Abstract
In the present study, the software Aspen Plus® was used to analyse two different systems for CO2 recycle in a SFE process for extraction of more polar compounds using ethanol as co-solvent, the most common co-solvent used due to its environment-friendly nature. The extraction process of β-ecdysone from Brazilian ginseng roots was considered as example in the computational simulations. The first CO2 recycle system, named Recycle A, considered the compression of the CO2 separated in the second flash to the recycle pressure assumed at the first flash tank, its cooling to 25 °C and recirculation, while the second recycle system, named Recycle B, considered the cooling and pumping of the CO2 separated in the second flash, its heating to 25 °C and recirculation. The best techno-economic condition to operate the recycling step would be using Recycle A at 40 bar and 30 °C considering a stand-alone SFE process; and using Recycle B at 40 bar and 40 °C, considering this process in close proximity of a hypothetical sugarcane biorefinery. Therefore, these results suggest that the selection where would be located the SFE plant should be taken into account during the first steps of the process design.
Keywords:
pinch analysis; process design; biomass processing; biorefinering
1 Introduction
Supercritical fluid extraction (SFE) is a process that takes advantage of the increase in the solvation power of fluids near or above their critical points. In spite of the possibility of using different supercritical fluids, carbon dioxide is the solvent usually used in applications related to the cosmetic, food and pharmaceutical industries. CO2 has a low critical temperature (30.4 °C) and a mild critical pressure (78 bar); it is non-toxic, relatively inert to several mediums, and, can be obtained at high purity at a reasonable cost. The power of supercritical carbon dioxide for selectively extract some substances from different vegetable matrixes is widely recognized. On the other hand, in some cases the solubility of some specific compounds is not good. This can be overcome by the addition of cosolvent, usually a more polar solvent such as ethanol, for example, to the supercritical solvent; this affects the properties of the fluid phase because of the strong interactions among the solute, the solvent, and the cosolvent (Pereira & Meireles, 2010Pereira, C. G., & Meireles, M. A. A. (2010). Supercritical fluid extraction of bioactive compounds: fundamentals, applications and economic perspectives. Food and Bioprocess Technology, 3(3), 340-372. http://dx.doi.org/10.1007/s11947-009-0263-2.
http://dx.doi.org/10.1007/s11947-009-026...
; Santos & Meireles, 2011Santos, D. T., & Meireles, M. A. A. (2011). Extraction of volatile oils by supercritical fluid extraction. Recent Patents on Engineering, 5(1), 17-22. http://dx.doi.org/10.2174/1872212111105010017.
http://dx.doi.org/10.2174/18722121111050...
).
The SFE process typically requires a pressurization step, a heating or cooling step, an extraction step, and a subsequent separation and solvent recycle step. The design of the recycle step is important as the costs of recompression of gaseous CO2 to liquid or supercritical is high, as a powerful compression equipment and often a refrigeration step prior to compression are required (Carlson et al., 2005Carlson, L. H. C., Bolzan, A., & Machado, R. A. F. (2005). Separation of d-limonene from supercritical CO by means of membranes. 2The Journal of Supercritical Fluids, 34(2), 143-34147. http://dx.doi.org/10.1016/j.supflu.2004.11.007.
http://dx.doi.org/10.1016/j.supflu.2004....
; Rosa & Meireles, 2009Rosa, P. T. V., & Meireles, M. A. A. (2009). Fundamentals of supercritical extraction from solid matrices. In M. A. A. Meireles (Ed.), Extracting bioactive compounds for food products: theory and applications (chap. 6.1, pp. 272-287). Boca Raton: CRC Press.).
In the last 10 years, a new concept has been developed by the researchers that work with sub/supercritical fluids: Integration of sub/supercritical fluids into existing processing concepts such as biomass conversion and biorefineries (Schacht et al., 2008Schacht, C., Zetzl, C., & Brunner, G. (2008). From plant materials to ethanol by means of supercritical fluid technology. The Journal of Supercritical Fluids, 46(3), 299-321. http://dx.doi.org/10.1016/j.supflu.2008.01.018.
http://dx.doi.org/10.1016/j.supflu.2008....
