Abstract
To investigate effect and mechanism of maternal exposure to trace cadmium on the differentiation and development in testis of male offspring rats. Thirty-two pregnant female SD rats were randomly divided into 4 groups: CdCl2 treated group (group A) was intraperitoneally injected with CdCl2 (1.5 mg/kg.d); DES group(group B) was subcutaneously injected with DES (100 μg/kg.d); CdCl2 +TMX group(group C) was treated with CdCl2 (same as above)+TMX (50 mg/kg.d); control group (group D) was treated with the same dose normal saline instead of CdCl2. On day 30th after parturition, the weight and sex ratio of off-spring male rats were recorded; Offspring testes were used for ultrastructural observation. Ultrastructural changes including swollen mitochondria and expanded endoplasmic reticulum in Sertoli cells, gonocyte and Leydig cells, moreover, lipid droplets cumulated in Leydig cells were observed in group A and group B, and so changes in group C were slightly more than those in group A or group B. The expression of StAR and inhibin-B in Leydig cells decreased significantly in group A and group B (p < 0.05), but there were no difference between group C and group D (P > 0.05). Cadmium has similar effect as estrogen which would affect gonadal differentiation and development in male fetal rats.
Keywords:
cadmium; testis; offspring rats
1 Introduction
Cadmium (Cd) is a widely distributed toxic heavy transition medal found in earth’s crust that used for making batteries and pigments. About 5-50% of the cadmium enter our body through our lungs and about 1-10% of the cadmium enter our body through the digestive tract (Ashizawa et al., 2012Ashizawa, A., Faroon, O., Wright, S., Tucker, P., Jenkins, K., Ingerman, L., & Rudisill, C. (2012). Toxicological profile for cadmium. Atlanta: Agency for Toxic Substances and Disease Registry.). Cd is harmful to personal health and will cause multi-organ damage (Huang et al., 2017Huang, Y., He, C., Shen, C., Guo, J., Mubeen, S., Yuan, J., & Yang, Z. (2017). Toxicity of cadmium and its health risks from leafy vegetable consumption. Food & Function, 8(4), 1373-1401. http://dx.doi.org/10.1039/C6FO01580H. PMid:28232985.
http://dx.doi.org/10.1039/C6FO01580H...
). Cd contamination of soils and foods arouses great public concerns owing to the massive use of fertilizer (Dharma-Wardana, 2018Dharma-Wardana, M. W. C. (2018). Fertilizer usage and cadmium in soils, crops and food. Environmental Geochemistry and Health, 40(6), 2739-2759. http://dx.doi.org/10.1007/s10653-018-0140-x. PMid:29936671.
http://dx.doi.org/10.1007/s10653-018-014...
). The US Environmental Protection Agency (EPA) has already classified Cd as a priority pollutant (Jin et al., 1998Jin, T., Lu, J., & Nordberg, M. (1998). Toxicokinetics and biochemistry of cadmium with special emphasis on the role of metallothionein. Neurotoxicology, 19(4-5), 529-535. PMid:9745907.). According to the Ministry of Environmental Protection (MEP) and the Ministry of Land and Resources (MLR) of China, Cd is the most severe contaminant in soil among all the heavy metals. Some of the investigated soils in China contains excessive Cd which is above the normal level.
Cadmium accumulated in cereals can enter the food chain and thus create a public health hazard. Rice is a popular staple food around world especially in Asia (Liu et al., 2014Liu, J. G., Qu, P., Zhang, W., Dong, Y., Li, L., & Wang, M. X. (2014). Variations among rice cultivars in subcellular distribution of Cd: The relationship between translocation and grain accumulation. Environmental and Experimental Botany, 107, 25-31. http://dx.doi.org/10.1016/j.envexpbot.2014.05.004.
http://dx.doi.org/10.1016/j.envexpbot.20...
). However, the soil subjected to the Cd contamination heavily compromises the rice quality (Hu et al., 2009Hu, Y., Ge, Y., Zhang, C., Ju, T., & Cheng, W. (2009). Cadmium toxicity and translocation in rice seedlings are reduced by hydrogen peroxide pretreatment. Plant Growth Regulation, 59(1), 51-61. http://dx.doi.org/10.1007/s10725-009-9387-7.
http://dx.doi.org/10.1007/s10725-009-938...
). China is one of the world's largest producers and consumers of rice. Although Cd is a small quantity of components in rice, its biological half-life in human body can be as long as 10 to 40 years. Moreover, it will accumulate in various tissues and organs for a long time, especially in liver and kidney (Wang et al., 2017Wang, L., Zhang, S., Wang, Z., Xu, M., Yuan, L., Cui, J., & Liu, S. (2017). A protective role of Heme-regulated eIF2alpha kinase in cadmium-induced liver and kidney injuries. Chemosphere, 185, 284-289. http://dx.doi.org/10.1016/j.chemosphere.2017.07.018. PMid:28700957.
http://dx.doi.org/10.1016/j.chemosphere....
; World Health Organization, 2000World Health Organization – WHO. (2000). Air quality guidelines. Geneva: WHO.). It will also have a series of adverse effects on embryonic and reproductive system development (Siu et al., 2009Siu, E. R., Mruk, D. D., Porto, C. S., & Cheng, C. Y. (2009). Cadmium-induced testicular injury. Toxicology and Applied Pharmacology, 238(3), 240-249. http://dx.doi.org/10.1016/j.taap.2009.01.028. PMid:19236889.
http://dx.doi.org/10.1016/j.taap.2009.01...
). Therefore, it is important to clarify the interference mechanism of trace cadmium exposure on reproductive system development.
Pregnancy and nursing are critical periods for differentiation and development of reproductive system. Exposure to a certain amount of exogenous estrogen during these periods will lead to abnormal development of the reproductive system or impairment of the spermatogenesis and maturation of male offspring (Sharpe, 2003Sharpe, R. (2003). The ‘oestrogen hypothesis’- where do we stand now? International Journal of Andrology, 26(1), 2-15. http://dx.doi.org/10.1046/j.1365-2605.2003.00367.x. PMid:12534932.
http://dx.doi.org/10.1046/j.1365-2605.20...
). As a result, hypospadias, cryptorchidism and reduction of the sperm density in adulthood may occur (Sharma et al., 2014Sharma, T., Banerjee, B. D., Yadav, C. S., Gupta, P., & Sharma, S. (2014). Heavy metal levels in adolescent and maternal blood: association with risk of hypospadias. ISRN Pediatrics, 2014, 714234. http://dx.doi.org/10.1155/2014/714234. PMid:24729887.
http://dx.doi.org/10.1155/2014/714234...
