Diabetes mellitus is a common chronic metabolic disease worldwide whose prevalence has increased during the last decades. Besides its more commonly recognized complications, such as macrovascular disease, retinopathy, nephropathy and neuropathy, diabetes related bone disease has gained growing attention. Diabetic patients are more prone to fracture than the general population as well as to low turnover bone disease in the chronic kidney disease setting. In this review, we discuss the relationship between diabetes and bone as well as the pathogenesis of bone fragility in T2D.
Keywords:
bone fragility; diabetes mellitus; kidney failure, chronic
Resumos
O diabetes mellitus é uma desordem metabólica crônica cuja prevalência tem aumentado no mundo todo ao longo das últimas décadas. Além de suas complicações mais reconhecidas, como a doença macrovascular, retinopatia, nefropatia e neuropatia, as alterações ósseas relacionadas ao diabetes têm ganhado um crescente interesse. Os pacientes diabéticos são mais suscetíveis a fraturas do que a população geral e à doença de baixa remodelação entre os pacientes com doença renal crônica. Nesta revisão, iremos discutir a relação entre diabetes e o tecido ósseo, assim como a patogênese da fragilidade óssea no diabetes tipo 2.
Palavras-chave:
diabetes mellitus; fragilidade óssea; insuficiência renal crônica
Introduction
Diabetes mellitus (DM), in particular type 2DM, is a common chronic metabolic disease worldwide. Its prevalence has increased along with the increase in obesity resulting from lifestyle changes of modern life. Patients with type 2 diabetes (T2D) are at significant risk for well recognized diabetic complications, including macrovascular disease, retinopathy, nephropathy, and neuropathy. However, recently, one more complication has been associated with DM, an increased risk of fragility fractures which appears to be somewhat independent of bone mineral density (BMD).11 Liao CC, Lin CS, Shih CC, Yeh CC, Chang YC, Lee YW, et al. Increased risk of fracture and postfracture adverse events in patients with diabetes: two nationwide population-based retrospective cohort studies. Diabetes Care 2014;37:2246-52. PMID: 24804698 DOI: http://dx.doi.org/10.2337/dc13-2957
http://dx.doi.org/10.2337/dc13-2957...
,22 Jackuliak P, Payer J. Osteoporosis, fractures, and diabetes. Int J Endocrinol 2014;2014:820615. DOI: http://dx.doi.org/10.1155/2014/820615
http://dx.doi.org/10.1155/2014/820615...
In fact, T2D patients have, in general, normal BMD, implicating abnormalities in bone material strength and/or bone microarchitecture.33 Farr JN, Khosla S. Determinants of bone strength and quality in diabetes mellitus in humans. Bone 2015; pii: S87563282(15)00301-4. [Epub ahead of print] DOI: http://dx.doi.org/10.1016/j.bone.2015.07.027
http://dx.doi.org/10.1016/j.bone.2015.07...
In this regard, a study using high resolution peripheral quantitative computed tomography (HRpQCT) has demonstrated an increase of cortical porosity in T2D.44 Farr JN, Drake MT, Amin S, Melton LJ 3rd, McCready LK, Khosla S. In vivo assessment of bone quality in postmenopausal women with type 2 diabetes. J Bone Miner Res 2014;29:787-95. DOI: http://dx.doi.org/10.1002/jbmr.2106
http://dx.doi.org/10.1002/jbmr.2106...
Moreover, a large study using trabecular bone score (TBS), which is a texture parameter that evaluates pixel gray-level variations in the spine DXA, demonstrated low lumbar spine TBS which is associated with worse bone structure.55 Leslie WD, Aubry-Rozier B, Lamy O, Hans D; Manitoba Bone Density Program. TBS (trabecular bone score) and diabetes-related fracture risk. J Clin Endocrinol Metab 2013;98:602-9. DOI: http://dx.doi.org/10.1210/jc.2012-3118
http://dx.doi.org/10.1210/jc.2012-3118...