; Temelli & Ciftci, 2015Temelli, F., & Ciftci, O. N. (2015). Developing an integrated supercritical fluid biorefinery for theprocessing of grains. The Journal of Supercritical Fluids, 96, 77-85. http://dx.doi.org/10.1016/j.supflu.2014.09.028.
http://dx.doi.org/10.1016/j.supflu.2014....
). The possibility of constructing a Supercritical CO2 plant in close proximity to an alcoholic fermentation facility that produces high purity CO2 as a by-product and ethanol, preferred co-solvent for coupling with CO2 was mentioned by some researchers (King & Srinivas, 2009King, J. W., & Srinivas, K. (2009). Multiple unit processing using sub- and supercritical fluids. The Journal of Supercritical Fluids, 47(3), 598-610. http://dx.doi.org/10.1016/j.supflu.2008.08.010.
http://dx.doi.org/10.1016/j.supflu.2008....
), on the other hand, few evaluations was done until the present date. Recently, we demonstrated that this strategy could increase the economic potential of the supercritical fluid extraction (SFE) process up to 57% (Santos et al., 2014Santos, D. T., Albarelli, J. Q., Rostagno, M. A., Ensinas, A. V., Maréchal, F., & Meireles, M. A. A. (2014). New proposal for production of bioactive compounds by supercritical technology integrated to a sugarcane biorefinery. Clean Technologies and Environmental Policy, 16(7), 1455-1468. http://dx.doi.org/10.1007/s10098-014-0760-5.
http://dx.doi.org/10.1007/s10098-014-076...
; Albarelli et al., 2016Albarelli, J. Q., Santos, D. T., Cocero, M. J., & Meireles, M. A. A. (2016). Economic analysis of an integrated annatto seeds-sugarcane biorefinery using supercritical CO extraction as a first step. 2Materials, 9(6), 494. http://dx.doi.org/10.3390/ma9060494.
http://dx.doi.org/10.3390/ma9060494...
). Such integration is a win–win situation creating new uses for CO2 generated as a result of fermentation. Since the ethanol sector in is one of the major activities for the Brazilian economy, this sector has experienced major modernization, and different alternatives are considered to compose the future scenario of sugarcane industry in Brazil. The studies of the recent created research Institute called Brazilian Bioethanol Science and Technology Laboratory (CTBE) at Campinas have demonstrated that the integrated first and second generation ethanol production process from sugarcane leads to better energetic and economic results when compared with the stand-alone plant (Dias et al., 2009Dias, M. O. S., Junqueira, T. L., Cavalett, O., Cunha, M. P., Jesus, C. D. F., Mantelatto, P. E., Rossell, C. E. V., Maciel, R., Fo., & Bonomi, A. (2009). Integrated first and second generation ethanol production from sugarcane. Chemical Engineering Research & Design, 87(9), 1206-1216. http://dx.doi.org/10.1016/j.cherd.2009.06.020.
http://dx.doi.org/10.1016/j.cherd.2009.0...
, 2012Dias, M. O. S., Junqueira, T. L., Cavalett, O., Cunha, M. P., Jesus, C. D. F., Rossell, C. E. V., Maciel, R., Fo., & Bonomi, A. (2012). Integrated versus stand-alone second generation ethanol production from sugarcane bagasse and trash. Bioresource Technology, 103(1), 152-161. PMid:22019267. http://dx.doi.org/10.1016/j.biortech.2011.09.120.
http://dx.doi.org/10.1016/j.biortech.201...
, 2013Dias, M. O. S., Ensinas, A. V., Nebra, S. A., Maciel, R., Fo., Rossell, C. E. V., & Maciel, M. R. W. (2013). Cogeneration in integrated first and second generation ethanol from sugarcane. Chemical Engineering Research & Design, 91(8), 1411-1417. http://dx.doi.org/10.1016/j.cherd.2013.05.009.
http://dx.doi.org/10.1016/j.cherd.2013.0...