; Balabanic et al., 2011Balabanic, D., Rupnik, M., & Klemencic, A. K. (2011). Negative impact of endocrine-disrupting compounds on human reproductive health. Reproduction, Fertility, and Development, 23(3), 403-416. http://dx.doi.org/10.1071/RD09300. PMid:21426858.
http://dx.doi.org/10.1071/RD09300...
; Oliveira et al., 2009Oliveira, H., Spanò, M., Santos, C., & Pereira, M. L. (2009). Adverse effects of cadmium exposure on mouse sperm. Reproductive Toxicology, 28(4), 550-555. http://dx.doi.org/10.1016/j.reprotox.2009.08.001. PMid:19695322.
http://dx.doi.org/10.1016/j.reprotox.200...
; Zhao et al., 2015Zhao, X., Cheng, Z., Zhu, Y. I., Li, S., Zhang, L., & Luo, Y. (2015). Effects of paternal cadmium exposure on the sperm quality of male rats and the neurobehavioral system of their offspring. Experimental and Therapeutic Medicine, 10(6), 2356-2360. http://dx.doi.org/10.3892/etm.2015.2777. PMid:26668641.
http://dx.doi.org/10.3892/etm.2015.2777...
). Previous studies have confirmed that cadmium is an endocrine disrupter (EDs) (Zhao et al., 2015Zhao, X., Cheng, Z., Zhu, Y. I., Li, S., Zhang, L., & Luo, Y. (2015). Effects of paternal cadmium exposure on the sperm quality of male rats and the neurobehavioral system of their offspring. Experimental and Therapeutic Medicine, 10(6), 2356-2360. http://dx.doi.org/10.3892/etm.2015.2777. PMid:26668641.
http://dx.doi.org/10.3892/etm.2015.2777...
), which interferes with the development of the male reproductive system. However, the mechanism of cadmium reproductive toxicity has not been fully explored. Based on our previous experiments, the present study was designed and established a pregnant and nursing Cd model to investigate the effects of trace cadmium exposure on offspring gonadal development and possible endocrine disruption mechanisms.
2 Materials and methods
2.1 Experimental animals
Thirty-two sexually-mature Sprague-Dawley rats weighed between 230-260 g were used in this study. They were provided with free access to food and water in pathogen-free environment (temperature: 20-22 °C, humidity: 40-70%, day/night cycle: 12/12 h). All of the rats were fed 7 days to adapt the environment. Male and female rats were ed mated at 6:00 pm. The ratio of male to female rats was kept in 2:1. The first day that sputum was observed was recorded as GD0 (Gestation day, GD0).
After pregnancy, rats were randomly divided into 4 groups: CdCl2 treated group (group A, n=8) was intraperitoneally injected with CdCl2 (1.5 mg/kg.d) on 9th, 11th, 13th day of the gestation period and every other day during lactation respectively; DES group (group B, n=8) was subcutaneously injected with DES (100 μg/kg.d) from day 9th to 17th of gestation period and every other day during lactation respectively; CdCl2 +TMX group (group C, n=8) was treated with CdCl2 (same as above)+TMX (50 mg/kg.d); Control group (group D, n=8) was treated with normal saline instead of CdCl2 with same dose. During the administration period, the weight of the pregnant rats was recorded daily, and the presence or absence of abortion and death was observed. After the production, the pregnant rats were weighed every 3 days to test their growth. The number of male offspring rats was observed 30 days after their birth. The weights of male rats and their testis were recorded. Welfare of the experimental animals was guaranteed under the supervision of our university Animal Experiment Center (license number: SCXK(Yu)20070001).
2.2 Observation with Transmission Electron Microscopy (TEM)
The ultrastructure in the testis of male rats was observed under transmission electron microscope. First, 4% glutaraldehyde was injected into the tunica albuginea testis for fixation in situ. After 20 minutes, the testes were taken out and sliced into 1 mm3 tissue blocks. Then the blocks were placed in 4% glutaraldehyde for 2 h. Next, the sections were dehydrated by graded ethanol and acetone after 2 h fixation in 1% citric acid. Finally, the sections were immersed with epoxy resin, embedded and then sliced into ultraslices. After stained with both uranyl acetate and lead citrate, the slices were observed in TEM.
2.3 HE analysis
The testes of 18-day-old male rats were quickly removed under a microscope and placed into a fixed solution of glacial acetic acid, formaldehyde, and picric acid in a volume ratio of 1:5:15. Followed by graded ethanol dehydration, paraffin embedding, sectioning, and finally HE staining, the morphological structure, supporting cells and seminiferous tubules of the rat testes were observed under a light microscope.
2.4 Determination of StAR activity in testis of rats by immunofluorescence
The frozen sections were fixed in 4° acetone for 10 minutes. After dried, the sections were washed three times with 0.01 mol/L, pH 7.4 PBS for 3 min each time and treated with 0.1% Tritonx-100 for 15 min; Then the sections were washed by PBS to be kept at a certain humidity; Next, the goat antiserum blocking solution was added for 30 min in a 37 °C constant temperature water bath. After removing the serum, 1:200 mouse anti-rat StAR primary antibody was added at 4 °C overnight. The next day, the section was taken out and placed at 37°C constant temperature water bath for 1h; Then rinsed 4 times in PBS for 5 min each time. The sections were put into water bath for 1h after the second antibody was added in the dark environment. The sections were rinsed 3 times with PBS for 5 min each time and covered with a coverslip. Immediately observe and photograph the sections with a fluorescence microscope. For image analysis, six randomly selected fields per section were photographed and recorded. Quantitative image analysis was performed using the IPP software.
2.5 Quantitative Real-time Polymerase Chain Reaction (qRTPCR)
Total RNA was isolated from at least 1 × 105 cells or 0.1 g of testis using a Trizol reagent (Invitrogen, USA) according to the manufacturer’s protocol. After quantification, RNA samples with an A260/A280 nm ratio of more than 1.8 were retained and used for RT-PCR. Briefly, 2 μg of total RNA was reverse transcribed into 1ststrand cDNA using the Prime-Script RT reagent kit (Takara, Shiga, Japan). The obtained cDNA was then subjected to qRTPCR analysis and the sequence of the primers used for PCR was as follows: actin: 5-CGTTGACATCCGTAAAGACCTC-3' (sense) and 5'-TAGGAGCCAGGGCAGTAATCT-3' (antisense); inhibin-B: 5’- GCTCAGCCCCTGTTTTTGG -3’(sense) and 5’- GGAGGAGACGAGGTGCTTTTAG -3’ (antisense); MIS: 5- AACTGACCAATACCAGGGGC -3' (sense) and 5'- TGCTGTTCCCCAGTCTCTCC -3' (antisense).