Taken together, it seems that microarchitecture including trabecular and cortical bone are disrupted in T2D and may contribute to bone fragility. In addition to a disrupted architecture, a decrease in bone formation and turnover demonstrated by histomorphometric analysis of bone may play a role in the increase of risk of fragility fractures seen in T2D.66 Moreira CA, Dempster DW. Bone histomorphometry in diabetes mellitus. Osteoporosis Int 2015 Aug 5. [Epub ahead of print] DOI:http://dx.doi.org/10.1007/s00198-015-3258-z
http://dx.doi.org/10.1007/s00198-015-325...
The aim of this review is to discuss the relationship between diabetes and bone as well as the pathogenesis of bone fragility in T2D.
Epidemiology of T2D and fractures
There is convincing evidence that older adults with T2D have an elevated risk for all clinical fractures, particularly in African-American and Latino populations. In this regard, a meta-analysis demonstrated a relative risk (RR) of fracture of 1.2 (95% CI 1.0 to 1.5) in patients with T2D.77 Janghorbani M, Van Dam RM, Willett WC, Hu FB. Systematic review of type 1 and type 2 diabetes mellitus and risk of fracture. Am J Epidemiol 2007;166:495-505. PMID: 17575306 DOI: http://dx.doi.org/10.1093/aje/kwm106
http://dx.doi.org/10.1093/aje/kwm106...
Longer duration of diabetes appears to increase fracture risk; however, newly diagnosed T2D was related to a significantly increased risk of any fracture (adjusted hazard ratio, HR, 95% CI: 1.36, 1.32-1.40), as well as for hip, spine, wrist/hand, forearm, and upper arm/shoulder fractures. Furthermore, diabetes control also influences bone fragility as showed in a recent meta-analysis indicating that poor glycemic control contributes to increased fracture risk. However, aggressive lowering of A1C does not appear to be effective in preventing fracture.
The increased fracture risk in T2D is thought to be due to both an increased falling frequency and decreased bone strength. The increased falling frequency is mainly a result of complications of the disease such as a retinopathy and polyneuropathy.
The pathogenesis of bone changes in diabetes
Hyperglycemia
Hyperglycemia affects the skeleton at both cellular and extracellular bone matrix levels. In vitro studies have shown that high glucose levels augment osteoclast differentiation/fusion resulting in a more resorptive environment.88 Larsen KI, Falany ML, Ponomareva LV, Wang W, Williams JP. Glucose-dependent regulation of osteoclast H(+)-ATPase expression: potential role of p38 MAP-kinase. J Cell Biochem 2002;87:75-84. PMID: 12210724 DOI: http://dx.doi.org/10.1002/jcb.10252
http://dx.doi.org/10.1002/jcb.10252...
At the tissue level, hyperglycemia affects the organic bone matrix through the accumulation of advanced glycation end products (AGEs) leading to inferior bone strength.99 Hernandez CJ, Tang SY, Baumbach BM, Hwu PB, Sakkee AN, van der Ham F, et al. Trabecular microfracture and the influence of pyridinium and non-enzymatic glycation-mediated collagen cross-links. Bone 2005;37:825-32. PMID: 16140600 DOI: http://dx.doi.org/10.1016/j.bone.2005.07.019
http://dx.doi.org/10.1016/j.bone.2005.07...
Indeed, the contribution of AGEs to the development and progression of complications of diabetes is well demonstrated in the literature.1010 Ahmed N. Advanced glycation endproducts-role in pathology of diabetic complications. Diabetes Res Clin Pract 2005;67:3-21. PMID: 15620429 DOI:http://dx.doi.org/10.1016/j.diabres.2004.09.004
http://dx.doi.org/10.1016/j.diabres.2004...
In general, the pathological effects of AGEs are related the ability of these compounds to modify the chemical and functional properties of several biological structures. In all tissues, AGEs generate free radicals and promote oxidative stress, and increased expression of inflammatory mediators.1111 Huebschmann AG, Regensteiner JG, Vlassara H, Reusch JE. Diabetes and advanced glycoxidation end products. Diabetes Care 2006;29:1420-32. PMID: 16732039 DOI: http://dx.doi.org/10.2337/dc05-2096
http://dx.doi.org/10.2337/dc05-2096...