). Computational modeling and simulation are essential tools to perform these evaluations and create new ones. Thus, the development of a computational framework that allows a consistent comparison of the different pathways is very important for the further decision-making process. To the best of our knowledge, there is no other research project under development focusing on the development of an energetic self-sufficient and economically viable process that uses sub/supercritical fluid-based technologies during biomass processing steps and integrates a mix of biomasses in the analyses with the aim of increasing sustainable revenue generation.
In the present study, we aim to evaluate the other impacts related to the integration of a supercritical fluid extraction plant to a sugarcane biorefinery, specifically focusing on the CO2 recycle step. The software Aspen Plus® was used to analyze two different systems for CO2 recycle in a SFE process for extraction of more polar compounds using ethanol as co-solvent considering the SFE process integrated to a sugarcane biorefinery or as a stand-alone process. The extraction process of β-ecdysone from Brazilian ginseng roots was considered as example in the computational simulations. The economic analysis for each scenario was evaluated regarding the operational cost of the process and total investment cost and at each configuration evaluated the process was thermal integrated using the Pinch Method, aiming at the reduction of process heat requirements.
2 Materials and methods
2.1 Process description
The flowsheet of the analyzed SFE process is shown in Figure 1. The simulation of the SFE plant was performed using the commercial simulator Aspen Plus®. For the Brazilian ginseng (Pfaffia glomerata) roots extraction, it was considered a prior preparation of the material (mass flow of 29.9 kg/h) in which the roots were cleaned, air dried using heated air and milled. The preparation system was simulated as a separation block, to represent the cleaning system (block CLEANING), and a flash to simulate the drying in which the mass flow of air was adjusted to enable 10% of biomass final moisture (block DRYER). It was considered a prior milling of the biomass, this unit operation was not simulated in Aspen Plus® software but the electricity demand was taken in to account. The prepared roots were introduced in an extraction reactor were ethanol and CO2 was pumped at the desired proportions, pressure and temperature. The extractor was simulated as an extraction block (block EXTRACTOR) in which it was imposed the extraction conditions and its respective yields according to the data from the experimental results (Santos et al., 2014Santos, D. T., Albarelli, J. Q., Rostagno, M. A., Ensinas, A. V., Maréchal, F., & Meireles, M. A. A. (2014). New proposal for production of bioactive compounds by supercritical technology integrated to a sugarcane biorefinery. Clean Technologies and Environmental Policy, 16(7), 1455-1468. http://dx.doi.org/10.1007/s10098-014-0760-5.
http://dx.doi.org/10.1007/s10098-014-076...
). Although the extraction process is a non-continuous process, it can be modeled as a steady-state process since it was considered different extraction reactors operating in parallel, which enables a continuous production of extract.
Flowsheet of the supercritical fluid extraction (SFE) process with the two evaluated CO2 recycle cycles developed in the Aspen Plus® software.
After extraction, CO2 and ethanol need to be recovered and separated from the extract. It was considered two different systems for CO2 recovery and recycle. In both systems, the CO2 was recovered through 2 flash tanks. For the first flash tank it was considered the pressure of 70, 60, 50 and 40 bar, and temperature ranging from 25 to 60 °C. The second flash tank operates at 1 bar, 25 °C. The CO2 separated in the first flash tank was cooled to 25 °C, when necessary, and recycled to the process. The first CO2 recovery and recycle system, named Recycle A, considered the compression of the CO2 separated in the second flash to the recycle pressure assumed at the first flash tank, its cooling to 25 °C and recirculation. The second recovery system considered the CO2 separated in the second flash, cooling until it was in liquid phase, the pumping of this liquid to the recycle pressure assumed at the first flash tank, the heating of it to 25 °C and recirculation (named Recycle B). The flash tanks were simulated as a flash equipment (blocks F-C1 and F-C2), the compression and cooling/heating operations were simulated as compressor equipment (block COMP-C1) and as heat exchangers (blocks H-C3s) (Figure 1).