2.6 Statistical analysis
All data are calculated in forms of the mean ± standard deviation. SNK test were performed to determine significance difference in the software of GraphPad Prism 8.01 (GraphPad Software Inc., San Diego, CA, USA). A p value less than 0.05 indicates statistical significance.
3 Results
3.1 Numbers and weights of male offspring rats
The number male offspring rats was recorded and the weights of the male offspring rats and their testes were measured 30 days after birth. As shown in Table 1.
The results showed that, compared with the group D, the weights of the group A, group B and group C were all reduced. The reduction of body length of fetus, the weight loss of placenta and male rats between the group A and the group B was statistically significant (p < 0.05). There was no significant difference in the number of fetuses per pregant rat in each group (p > 0.05). The results showed that cadmium and diethylstilbestrol had certain effects on the body weight of the male fetus, and had no significant effect on the fecundity of the pregnant rats.
3.2 The proportion of male rats in all offspring rats
The proportion of male fetus in each group is shown in Table 2. Statistical analysis showed that there was no significant difference in sex ratio between male and female rats in the group D (p > 0.05), suggesting that trace cadmium or diethylstilbestrol exposure during pregnancy and lactation did not have a serious impact on the gonadal differentiation of fetal rats.
3.3 The ultrastructure of the testis of male offspring rats
As shown in Figure 1, under electron microscope, the normal testis had normal testicular morphology, and the Sertoli cells and spermatogenic cells in the seminiferous tubules were well-defined and closely arranged. There are lots of organelles especially smooth endoplasmic reticulum and mitochondria in Leydig cells. The ultrastructural damage of the testis in the group A and the group B was obvious. The mitochondria in the Leydig cells were swollen and the myeloid structure was formed. The Sertoli cells and spermatogenic cells in the seminiferous tubules also showed worse ultrastructural degeneration.
Representative images of TEM. The testes of rats in group D (control group) have a normal ultrastructure as shown in (A) (B). Myelin figure was observed in group A as shown in (C), mitochondrial swelling and acrosome destruction was also observed in (D) and (E) respectively. As for group B, myeloid formation and mitochondrial structure destruction in spermatogenic cells was observed in (F) and (G). The macrophage was shown in (H). The structure of Sertoli cells and spermatogenic cells was normal in group C as shown in (I).
3.4 HE analysis
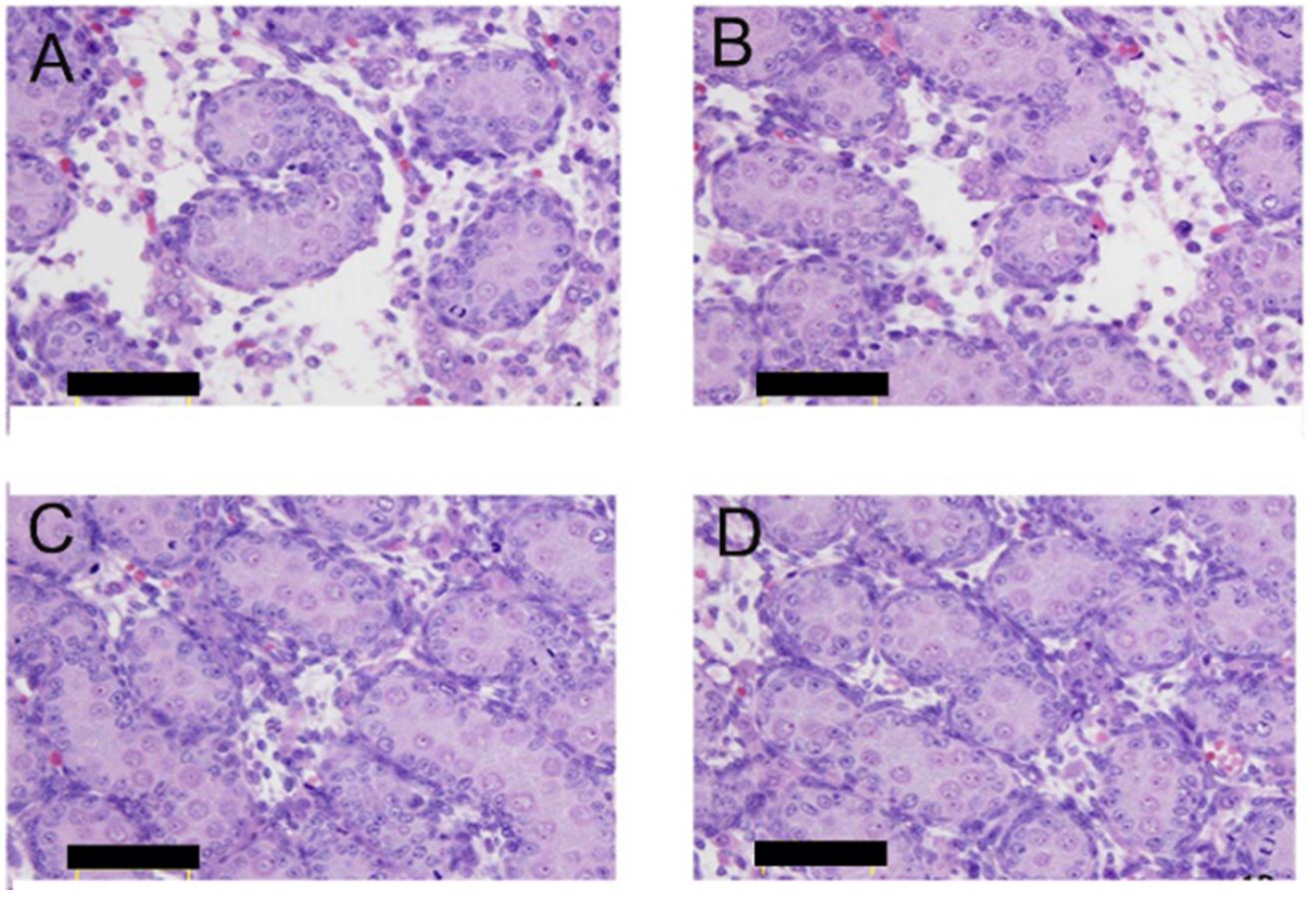
As shown in Figure 2, a great number of seminiferous tubules have been observed in the fetal testes of the group D at 18 days of gestation. It can be seen that the single-layer Sertoli cells are closely attached to the basement membrane and arranged neatly. In the group A, the arrangement of the Sertoli cells and gonocytes in the seminiferous tubules were disordered. Some of the Sertoli cells in the luminal were clumped, gonocytes were reduced or absent, and the stromal fibroblasts and collagen were abnormally proliferated. The results of the group B were similar to those of the group A, while the group C exhibits a relatively normal structure.
