singh, A., & Jalgaonkar, K. (2012). Utility of apple pomace as a substrate for various products: A review. Food and Bioproducts Processing, 90(4), 597-605. http://dx.doi.org/10.1016/j.fbp.2012.04.007
» http://dx.doi.org/10.1016/j.fbp.2012.04.007 - Martins, E. M. F., Ramos, A. M., Martins, M. L., & Rodrigues, M. Z. (2015). Research and development of probiotic products from vegetable bases: a new alternative for consuming functional food. In V. R. Rai & J. A. Bai. Beneficial microbes in fermented and functional foods (pp. 207-223). Boca Raton: CRC Press.
- Martins, E. M. F., Ramos, A. M., Vanzela, E. S. L., Stringheta, P. C., Pinto, C. L. O., & Martins, J. M. (2013). Products of vegetable origin: a new alternative for the consumption of probiotic bacteria. Food Research International, 51(2), 764-770. http://dx.doi.org/10.1016/j.foodres.2013.01.047
» http://dx.doi.org/10.1016/j.foodres.2013.01.047 - Mitsou, E. K., Kougia, E., Nomikos, T. Z., Yannakoulia, M., Mountzouris, K. C., & Kyriacou, A. (2011). Effect of banana consumption on faecal microbiota: a randomised, controlled trial. Anaerobe, 17(6), 384-387. http://dx.doi.org/10.1016/j.anaerobe.2011.03.018 PMid:21524710.
» http://dx.doi.org/10.1016/j.anaerobe.2011.03.018 - Morais, E. C., Morais, A. R., Cruz, A. G., & Bolini, H. M. A. (2014). Development of chocolate dairy dessert with addition of prebiotics and replacement of sucrose with different high-intensity sweeteners. Journal of Dairy Science, 97(5), 2600-2609. http://dx.doi.org/10.3168/jds.2013-7603 PMid:24612793.
» http://dx.doi.org/10.3168/jds.2013-7603 - Nicolescu, C. L., & Buruleanu, L. C. (2010). Correlation of some substrate parameters in growing on Vegetable and fruit cocktail juices. Lactobacillus acidophilusBulletin of the University of Agricultural Sciences and Veterinary Medicine, 67(2), 352-359.
- Oliveira, M. A., Souza, V. M., Bergamini, A. M. M., & Martinis, E. C. P. (2011). Microbiological quality of ready-to-eat minimally processed vegetables consumed in Brazil. Food Control, 22(8), 1400-1403. http://dx.doi.org/10.1016/j.foodcont.2011.02.020
» http://dx.doi.org/10.1016/j.foodcont.2011.02.020 - Pereira, A. L. F., Maciel, T. C., & Rodrigues, S. (2011). Probiotic beverage from cashew apple juice fermented with Lactobacillus casei.Food Research International, 44(5), 1276-1283. http://dx.doi.org/10.1016/j.foodres.2010.11.035
» http://dx.doi.org/10.1016/j.foodres.2010.11.035 - Pereira, E. P. R., Faria, J. A. F., Cavalcanti, R. N., Garcia, R. K. A., Silva, R., Esmerino, E. A., Cappato, L. P., Arellano, D. B., Raices, R. S. L., Silva, M. C., Padilha, M. C., Meireles, M. A., Bolini, H. M. A., & Cruz, A. G. (2016). Oxidative stress in probiotic Petit Suisse: is the jabuticaba skin extract a potential option? Food Research International, 81, 149-156. http://dx.doi.org/10.1016/j.foodres.2015.12.034
» http://dx.doi.org/10.1016/j.foodres.2015.12.034 - Peres, C. M., Peres, C., Hernández-Mendoza, A., & Malcata, F. X. (2012). Review on fermented plant materials as carriers and sources of potentially probiotic lactic acid bacteria – with an emphasis on table olives. Trends in Food Science & Technology, 26(1), 31-42. http://dx.doi.org/10.1016/j.tifs.2012.01.006
» http://dx.doi.org/10.1016/j.tifs.2012.01.006 - Pithva, S., Ambalam, P., Dave, J. M., & Vyas, B. R. M. (2011). Antimicrobial peptides of probiotic Lactobacillus strains. In A. Méndez-Vilas (Ed.), Science against microbial pathogens: communicating current research and technological advances (pp. 987-991). Badajoz: Formatex.
- R Core Team2012R: A language and environment for statistical computingViennaR Foundation for Statistical Computing
- Ranadheera, R. D. C. S., Baines, S. K., & Adams, M. C. (2010). Importance of food in probiotic efficacy. Food Research International, 43(1), 1-7. http://dx.doi.org/10.1016/j.foodres.2009.09.009
» http://dx.doi.org/10.1016/j.foodres.2009.09.009 - Rivera-Espinoza, Y., & Gallardo-Navarro, Y. (2010). Non-dairy probiotic products. Food Microbiology, 27(1), 1-11. http://dx.doi.org/10.1016/j.fm.2008.06.008 PMid:19913684.
» http://dx.doi.org/10.1016/j.fm.2008.06.008 - Rodriguez-Amaya, D. B. (2001). A guide to carotenoid analysis in foods (pp. 41-45). Washington: ISLI Press.