Ethanol was separated from the extracted compounds by evaporation and recycled to the process. The evaporator was simulated as a set of a heat exchanger and a flash tank (blocks H-E1 and F-E1). It was considered a loss of 5% of ethanol and the cooling of ethanol in a heat exchanger to the ethanol inlet temperature (25 °C) prior to its reuse.
2.2 Thermal process integration
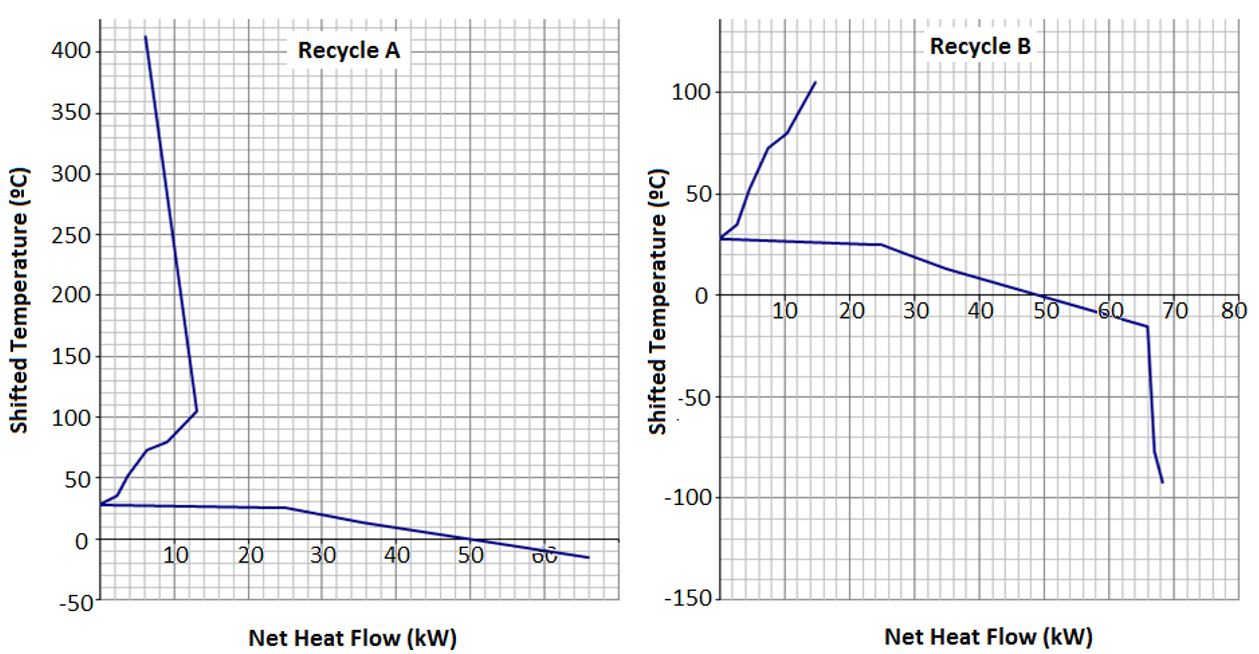
All the process design case studies were thermal integrated using the Pinch Method (Linnhoff et al., 1982Linnhoff, B., Towsend, D. W., Boland, D., Hewitt, G. F., Thomas, B. E. A., Guy, A. R., & Marsland, R. H. (1982). A user guide on process integration for the efficient use of energy. Rugby: The Institution of Chemical Engineers.), aiming at the reduction of process steam requirements. Based on the pinch analysis methodology, the optimal thermal process integration is computed after defining the maximum heat recovery potential between hot and cold streams and considering a minimum approach temperature ∆Tmin. Depending on the process alternative evaluated the temperature of the process heat flows ranged from 410 to -10 °C (Recycle A) or 105 to -93 °C (Recycle B). The energetic model was constructed and thermal integration calculation was accomplished using the spreadsheet software Excel®. The energetic model considered the heat flows calculated by the energy and mass balance model developed in Aspen Plus.