Fetal testicular histomorphology characteristic of male rats on GD18. HE images of group A, group B, group C and group D were displaced as (A), (B), (C) and (D).
3.5 StAR expression
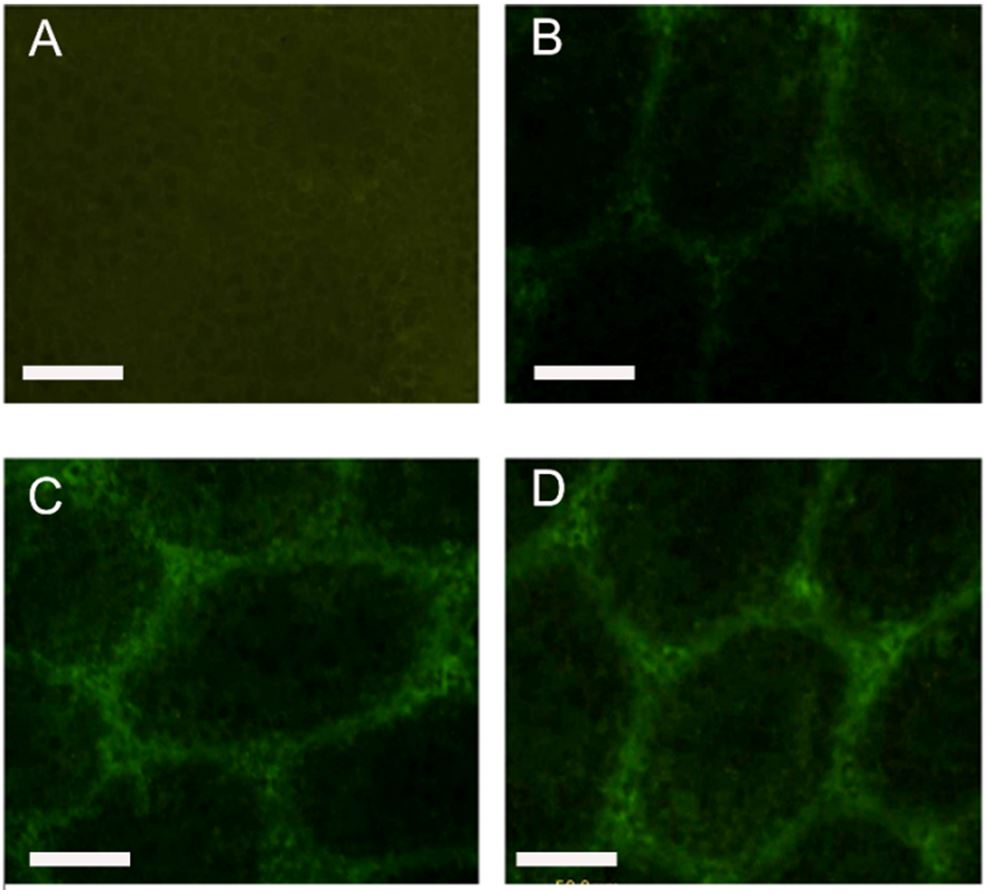
As shown in Figure 3, under the fluorescence microscope, the StAR positive reactants were stained green and expressed specifically in the seminiferous tubules. The StAR positive reactants in the testis tissue of the normal group were strongly expressed within 30 days. Compared with the group D (0.368 ± 0.026), the fluorescence intensity of the group A (0.214 ± 0.038), group B (0.215 ± 0.0 41) and group C (0.356 ± 0.027) significantly decreased (p < 0.05), while the group A and group B decreased more significantly than group C (p < 0.05).
Positive products of StAR in testes of off-spring male rats on PND30, which were expressed in cytoplasm of Leydig cells. Immunofluorescence images of group A, group B, group C and group D were displaced as (A), (B), (C) and (D).
3.6 Inhibin-B and MIS expression
SYBR Green I fluorescent dye is a double-stranded DNA-specific binding dye that binds non-specifically to all double-stranded DNA. Therefore, if non-specific amplification occurs in the reaction system, it will inevitably affect the repeatability and reliability of results. The melting curve of the PCR product can be analyzed to determine if the reaction is specific. In this experiment, the inhibin-B and MIS melting curve showed a single peak, indicating that the inhibin-B and MIS have good specificity.