- Rodriguez-Amaya, D. B., Kimura, M., & Amaya-Farfan, J. (2008). Fontes brasileiras de carotenóides: tabela brasileira de composição de carotenóides em alimentos. Brasília: MMA/SBF.
- Rößle, C., Auty, M. A. E., Brunton, N., Gormley, R. T., & Butler, F. (2010). Evaluation of fresh-cut apple slices enriched with probiotic bacteria. Innovative Food Science & Emerging Technologies, 11(1), 203-209. http://dx.doi.org/10.1016/j.ifset.2009.08.016
» http://dx.doi.org/10.1016/j.ifset.2009.08.016 - Russo, P., Chiara, M. L. V., Vernile, A., Amodio, M. L., Arena, M. P., Capozzi, V., Massa, S., & Spano, G. (2014). Fresh-cut pineapple as a new carrier of probiotic lactic acid bacteria. BioMed Research International, 2014, 1-9. http://dx.doi.org/10.1155/2014/309183
» http://dx.doi.org/10.1155/2014/309183 - Saad, S. M. I., Komatsu, T. R., Granato, D., Branco, G. F., & Buriti, F. C. A. (2011). Probióticos e prebióticos em alimentos: aspectos tecnológicos, legislação e segurança no uso. In S. M. I. Saad, A. G. Cruz & J. A. F. Faria (Eds.), Probióticos e prebióticos em alimentos: fundamentos e aplicações tecnológicas (pp. 23-49). São Paulo: Editora Varela.
- Santos, B. A., Campagnol, P. C. B., Cruz, A. G., Morgano, M. A., Wagner, R., & Pollonio, M. A. R. (2015). Is there a potential consumer market for low-sodium fermented sausages? Journal of Food Science, 80(5), 1093-S1099. http://dx.doi.org/10.1111/1750-3841.12847 PMid:25808547.
» http://dx.doi.org/10.1111/1750-3841.12847 - Sañudo-Barajas, J. A., Labavitch, J., Greve, C., Osuna-Enciso, T., Muy-Rangel, D., & Siller-Cepeda, J. (2009). Cell wall disassembly during papaya softening: role of ethylenein changes in composition, pectin-derived oligomers (PDOs) production and wall hydrolases. Postharvest Biology and Technology, 51(2), 158-167. http://dx.doi.org/10.1016/j.postharvbio.2008.07.018
» http://dx.doi.org/10.1016/j.postharvbio.2008.07.018 - Sheehan, V. M., Ross, P., & Fitzgerald, G. F. (2007). Assessing the acid tolerance and the technological robustness of probiotic cultures for fortification in fruit juices. Innovative Food Science & Emerging Technologies, 8(2), 279-284. http://dx.doi.org/10.1016/j.ifset.2007.01.007
» http://dx.doi.org/10.1016/j.ifset.2007.01.007 - Silveira, M. (1989). Preparo de amostras biológicas para microscopia eletrônica de varredura. In W. Souza, A. Haddad, A. Sesso, M. Silveira, O. M. Barth, R. D. Machado & T. Souto-Padrón (Eds.), Manual sobre técnicas básicas em microscopia eletrônica (pp. 71-79). Rio de Janeiro: Sociedade Brasileira de Microscopia Eletrônica.
- Soccol, C. R., Vandenberghe, L. P. S., Spier, M. R., Medeiros, A. B. P., Yamaguishi, C. T., Lindner, J. D., Pandey, A., & Soccol, V. T. (2010). The potential of probiotics. Food Technology and Biotechnology, 48(4), 413-434.
- Tapia, M. S., Rojas-Grau, M. A., Carmona, A., Rodriguez, F. J., Soliva-Fortuny, R., & Martin-Belloso, O. (2008). Use of alginate and gellan-based coatings for improving barrier, texture and nutritional properties of fresh-cut papaya. Food Hydrocolloids, 22(8), 1493-1503. http://dx.doi.org/10.1016/j.foodhyd.2007.10.004
» http://dx.doi.org/10.1016/j.foodhyd.2007.10.004 - Tortora, G. J., Funke, B. R., & Case, C. L. (2006). Microbiology an introduction. San Francisco: Benjamin Cummings.
- Yoon, K. Y., Woodams, E. E., & Hang, Y. D. (2004). Probiotication of tomato juice by lactic acid bacteria. Journal of Microbiology (Seoul, Korea), 42(4), 315-318. PMid:15650688.
- Yu, Z. H., Zhang, X., Li, S. Y., Li, C. Y., Li, D., & Yang, Z. N. (2012). In vitro evaluation of probiotic properties of strains isolated from Chinese Sauerkraut. Lactobacillus plantarumAfrican Journal of Biotechnology, 11(21), 4868-4875.
- Zenebon, O., & Pascuet, N. S. (2004). Métodos físico-químicos para análise de alimentos. São Paulo: Instituto Adolfo Lutz.
Publication Dates
-
Publication in this collection
07 July 2016 -
Date of issue
Jul-Sep 2016
History
-
Received
18 Feb 2016 -
Accepted
07 June 2016