There are several AGEs receptors or AGE -binding proteins, but the RAGE receptor is probably the best characterized.1212 Lin L. RAGE on the Toll Road? Cell Mol Immunol 2006;3:351-8. There is evidence for involvement of RAGE in the development of diabetic macro- and microangiopathy.1313 Shoji T, Koyama H, Morioka T, Tanaka S, Kizu A, Motoyama K, et al. Receptor for advanced glycation end products is involved in impaired angiogenic response in diabetes. Diabetes 2006;55:2245-55. PMID: 16873687 DOI: http://dx.doi.org/10.2337/db05-1375
http://dx.doi.org/10.2337/db05-1375...
In the skeleton, the accumulation of AGEs leads to more brittle bone with reduced toughness and therefore, less ability to deform before fracturing. The most studied AGE is pentosidine, the concentrations of which in cortical and trabecular bone are negatively associated with bone strength.1414 Saito M, Fujii K, Soshi S, Tanaka T. Reductions in degree of mineralization and enzymatic collagen cross-links and increases in glycation-induced pentosidine in the femoral neck cortex in cases of femoral neck fracture. Osteoporos Int 2006;17:986-95. DOI: http://dx.doi.org/10.1007/s00198-006-0087-0
http://dx.doi.org/10.1007/s00198-006-008...
Patients with fracture present higher concentrations of pentosidine than nonfractured controls.1515 Yamamoto M, Yamaguchi T, Yamauchi M, Yano S, Sugimoto T. Serum pentosidine levels are positively associated with the presence of vertebral fractures in postmenopausal women with type 2 diabetes. J Clin Endocrinol Metab 2008;93:1013-9. PMID: 18160470 DOI:http://dx.doi.org/10.1210/jc.20071270
http://dx.doi.org/10.1210/jc.20071270...
It was demonstrated in vitro that incubation of osteoblasts with pentosidine caused a significant decrease in alkaline phosphatase, collagen 1α1, osteocalcin, and RAGE gene expression. These data suggest a detrimental effect of AGEs on bone that leads to functional alterations in osteoblasts and in the bone mineralization process.1616 Sanguineti R, Storace D, Monacelli F, Federici A, Odetti P. Pentosidine effects on human osteoblasts in vitro. Ann N Y Acad Sci 2008;1126:166-72. PMID: 18448811 DOI: http://dx.doi.org/10.1196/annals.1433.044
http://dx.doi.org/10.1196/annals.1433.04...
In addition, serum concentrations of pentosidine in T2D were shown to be higher than those in control subjects and were correlated with cortical bone pentosidine. One Japanese study evaluated serum pentosidine levels in postmenopausal women with diabetes and demonstrated an association with prevalent vertebral fractures, which was independent of BMD.1515 Yamamoto M, Yamaguchi T, Yamauchi M, Yano S, Sugimoto T. Serum pentosidine levels are positively associated with the presence of vertebral fractures in postmenopausal women with type 2 diabetes. J Clin Endocrinol Metab 2008;93:1013-9. PMID: 18160470 DOI:http://dx.doi.org/10.1210/jc.20071270
http://dx.doi.org/10.1210/jc.20071270...
This study suggested that serum pentosidine was more sensitive than BMD in assessing the risk of prevalent vertebral fractures in women with diabetes. The relationship between pentosidine and bone fragility has been also demonstrated in type 1 diabetes (T1D).1717 Neumann T, Lodes S, Kästner B, Franke S, Kiehntopf M, Lehmann T, et al. High serum pentosidine but not esRAGE is associated with prevalent fractures in type 1 diabetes independent of bone mineral density and glycaemic control. Osteoporos Int 2014;25:1527-33. PMID: 24599273 DOI:http://dx.doi.org/10.1007/s00198-014-2631-7
http://dx.doi.org/10.1007/s00198-014-263...
,1818 Farlay D, Armas LA, Gineyts E, Akhter MP, Recker RR, Boivin G. Nonenzymatic Glycation and Degree of Mineralization Are Higher in Bone From Fractured Patients With Type 1 Diabetes Mellitus. J Bone Miner Res 2015 Aug 1. [Epub ahead of print] DOI: http://dx.doi.org/10.1002/jbmr.2607
http://dx.doi.org/10.1002/jbmr.2607...