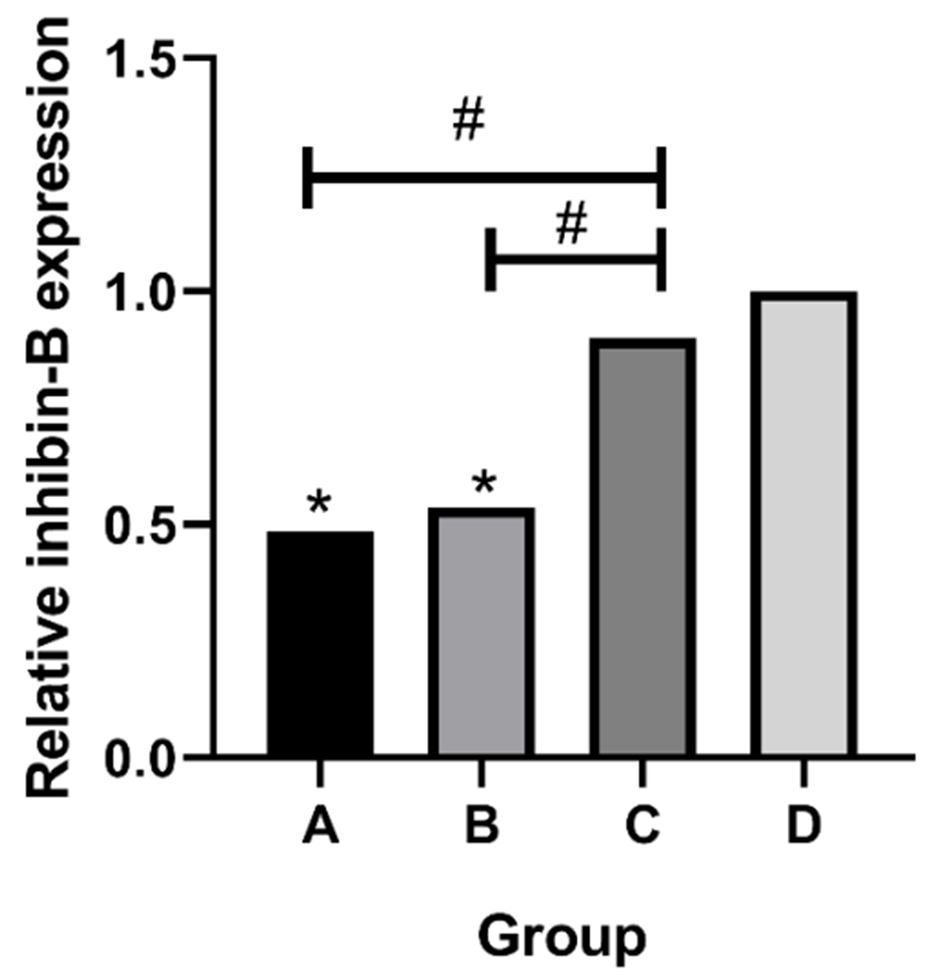
The amplification results of inhibin-B are shown in Figure 4. The expression of inhibin-B in the testis of the group A and group B was decreased in the 30-day-old rats, which was statistically significant when compared with the normal group (P < 0.05). There was no significant difference in the normal group (P > 0.05). Compared with the group C, the group A and group B (P < 0.05). The amplification results of MIS are shown in Figure 5. There was no significant difference of MIS expression in the four groups (P > 0.05).
PCR analysis of inhibin-B expression. *p < 0.05, compared with the group D; #p < 0.05, compared with the group C.
4 Discussion
Recent studies have shown that the number of sperm in men has decreased worldwide by 50% in the past half century, and the incidence of genital diseases such as cryptorchidism, hypospadias, testicular tumors, and prostate cancer has doubled (Ma et al., 2008Ma, A., Yang, X., Wang, Z., Shi, D., & Chen, Y. (2008). Adult exposure to diethylstilbestrol induces spermatogenic cell apoptosis in vivo through increased oxidative stress in male hamster. Reproductive Toxicology, 25(3), 367-373. http://dx.doi.org/10.1016/j.reprotox.2007.12.007. PMid:18296022.
http://dx.doi.org/10.1016/j.reprotox.200...
). Infertility also tends to rise with the diseases above. Increased incidence of male reproductive system malformations is associated with exposure factors for environmental endocrine disruptors in pregnant women and fetuses (Irvine, 2000Irvine, D. S. (2000). Male reproductive health: cause for concern? Andrologia, 32(4-5), 195-208. http://dx.doi.org/10.1046/j.1439-0272.2000.00388.x. PMid:11021510.
http://dx.doi.org/10.1046/j.1439-0272.20...
).
Cadmium acts as a potential non-steroidal environmental endocrine disruptor whose biological activity was similar to that of estrogen (Darbre, 2006Darbre, P. D. (2006). Metalloestrogens: an emerging class of inorganic xenoestrogens with potential to add to the oestrogenic burden of the human breast. Journal of Applied Toxicology, 26(3), 191-197. http://dx.doi.org/10.1002/jat.1135. PMid:16489580.
http://dx.doi.org/10.1002/jat.1135...
). In this study, rats were exposed to Cd during a sensitive window period. The dose and time of intervention were selected based on previous studies (Holt & Webb, 1987Holt, D., & Webb, M. (1987). Teratogenicity of ionic cadmium in the Wistar rat. Archives of Toxicology, 59(6), 443-447. http://dx.doi.org/10.1007/BF00316212. PMid:3606391.
http://dx.doi.org/10.1007/BF00316212...
), and the DES and Cd combined with estrogen receptor antagonist tamoxifen were added for comparison. Our results revealed that the body weights of female rats and male rats in the group A and group B were decreased, suggesting that cadmium and DES adversely affected the growth and development of rats. On the one hand, it may due to the toxic effects of Cd and DES on the maternal rats, which may cause poor intrauterine fetal growth in offspring rats. On the other hand, Cd and DES inhibited the activity of some metabolic enzymes in rats, resulting in a decrease in body weight of fetal rats. Versloot et al. (1998)Versloot, P., Van der Heide, D., Schroder-van der Elst, J., & Boogerd, L. (1998). Maternal thyroxine and 3,5,3\”-tri-iodothyronine kinetics in near-term pregnant rats at two different levels of hypothyroidism. European Journal of Endocrinology, 138(1), 113-119. http://dx.doi.org/10.1530/eje.0.1380113. PMid:9461326.
http://dx.doi.org/10.1530/eje.0.1380113...
found that decreased serum thyroid hormone levels in pregnant rats can lead to weight loss in fetal rats. Mushtaq et al. (1981)Mushtaq, M., Mukhtar, H., Datta, K. K., Tandon, S. G., & Seth, P. K. (1981). Toxicological studies of a leachable stabilizer di-n-butyltin dilaurate(DBTL): effects on hepatic drug metabolizing enzyme activities. Drug and Chemical Toxicology, 4(1), 75-88. http://dx.doi.org/10.3109/01480548109066373. PMid:7261948.
http://dx.doi.org/10.3109/01480548109066...
demonstrated that the treatment of Di-n-butyltin dilaurate (DBTL) reduced the activity of 6-phosphate glucose dehydrogenase and cytochrome P450 in rat hepatocytes. These two enzymes will affect the biotransformation function of hepatocytes and the heme metabolism, resulting in weight loss of rats. Whether Cd and DES cause the weight loss of fetal rats through these mechanisms needs a further study.
Our study also found that the morphology of the testes in fetal rats was significantly changed. Under the electron microscope, fetal rats in group A and group B showed that mitochondrial and nucleus of Leydig cell is swelling. Myeloid structure formation and excessive lipid droplets accumulation can also be observed. Leydig cells are primarily responsible for the synthesis and secretion of androgen testosterone. More than 90% of the body's testosterone is secreted by Leydig cells. Degeneration of the morphological structure of Leydig cell and subsequent dysfunction are bound to cause abnormalities in male gonadal differentiation and development. There is also necrosis of Sertoli cells and spermatogonia that found in group A and group B. Sertoli cell plays an important part in blood-testis barrier (BBB), and its destruction will inevitably cause damage to the BBB.