2.3 Economical evaluation
Table 1 shows the input data used for the economical evaluation. The economic analysis for each scenario was evaluated regarding the operational cost of the process and total investment cost. To calculate the total investment cost, the major process equipments were roughly sized and their purchase cost were calculated and adjusted to account for specific process pressures and materials using correlations from literature (Turton et al., 2009Turton, R. B., Wallace, B., Whiting, J. S., & Bhattacharyya, D. (2009). Analysis, synthesis and design of chemical processes. Upper Saddle River: Prentice Hall.; Ulrich & Vasudevan, 2003Ulrich, G., & Vasudevan, P. (2003). A guide to chemical engineering process design and economics a practical guide. Boca Raton: CRC Press.). The total investment cost was then calculated using multiplication factors to take into account indirect expenses like installation costs, contingencies and auxiliary facilities. All costs had been updated by using the Marshall and Swift Index. The economic model was developed in the OSMOSE platform collecting relevant data from the Aspen Plus model (e.i. mass and volume flows, temperature, pressure, power demand and other data depending on the equipment analysed). OSMOSE (OptimiSation Multi-Objectifs de Systemes Energetiques integres, which means “Multi-Objective OptimiZation of integrated Energy Systems”) is a computation platform that was built in MATLAB, developed and continuously improved at École Polytechnique Fédérale de Lausanne in Switzerland for the design and analysis of integrated energy systems. The platform allows one to link Aspen Plus® software for a complete suite of computation and result analysis tools (École Polytechnique Fédérale de Lausanne, 2013École Polytechnique Fédérale de Lausanne – EPFL. (2013, June 01). OSMOSE Platform: a tool for the design and analysis of integrated energy systems. Lausanne: EPFL. Retrieved from http://leni.epfl.ch/osmose
http://leni.epfl.ch/osmose...
).
3 Results and discussion
Table 2 shows that CO2 recycle ration is around 100% (CO2 recovery ratio = 0.999-1), being separated almost entirely in the second flash tank (CO2 separation ratio at the first flash tank being 0.000) under certain conditions (runs 1,2). On the other hand, under these conditions ethanol is not separated completely from CO2, only less than 30% (ethanol recycle ratio < 0.3) is sent to ethanol separation step, being more than 70% recycled with CO2. It increases the power demand of the CO2 recirculation system (Table 3). Higher CO2 separation ratios in the first flash thank is achieved increasing the temperature under a fixed pressure, achieving a ratio up to 95.6% (0.959), under pressure of 40 bar.
At each configuration evaluated the process was thermal integrated using the Pinch Method, aiming at the reduction of process heat requirements. The Pinch analysis (Linnhoff et al., 1982Linnhoff, B., Towsend, D. W., Boland, D., Hewitt, G. F., Thomas, B. E. A., Guy, A. R., & Marsland, R. H. (1982). A user guide on process integration for the efficient use of energy. Rugby: The Institution of Chemical Engineers.) is a thermal integration tool that aims to minimize the energy consumption of a process by analyzing its energy flows. This analysis is based on the first and second law of thermodynamics, in which energy must be conserved and heat will flow in only one direction. In this analysis, the heat flux streams are combined into groups of hot and cold streams and composite curves are formed. The closest point between these curves is the Pinch temperature, which is the best starting point for design studies (Kemp, 2007Kemp, I. C. (2007). Pinch analysis and process integration: a user guide on process integration for the efficient use of energy. Oxford: Elsevier. 415 p.).
The thermal integration of the process played an important role in minimizing the need of hot utility and promoting a global system view, which showed that the required high temperatures for the SFE process is not a road block if the overall picture of the process is considered. After thermal process integration, no heat (hot utility) was necessary for the CO2 recycle system Recycle A at the pressures of 70, 60 and 50 bar at 25 and 30 °C (Table 2, runs 1-4). This was due to the large amount of thermal energy at high temperature available at CO2 cooling prior recycle. Figure 2 shows the grand composite curves for each configuration evaluated at Run 9. It can be seen by the diagrams that the main demand for both Recycle A and B is of cold demand. The Pinch point is found at 30 °C, and after thermal integration of this run the heat demand decreased 4.6 and 2.4 times for Recycle A and B, respectively.