Recently, bone biopsies from T1D patients with fracture were analyzed by high-performance liquid chromatography to assess pentosidine concentrations in trabecular and cortical bone.1818 Farlay D, Armas LA, Gineyts E, Akhter MP, Recker RR, Boivin G. Nonenzymatic Glycation and Degree of Mineralization Are Higher in Bone From Fractured Patients With Type 1 Diabetes Mellitus. J Bone Miner Res 2015 Aug 1. [Epub ahead of print] DOI: http://dx.doi.org/10.1002/jbmr.2607
http://dx.doi.org/10.1002/jbmr.2607...
In addition, the degree of mineralization of bone (DMB) was assessed by digitized microradiography, and mechanical properties by micro- and nanohardness tests. Positive correlations were found between HbA1c and pentosidine and between HbA1c and DMB. Both modifications resulted in less flexible bone (reduced modulus of elasticity) increasing the probability of low-energy fractures in T1D patients. Based on the correlation between pentosidine and fractures, it is reasonable to speculate that serum pentosidine levels could serve as a marker for fracture risk in diabetic patients, since BMD is less effective in the identification of those patients with diabetes at risk for fragility fractures.
Insulin and IGF-1
Insulin is an anabolic hormone which has effects on the skeleton. It acts on bone tissue through insulin receptors (IRS-1 and IRS-2) expressed by osteoblasts. In normal physiological conditions, stimulation of these receptors stimulates bone formation by increasing osteoblast proliferation and promoting collagen synthesis. In the same way, insulin growth factor-1 (IGF-1) is a key regulator of bone and acts to increase osteoblast recruitment and bone matrix deposition and reduce collagen degradation.1919 Niu T, Rosen CJ. The insulin-like growth factor-I gene and osteoporosis: a critical appraisal. Gene 2005;361:38-56. PMID: 16183214 DOI:http://dx.doi.org/10.1016/j.gene.2005.07.016
http://dx.doi.org/10.1016/j.gene.2005.07...
Indeed, studies have demonstrated a positive correlation between IGF-1 and BMD and also a negative correlation with hip and vertebral fractures.2020 Garnero P, Sornay-Rendu E, Delmas PD. Low serum IGF-1 and occurrence of osteoporotic fractures in postmenopausal women. Lancet 2000;355:898-9. PMID: 10752709 DOI: http://dx.doi.org/10.1016/S0140-6736(99)05463-X
http://dx.doi.org/10.1016/S0140-6736(99)...
Bone turnover
Studies have shown that serum markers of bone turnover (BTM), especially the formation markers (osteocalcin and P1NP) are decreased in patients with diabetes.2121 Rubin MR. Bone cells and bone turnover in diabetes mellitus. Curr Osteoporos Rep 2015;13:186-91. DOI: http://dx.doi.org/10.1007/s11914-015-0265-0
http://dx.doi.org/10.1007/s11914-015-026...
,2222 Dobnig H, Piswanger-Sölkner JC, Roth M, Obermayer-Pietsch B, Tiran A, Strele A, et al. Type 2 diabetes mellitus in nursing home patients: effects on bone turnover, ß, and fracture risk. J Clin Endocrinol Metab 2006;91:3355-63. DOI: http://dx.doi.org/10.1210/jc.2006-0460
http://dx.doi.org/10.1210/jc.2006-0460...
Moreover, bone histomorphometry has demonstrated that remodeling parameters such as bone formation rate and mineralizing surface are significantly lower in T2D than controls indicating a low turnover state.2323 Krakauer JC, McKenna MJ, Buderer NF, Rao DS, Whitehouse FW, Parfitt AM. Bone loss and bone turnover in diabetes. Diabetes 1995;44:775-82. PMID: 7789645 DOI: http://dx.doi.org/10.2337/diab.44.7.775
http://dx.doi.org/10.2337/diab.44.7.775...
,2424 Manavalan JS, Cremers S, Dempster DW, Zhou H, Dworakowski E, Kode A, et al. Circulating osteogenic precursor cells in type 2 diabetes mellitus. J Clin Endocrinol Metab 2012;97:3240-50. PMID: 22740707 DOI: http://dx.doi.org/10.1210/jc.2012-1546
http://dx.doi.org/10.1210/jc.2012-1546...