Steroid hormone synthesis acute regulatory protein (StAR) is a transporter of cholesterol that mainly involved in the metabolism of cholesterol and the synthesis of steroid hormones. During hormone synthesis, free cholesterol needs to be transferred to the steroidogenic tissue via the outer mitochondrial membrane. The steroid hormone synthesis signal allows StAR to be rapidly expressed in the testis tissue. StAR may be involved in cholesterol transmembrane transport mediating and promoting cholesterol (Ning et al., 2006Ning, Y., Chen, S., Li, X., Ma, Y., Zhao, F., & Yin, L. (2006). Cholesterol, LDL, and 25-hydroxycholesterol regulate expression of the steroidogenic acute regulatory protein in microvascular endothelial cell line (bEnd.3). Biochemical and Biophysical Research Communications, 342(4), 1249-1256. http://dx.doi.org/10.1016/j.bbrc.2006.02.093. PMid:16516145.
http://dx.doi.org/10.1016/j.bbrc.2006.02...
). It mediates and promotes the transportation of cholesterol from the outer mitochondrial membrane to the inner mitochondrial membrane, which in turn to form pregnenolone under the action of P450scc, and finally converts into testosterone and other steroids under the action of related steroidogenic synthase. This is the rate-limiting step of testosterone synthesis (Miller, 2007Miller, W. L. (2007). Steroidogenic acute regulatory protein (StAR), a novel mitochondrial cholesterol transporter. Biochimica et Biophysica Acta, 1771(6), 663-676. http://dx.doi.org/10.1016/j.bbalip.2007.02.012. PMid:17433772.
http://dx.doi.org/10.1016/j.bbalip.2007....
). Our results have shown that the expression of StAR positive products in the testes of fetal rats is decreased both in the group A and group B. In addition, electron microscopy showed that more macrophages were found in the testis of rats in the group A and group B, while macrophages secreted inflammatory mediators such as cytokines and reactive oxygen species (ROS) which could bind to some biomacromolecules. Hales et al. (1987)Hales, D. B., Sha, L. L., & Payne, A. H. (1987). Testosterone inhibits cAMP-induced de Novo synthesis of Leydig cell cytochrome P-450(17 alpha) by an androgen receptor-mediated mechanism. Journal of Biological Chemistry, 262(23), 11200-11206. PMid:3038910. believe that ROS secreted by macrophages can inhibit the expression of StAR protein by interfering with mitochondria of Leydig cells, thereby regulating the synthesis of androgen in Leydig cells. Therefore, we speculate that the expression of StAR positive products in the group A and group B may be related to not only the direct effect of Cd and DES but also the functional change in macrophages caused by them.
Inhibin B is a testis-derived glycoprotein hormone composed of two subunits, α and β. It is secreted by Sertoli cells before puberty, and then secreted by a group of spermatogenic cells at various stages in combination with Sertoli cells after puberty (Christiansen et al., 2002Christiansen, P., Andersson, A., Skakkebaek, N., & Juul, A. (2002). Serum inhibin B, FSH, LH and testosterone levels before and after human chorionic gonadotropin stimulation in prepubertal boys with cryptorchidism. European Journal of Endocrinology, 147(1), 95-101. http://dx.doi.org/10.1530/eje.0.1470095. PMid:12088925.
http://dx.doi.org/10.1530/eje.0.1470095...
; Marchetti et al., 2003Marchetti, C., Hamdane, M., Mitchell, V., Mayo, K., Devisme, L., Rigot, J. M., Beauvillain, J. C., Hermand, E., & Defossez, A. (2003). Immunolocalization of inhibin and activin α and βB subunits and expression of corresponding messenger RNAs in the human adult testis. Biology of Reproduction, 68(1), 230-235. http://dx.doi.org/10.1095/biolreprod.102.004424. PMid:12493718.
http://dx.doi.org/10.1095/biolreprod.102...
). It is a representative marker of the testicular seminiferous tubules, and the changes in inhibin B levels will affect spermatogenic function. Studies have found that serum inhibin B levels in male patients with low spermatogenic function are significantly lower than those have normal normal spermatogenesis function. Serum inhibin B levels were also significantly associated with testicular volume and total sperm count. In adult male rhesus monkeys, serum inhibin B levels decreased by 50% after performed with unilateral orchiectomy (Ramaswamy et al., 2000Ramaswamy, S., Marshall, G. R., Mcneilly, A. S., & Plant, T. M. (2000). Dynamics of the follicle-stimulating hormone (FSH)-inhibin B feedback loop and its role in regulating spermatogenesis in the adult male rhesus monkey (Macaca mulatta) as revealed by unilateral orchidectomy. Endocrinology, 141(1), 18-27. http://dx.doi.org/10.1210/endo.141.1.7276. PMid:10614619.
http://dx.doi.org/10.1210/endo.141.1.727...
). In this study, the expression of testosterone B in the group A and group B was decreased which may be related to the destruction of Sertoli cells by Cd and DES and may also be related to the reduction of FSH. Decreased expression of inhibin B will give rise to disorder of spermatogenic process in the testis, resulting in a decrease in sperm production and infertility.
MIS is a homodimeric glycoprotein secreted by fetal and adult Sertoli cells as well as ovarian granulosa cells with a relative molecular weight of 140 kD. MIS can be used both as autocrine and sideThe secretory factor acts locally, and it can also act as a hormone to enter the circulatory system and play a role in other target cells. In the testis, MIS can promote the Mullerian tube degeneration and inhibit the proliferation of Leydig cells and Sertoli cells, thereby prompting the male genitourinary system formation. However, there was no significant difference in MIS expression among the four groups in our study (P > 0.05), suggesting that the effect of Cd exposure during pregnancy on the development of gonads in male fetuses may not be caused by Cd induced MIS pathway.