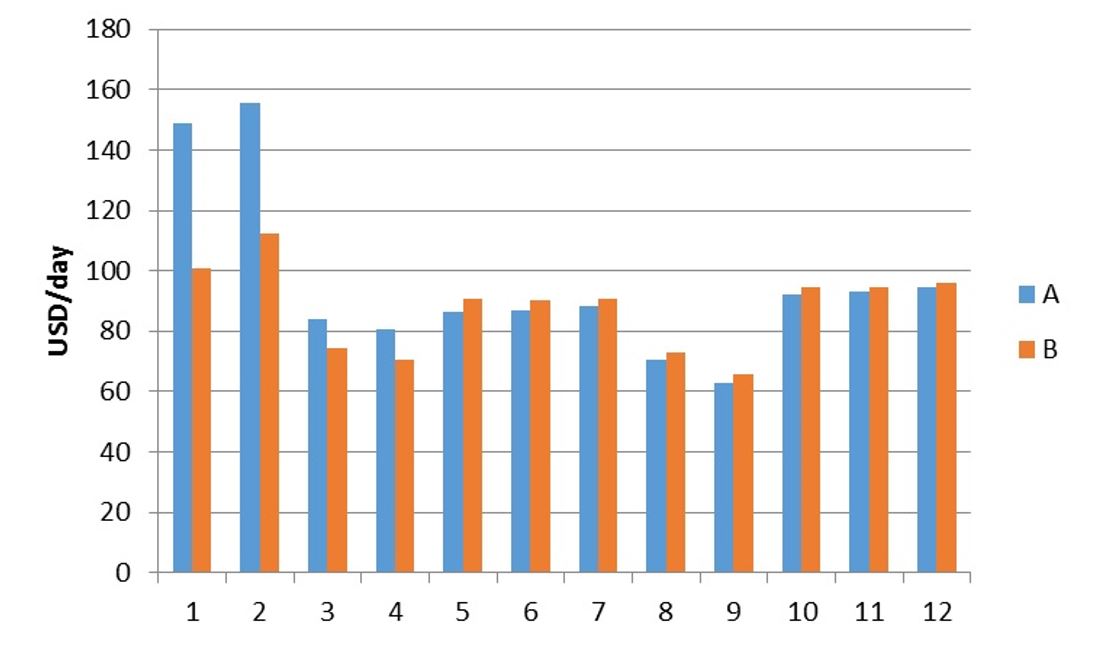
The economic analysis for each scenario was evaluated regarding the operational cost of the process and total investment cost. The best operational costs were found at 40 bar at temperatures 30 °C (run 9), for both recycle systems (Figure 3), meanwhile it is possible to detect that the best recycling option would be using Recycle A (9A). However, the results shows only the operational costs of each system, and, for 9A it would be necessary to buy a compressor, what would lead to an increase in the investment cost and therefore in the economic attractiveness of the process. The difference between 9A and 9B is approximately 5%, this difference could be decreased to around 1% by decreasing the steam at low pressure cost by 23%. Lower operational cost for 9B comparing with 9A would be possible if the steam at low pressure cost decreased more than 28.9%. It would be possible if it was considered the use of process wastes as fuel in a burner with a steam cycle system. Consequently, the steam cost could be reduced if it was considered the insertion of this process in a conglomerate of different production plants sharing a cogeneration system, in which electricity and thermal energy is produced to supply the processes and extra electricity could be sold.
Operational cost of the supercritical fluid extraction process consid-ering the two CO2 recycle systems.
In other to evaluate this new scenario we considered this process in close proximity of a hypothetical sugarcane biorefinery, being the Brazilian ginseng residue from the process directly used at the cogeneration system. At this new scenario, Recycle B would present the lowest operational costs. Regarding the investment cost, for the best conditions evaluated, Recycle A would demand a compressor 41% more expensive than the pump necessary for Recycle B. This difference was minimal (less than 1%) when evaluating the overall investment cost where the contribution of the compressor or pump is around 2% of the total, being the higher cost related to the extraction vessel (44%). From the evaluated results the best condition to operate the process would be consider Recycle B at 40 bar and 40 °C (run 10, 10B), as it presents low operational and total investment costs.