In this regard, sclerostin a regulator of bone formation, has emerged as an important player in this scenario. Sclerostin is an osteocyte product which inhibits the wnt B-catenin pathway by binding to LRP5 or 6 and, thereby, negatively regulates bone formation.2525 Canalis E. Wnt signalling in osteoporosis: mechanisms and novel therapeutic approaches. Nat Rev Endocrinol 2013;9:575-83. DOI:http://dx.doi.org/10.1038/nrendo.2013.154
http://dx.doi.org/10.1038/nrendo.2013.15...
Patients with T2D have been shown to have higher levels of circulating sclerostin that were associated with time and control of the disease.2626 Gennari L, Merlotti D, Valenti R, Ceccarelli E, Ruvio M, Pietrini MG, et al. Circulating sclerostin levels and bone turnover in type 1 and type 2 diabetes. J Clin Endocrinol Metab 2012;97:1737-44. PMID: 22399511 DOI: http://dx.doi.org/10.1210/jc.2011-2958
http://dx.doi.org/10.1210/jc.2011-2958...
,2727 García-Martín A, Rozas-Moreno P, Reyes-García R, Morales-Santana S, García-Fontana B, García-Salcedo JA, et al. Circulating levels of sclerostin are increased in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab 2012;97:234-41. PMID: 22031520 DOI: http://dx.doi.org/10.1210/jc.2011-2186
http://dx.doi.org/10.1210/jc.2011-2186...
A Chinese study evaluated 265 postmenopausal women with T2D and showed that the serum sclerostin level was significantly higher than that in a non-diabetic control group (48.2 ± 19.4 vs. 37.2 ± 18.6 pmol/L, p < 0.001). Serum sclerostin concentration was positively correlated with hemoglobin A1c level and negatively associated with biochemical bone turnover markers, intact parathyroid hormone and bone-specific alkaline phosphatase.2828 Zhou YJ, Li A, Song YL, Zhou H, Li Y, Tang YS. Role of sclerostin in the bone loss of postmenopausal chinese women with type 2 diabetes. Chin Med Sci J 2013;28:135-9. DOI: http://dx.doi.org/10.1016/S1001-9294(13)60038-3
http://dx.doi.org/10.1016/S1001-9294(13)...
In addition, sclerostin levels were associated with increased risk of vertebral fractures, independent of BMD,1515 Yamamoto M, Yamaguchi T, Yamauchi M, Yano S, Sugimoto T. Serum pentosidine levels are positively associated with the presence of vertebral fractures in postmenopausal women with type 2 diabetes. J Clin Endocrinol Metab 2008;93:1013-9. PMID: 18160470 DOI:http://dx.doi.org/10.1210/jc.20071270
http://dx.doi.org/10.1210/jc.20071270...
indicating that the low bone formation caused by high levels of sclerostin impaired bone quality. A significant association between bone formation markers and IGF-1 was demonstrated and confirmed that IGF-1 is linked to osteoblast function.2929 Ardawi MS, Akhbar DH, Alshaikh A, Ahmed MM, Qari MH, Rouzi AA, et al. Increased serum sclerostin and decreased serum IGF-1 are associated with vertebral fractures among postmenopausal women with type-2 diabetes. Bone 2013;56:355-62. DOI: http://dx.doi.org/10.1016/j.bone.2013.06.029
http://dx.doi.org/10.1016/j.bone.2013.06...
Moreover, an inverse association of IGF-1 and sclerostin was demonstrated in postmenopausal women with T2D and vertebral fractures. It, therefore, appears that the association of low IGF-1 and high sclerostin levels contribute to the bone fragility observed in T2D patients.
Osteocalcin is a non-collagenous matrix protein that is linked to glucose metabolism. It is a 49-amino acid peptide synthesized exclusively by the osteoblasts and stored in matrix.3030 Zanatta LC, Boguszewski CL, Borba VZ, Kulak CA. Osteocalcin, energy and glucose metabolism. Arq Bras Endocrinol Metabol 2014;58:444-51. PMID: 25166034 DOI: http://dx.doi.org/10.1590/0004-2730000003333
http://dx.doi.org/10.1590/0004-273000000...