The results of the group A and group B were similar which indicate that Cd can cause embryotoxicity like estrogen. And the results of the group C further confirm that cadmium has an estrogen-like effect. Martin et al. (2003)Martin, M. B., Reiter, R., Pham, T., Avellanet, Y. R., Camara, J., Lahm, M., Pentecost, E., Pratap, K., Gilmore, B. A., Divekar, S., Dagata, R. S., Bull, J. L., & Stoica, A. (2003). Estrogen-like activity of metals in MCF-7 breast cancer cells. Endocrinology, 144(6), 2425-2436. http://dx.doi.org/10.1210/en.2002-221054. PMid:12746304.
http://dx.doi.org/10.1210/en.2002-221054...
showed that Cd can bind to estrogen receptor (ER), resulting in increased uterine weight in adult ovariectomized rats and the proliferation of human breast cancer cells T47D (Wilson et al., 2004Wilson, S. V., Bobseine, K., & Gray, L. E. Jr. (2004). Development and characterization of a cell line that stably expresses an estrogen-responsive luciferase reporter for the detection of estrogen receptor agonist and antagonists. Toxicological Sciences, 81(1), 69-77. http://dx.doi.org/10.1093/toxsci/kfh180. PMid:15166400.
http://dx.doi.org/10.1093/toxsci/kfh180...
). This study proved that Cd exposure during pregnancy can directly cause damage to the testicular ultrastructure of fetal rats. It can also reduce the activity of testosterone synthesis rate-limiting enzyme StAR, which interferes with embryonic male gonad development. But there is no evidence that it has effect on gonadal differentiation. As for whether it jeopardizes the fertility of the rat during puberty or later, we are working hard to figure out the long-term effects in future study.
5 Conclusion
These results indicate Cd exposure during pregnancy can directly cause damage to the testicular ultrastructure of fetal rats. It can also reduce the activity of testosterone synthesis rate-limiting enzyme StAR, which interferes with embryonic male gonad development.
Acknowledgements
The study was supported by the Experimental study on insulin resistance mechanism of pancreatic islet B cells in high-fat rats Key projects of Sichuan Provincial Education Department (13ZA0225); the Effect of long-term High-fat diet on Morphology and Function of Pancreatic islet β cell of Strategic cooperation projects in science and technology between city and school of Nanchong (18SXHZ0285).
-
Practical Application: Cadmium exposure during pregnancy can directly cause damage to the testicular ultrastructure of fetal rats. It can also reduce the activity of testosterone synthesis rate-limiting enzyme StAR, which interferes with embryonic male gonad development.
References
- Ashizawa, A., Faroon, O., Wright, S., Tucker, P., Jenkins, K., Ingerman, L., & Rudisill, C. (2012). Toxicological profile for cadmium Atlanta: Agency for Toxic Substances and Disease Registry.
- Balabanic, D., Rupnik, M., & Klemencic, A. K. (2011). Negative impact of endocrine-disrupting compounds on human reproductive health. Reproduction, Fertility, and Development, 23(3), 403-416. http://dx.doi.org/10.1071/RD09300 PMid:21426858.
» http://dx.doi.org/10.1071/RD09300 - Christiansen, P., Andersson, A., Skakkebaek, N., & Juul, A. (2002). Serum inhibin B, FSH, LH and testosterone levels before and after human chorionic gonadotropin stimulation in prepubertal boys with cryptorchidism. European Journal of Endocrinology, 147(1), 95-101. http://dx.doi.org/10.1530/eje.0.1470095 PMid:12088925.
» http://dx.doi.org/10.1530/eje.0.1470095 - Darbre, P. D. (2006). Metalloestrogens: an emerging class of inorganic xenoestrogens with potential to add to the oestrogenic burden of the human breast. Journal of Applied Toxicology, 26(3), 191-197. http://dx.doi.org/10.1002/jat.1135 PMid:16489580.
» http://dx.doi.org/10.1002/jat.1135 - Dharma-Wardana, M. W. C. (2018). Fertilizer usage and cadmium in soils, crops and food. Environmental Geochemistry and Health, 40(6), 2739-2759. http://dx.doi.org/10.1007/s10653-018-0140-x PMid:29936671.
» http://dx.doi.org/10.1007/s10653-018-0140-x - Hales, D. B., Sha, L. L., & Payne, A. H. (1987). Testosterone inhibits cAMP-induced de Novo synthesis of Leydig cell cytochrome P-450(17 alpha) by an androgen receptor-mediated mechanism. Journal of Biological Chemistry, 262(23), 11200-11206. PMid:3038910.
- Holt, D., & Webb, M. (1987). Teratogenicity of ionic cadmium in the Wistar rat. Archives of Toxicology, 59(6), 443-447. http://dx.doi.org/10.1007/BF00316212 PMid:3606391.
» http://dx.doi.org/10.1007/BF00316212 - Hu, Y., Ge, Y., Zhang, C., Ju, T., & Cheng, W. (2009). Cadmium toxicity and translocation in rice seedlings are reduced by hydrogen peroxide pretreatment. Plant Growth Regulation, 59(1), 51-61. http://dx.doi.org/10.1007/s10725-009-9387-7
» http://dx.doi.org/10.1007/s10725-009-9387-7 - Huang, Y., He, C., Shen, C., Guo, J., Mubeen, S., Yuan, J., & Yang, Z. (2017). Toxicity of cadmium and its health risks from leafy vegetable consumption. Food & Function, 8(4), 1373-1401. http://dx.doi.org/10.1039/C6FO01580H PMid:28232985.
» http://dx.doi.org/10.1039/C6FO01580H - Irvine, D. S. (2000). Male reproductive health: cause for concern? Andrologia, 32(4-5), 195-208. http://dx.doi.org/10.1046/j.1439-0272.2000.00388.x PMid:11021510.
» http://dx.doi.org/10.1046/j.1439-0272.2000.00388.x - Jin, T., Lu, J., & Nordberg, M. (1998). Toxicokinetics and biochemistry of cadmium with special emphasis on the role of metallothionein. Neurotoxicology, 19(4-5), 529-535. PMid:9745907.
- Liu, J. G., Qu, P., Zhang, W., Dong, Y., Li, L., & Wang, M. X. (2014). Variations among rice cultivars in subcellular distribution of Cd: The relationship between translocation and grain accumulation. Environmental and Experimental Botany, 107, 25-31. http://dx.doi.org/10.1016/j.envexpbot.2014.05.004
» http://dx.doi.org/10.1016/j.envexpbot.2014.05.004 - Ma, A., Yang, X., Wang, Z., Shi, D., & Chen, Y. (2008). Adult exposure to diethylstilbestrol induces spermatogenic cell apoptosis in vivo through increased oxidative stress in male hamster. Reproductive Toxicology, 25(3), 367-373. http://dx.doi.org/10.1016/j.reprotox.2007.12.007 PMid:18296022.