4 Conclusions
The results showed that in both systems, CO2 is separated almost entirely in the second flash tank and ethanol is not separated completely from CO2, only less than 30% is sent to ethanol separation, increasing the power demand of the CO2 recirculation system.
The best techno-economic condition to operate the recycling step was influenced by the decision to have the SFE process as a stand-alone process or in close proximity of a hypothetical sugarcane biorefinery. The best operational costs were found at 40 bar and at temperature 30 °C for a stand-alone process using Recycle A (Run 9A) and at 40 °C for an integrated SFE-sugarcane biorefinery using Recycle B (Run 10B). When considering the SFE process integrated to a sugarcane biorefinery the use of the biomass residue from the SFE process at the cogeneration system significantly decreased the steam cost. At this new scenario, Recycle B would present the lowest operational costs. From the evaluated results the best condition to operate the process would be consider Recycle B at 40 bar and 40 °C in an integrated SFE-sugarcane biorefinery, as it presented low operational cost and total investment costs. Thus, the location of the SFE plant is an important parameter that should be taken into consideration. Since to date there is no industrial supercritical fluid extraction unit in Brazil this information should be very useful in order to provide comprehensive perspectives on the possibility of constructing the first industrial SFE unit in Brazil in close proximity to an alcoholic fermentation facility that produces high purity CO2 as a by-product and ethanol.
Acknowledgements
Juliana Q. Albarelli thanks FAPESP (processes 2013/18114-2, 2015/06954-1) for the post-doctoral fellowships. Diego T. Santos thanks CAPES (process 7545-15-0) and FAPESP (processes 2010/16485-5, 2012/19304-7) for the post-doctoral fellowships. M. Angela A. Meireles thanks CNPq for the productivity grant (301301/2010-7). The authors acknowledge the financial support from CNPq and FAPESP (processes 2012/10685-8, 2015/13299-0).
-
Practical Application: The design of the recycle step of a SFE process is an important step as it has a high-cost contribution.
References
- Albarelli, J. Q., Santos, D. T., Cocero, M. J., & Meireles, M. A. A. (2016). Economic analysis of an integrated annatto seeds-sugarcane biorefinery using supercritical CO extraction as a first step. 2Materials, 9(6), 494. http://dx.doi.org/10.3390/ma9060494
» http://dx.doi.org/10.3390/ma9060494 - Carlson, L. H. C., Bolzan, A., & Machado, R. A. F. (2005). Separation of d-limonene from supercritical CO by means of membranes. 2The Journal of Supercritical Fluids, 34(2), 143-34147. http://dx.doi.org/10.1016/j.supflu.2004.11.007
» http://dx.doi.org/10.1016/j.supflu.2004.11.007 - Dias, M. O. S., Ensinas, A. V., Nebra, S. A., Maciel, R., Fo., Rossell, C. E. V., & Maciel, M. R. W. (2013). Cogeneration in integrated first and second generation ethanol from sugarcane. Chemical Engineering Research & Design, 91(8), 1411-1417. http://dx.doi.org/10.1016/j.cherd.2013.05.009
» http://dx.doi.org/10.1016/j.cherd.2013.05.009 - Dias, M. O. S., Junqueira, T. L., Cavalett, O., Cunha, M. P., Jesus, C. D. F., Rossell, C. E. V., Maciel, R., Fo., & Bonomi, A. (2012). Integrated versus stand-alone second generation ethanol production from sugarcane bagasse and trash. Bioresource Technology, 103(1), 152-161. PMid:22019267. http://dx.doi.org/10.1016/j.biortech.2011.09.120
» http://dx.doi.org/10.1016/j.biortech.2011.09.120 - Dias, M. O. S., Junqueira, T. L., Cavalett, O., Cunha, M. P., Jesus, C. D. F., Mantelatto, P. E., Rossell, C. E. V., Maciel, R., Fo., & Bonomi, A. (2009). Integrated first and second generation ethanol production from sugarcane. Chemical Engineering Research & Design, 87(9), 1206-1216. http://dx.doi.org/10.1016/j.cherd.2009.06.020
» http://dx.doi.org/10.1016/j.cherd.2009.06.020 - École Polytechnique Fédérale de Lausanne – EPFL. (2013, June 01). OSMOSE Platform: a tool for the design and analysis of integrated energy systems. Lausanne: EPFL. Retrieved from http://leni.epfl.ch/osmose
» http://leni.epfl.ch/osmose - Kemp, I. C. (2007). Pinch analysis and process integration: a user guide on process integration for the efficient use of energy. Oxford: Elsevier. 415 p.