» http://dx.doi.org/10.1016/j.reprotox.2007.12.007 - Marchetti, C., Hamdane, M., Mitchell, V., Mayo, K., Devisme, L., Rigot, J. M., Beauvillain, J. C., Hermand, E., & Defossez, A. (2003). Immunolocalization of inhibin and activin α and βB subunits and expression of corresponding messenger RNAs in the human adult testis. Biology of Reproduction, 68(1), 230-235. http://dx.doi.org/10.1095/biolreprod.102.004424 PMid:12493718.
» http://dx.doi.org/10.1095/biolreprod.102.004424 - Martin, M. B., Reiter, R., Pham, T., Avellanet, Y. R., Camara, J., Lahm, M., Pentecost, E., Pratap, K., Gilmore, B. A., Divekar, S., Dagata, R. S., Bull, J. L., & Stoica, A. (2003). Estrogen-like activity of metals in MCF-7 breast cancer cells. Endocrinology, 144(6), 2425-2436. http://dx.doi.org/10.1210/en.2002-221054 PMid:12746304.
» http://dx.doi.org/10.1210/en.2002-221054 - Miller, W. L. (2007). Steroidogenic acute regulatory protein (StAR), a novel mitochondrial cholesterol transporter. Biochimica et Biophysica Acta, 1771(6), 663-676. http://dx.doi.org/10.1016/j.bbalip.2007.02.012 PMid:17433772.
» http://dx.doi.org/10.1016/j.bbalip.2007.02.012 - Mushtaq, M., Mukhtar, H., Datta, K. K., Tandon, S. G., & Seth, P. K. (1981). Toxicological studies of a leachable stabilizer di-n-butyltin dilaurate(DBTL): effects on hepatic drug metabolizing enzyme activities. Drug and Chemical Toxicology, 4(1), 75-88. http://dx.doi.org/10.3109/01480548109066373 PMid:7261948.
» http://dx.doi.org/10.3109/01480548109066373 - Ning, Y., Chen, S., Li, X., Ma, Y., Zhao, F., & Yin, L. (2006). Cholesterol, LDL, and 25-hydroxycholesterol regulate expression of the steroidogenic acute regulatory protein in microvascular endothelial cell line (bEnd.3). Biochemical and Biophysical Research Communications, 342(4), 1249-1256. http://dx.doi.org/10.1016/j.bbrc.2006.02.093 PMid:16516145.
» http://dx.doi.org/10.1016/j.bbrc.2006.02.093 - Oliveira, H., Spanò, M., Santos, C., & Pereira, M. L. (2009). Adverse effects of cadmium exposure on mouse sperm. Reproductive Toxicology, 28(4), 550-555. http://dx.doi.org/10.1016/j.reprotox.2009.08.001 PMid:19695322.
» http://dx.doi.org/10.1016/j.reprotox.2009.08.001 - Ramaswamy, S., Marshall, G. R., Mcneilly, A. S., & Plant, T. M. (2000). Dynamics of the follicle-stimulating hormone (FSH)-inhibin B feedback loop and its role in regulating spermatogenesis in the adult male rhesus monkey (Macaca mulatta) as revealed by unilateral orchidectomy. Endocrinology, 141(1), 18-27. http://dx.doi.org/10.1210/endo.141.1.7276 PMid:10614619.
» http://dx.doi.org/10.1210/endo.141.1.7276 - Sharma, T., Banerjee, B. D., Yadav, C. S., Gupta, P., & Sharma, S. (2014). Heavy metal levels in adolescent and maternal blood: association with risk of hypospadias. ISRN Pediatrics, 2014, 714234. http://dx.doi.org/10.1155/2014/714234 PMid:24729887.
» http://dx.doi.org/10.1155/2014/714234 - Sharpe, R. (2003). The ‘oestrogen hypothesis’- where do we stand now? International Journal of Andrology, 26(1), 2-15. http://dx.doi.org/10.1046/j.1365-2605.2003.00367.x PMid:12534932.
» http://dx.doi.org/10.1046/j.1365-2605.2003.00367.x - Siu, E. R., Mruk, D. D., Porto, C. S., & Cheng, C. Y. (2009). Cadmium-induced testicular injury. Toxicology and Applied Pharmacology, 238(3), 240-249. http://dx.doi.org/10.1016/j.taap.2009.01.028 PMid:19236889.
» http://dx.doi.org/10.1016/j.taap.2009.01.028 - Versloot, P., Van der Heide, D., Schroder-van der Elst, J., & Boogerd, L. (1998). Maternal thyroxine and 3,5,3\”-tri-iodothyronine kinetics in near-term pregnant rats at two different levels of hypothyroidism. European Journal of Endocrinology, 138(1), 113-119. http://dx.doi.org/10.1530/eje.0.1380113 PMid:9461326.
» http://dx.doi.org/10.1530/eje.0.1380113 - Wang, L., Zhang, S., Wang, Z., Xu, M., Yuan, L., Cui, J., & Liu, S. (2017). A protective role of Heme-regulated eIF2alpha kinase in cadmium-induced liver and kidney injuries. Chemosphere, 185, 284-289. http://dx.doi.org/10.1016/j.chemosphere.2017.07.018 PMid:28700957.
» http://dx.doi.org/10.1016/j.chemosphere.2017.07.018 - Wilson, S. V., Bobseine, K., & Gray, L. E. Jr. (2004). Development and characterization of a cell line that stably expresses an estrogen-responsive luciferase reporter for the detection of estrogen receptor agonist and antagonists. Toxicological Sciences, 81(1), 69-77. http://dx.doi.org/10.1093/toxsci/kfh180 PMid:15166400.
» http://dx.doi.org/10.1093/toxsci/kfh180 - World Health Organization – WHO. (2000). Air quality guidelines Geneva: WHO.
- Zhao, X., Cheng, Z., Zhu, Y. I., Li, S., Zhang, L., & Luo, Y. (2015). Effects of paternal cadmium exposure on the sperm quality of male rats and the neurobehavioral system of their offspring. Experimental and Therapeutic Medicine, 10(6), 2356-2360. http://dx.doi.org/10.3892/etm.2015.2777 PMid:26668641.
» http://dx.doi.org/10.3892/etm.2015.2777
Publication Dates
-
Publication in this collection
03 Feb 2021 -
Date of issue
Jul-Sep 2021
History
-
Received
18 May 2020 -
Accepted
08 Aug 2020