- King, J. W., & Srinivas, K. (2009). Multiple unit processing using sub- and supercritical fluids. The Journal of Supercritical Fluids, 47(3), 598-610. http://dx.doi.org/10.1016/j.supflu.2008.08.010
» http://dx.doi.org/10.1016/j.supflu.2008.08.010 - Linnhoff, B., Towsend, D. W., Boland, D., Hewitt, G. F., Thomas, B. E. A., Guy, A. R., & Marsland, R. H. (1982). A user guide on process integration for the efficient use of energy. Rugby: The Institution of Chemical Engineers.
- Pereira, C. G., & Meireles, M. A. A. (2010). Supercritical fluid extraction of bioactive compounds: fundamentals, applications and economic perspectives. Food and Bioprocess Technology, 3(3), 340-372. http://dx.doi.org/10.1007/s11947-009-0263-2
» http://dx.doi.org/10.1007/s11947-009-0263-2 - Rosa, P. T. V., & Meireles, M. A. A. (2009). Fundamentals of supercritical extraction from solid matrices. In M. A. A. Meireles (Ed.), Extracting bioactive compounds for food products: theory and applications (chap. 6.1, pp. 272-287). Boca Raton: CRC Press.
- Santos, D. T., & Meireles, M. A. A. (2011). Extraction of volatile oils by supercritical fluid extraction. Recent Patents on Engineering, 5(1), 17-22. http://dx.doi.org/10.2174/1872212111105010017
» http://dx.doi.org/10.2174/1872212111105010017 - Santos, D. T., Albarelli, J. Q., Rostagno, M. A., Ensinas, A. V., Maréchal, F., & Meireles, M. A. A. (2014). New proposal for production of bioactive compounds by supercritical technology integrated to a sugarcane biorefinery. Clean Technologies and Environmental Policy, 16(7), 1455-1468. http://dx.doi.org/10.1007/s10098-014-0760-5
» http://dx.doi.org/10.1007/s10098-014-0760-5 - Schacht, C., Zetzl, C., & Brunner, G. (2008). From plant materials to ethanol by means of supercritical fluid technology. The Journal of Supercritical Fluids, 46(3), 299-321. http://dx.doi.org/10.1016/j.supflu.2008.01.018
» http://dx.doi.org/10.1016/j.supflu.2008.01.018 - Temelli, F., & Ciftci, O. N. (2015). Developing an integrated supercritical fluid biorefinery for theprocessing of grains. The Journal of Supercritical Fluids, 96, 77-85. http://dx.doi.org/10.1016/j.supflu.2014.09.028
» http://dx.doi.org/10.1016/j.supflu.2014.09.028 - Turton, R. B., Wallace, B., Whiting, J. S., & Bhattacharyya, D. (2009). Analysis, synthesis and design of chemical processes. Upper Saddle River: Prentice Hall.
- Ulrich, G., & Vasudevan, P. (2003). A guide to chemical engineering process design and economics a practical guide. Boca Raton: CRC Press.
Publication Dates
-
Publication in this collection
08 May 2017 -
Date of issue
Jan-Mar 2018
History
-
Received
22 Nov 2016 -
Accepted
01 Apr 2017