In its undercarboxylated form, it has some hormonal features and has been associated with glucose metabolism and fat mass. Osteocalcin stimulates insulin secretion and enhances insulin sensitivity in adipose tissue and muscle (Figure 1). A negative association between osteocalcin and markers of metabolic syndrome, such as serum glucose, insulin, high-sensitivity C reactive protein, interleukin-6, body fat and body mass index (BMI) has been demonstrated,3131 Pittas AG, Harris SS, Eliades M, Stark P, Dawson-Hughes B. Association between serum osteocalcin and markers of metabolic phenotype. J Clin Endocrinol Metab 2009;94:827-32. PMID: 19088165 DOI: http://dx.doi.org/10.1210/jc.20081422
http://dx.doi.org/10.1210/jc.20081422...
suggesting that reduced osteocalcin levels may play a role in the pathophysiology of bone fragility in T2D.
Endocrine link between bone and energy and glucose metabolism. Insulin stimulates the secretion of undercarboxylated osteocalcin, which improves insulin secretion and adiponectin production by fat cells. Figure from reference 27, with permission.
Obesity and adipocytes in the bone marrow
Obesity was once believed to be protective for osteoporosis. An elevated BMI is very frequent in T2D patients and is strongly associated with higher BMD, however obesity is not protective against fractures.3232 Tanaka S, Kuroda T, Saito M, Shiraki M. Overweight/obesity and underweight are both risk factors for osteoporotic fractures at different sites in Japanese postmenopausal women. Osteoporosis Int 2013;24:69-76. DOI: http://dx.doi.org/10.1007/s00198-012-2209-1
http://dx.doi.org/10.1007/s00198-012-220...
Indeed, results from the GLOW study (Global Longitudinal study of Osteoporosis in Women) demonstrate that obesity is not protective against fracture in postmenopausal women and is associated with increased risk of ankle and upper leg fractures.3333 Compston JE, Watts NB, Chapurlat R, Cooper C, Boonen S, Greenspan S, et al. Obesity is not protective against fracture in postmenopausal women: GLOW. Am J Med 2011;124:1043-50. DOI: http://dx.doi.org/10.1016/j.amjmed.2011.06.013
http://dx.doi.org/10.1016/j.amjmed.2011....
In addition, interesting interactions between fat and bone may play a role in the pathophysiology of bone fragility.
There is growing interest in the relationship between bone marrow fat (BMF), BMD and fractures. This interaction occurs due to the fact of osteoblasts and adipocytes differentiate from the same mesenchymal stem cells (MSC).3434 Paccou J, Hardouin P, Cotten A, Penel G, Cortet B. The Role of Bone Marrow Fat in Skeletal Health: Usefulness and perspectives for clinicians. J Clin Endocrinol Metab 2015. 100:3613-21. PMID: 26244490 DOI: http://dx.doi.org/10.1210/jc.20152338
http://dx.doi.org/10.1210/jc.20152338...
Recent studies on bone marrow adipocytes have shown that they are not only lipid storage cells, but also secrete adipokines, such as leptin and adiponectin in an autocrine and paracrine manner. An inverse relationship between BMF and BMD was observed; however, no study has yet demonstrated an association with bone fragility.3535 Shen W, Chen J, Punyanitya M, Shapses S, Heshka S, Heymsfield SB. MRI-measured bone marrow adipose tissue is inversely related to DXA-measured bone mineral in Caucasian women. Osteoporosis Int 2007;18:641-7. DOI: http://dx.doi.org/10.1007/s00198-006-0285-9
http://dx.doi.org/10.1007/s00198-006-028...
BMF can be measured by sophisticated techniques such as magnetic resonance imaging, with or without spectroscopy, and by bone biopsy, in which the adipocyte volume, perimeter and density can be quantified.3636 Verma S, Rajaratnam JH, Denton J, Hoyland JA, Byers RJ. Adipocytic proportion of bone marrow is inversely related to bone formation in osteoporosis. J Clin Pathol 2002;55:693-8. PMID: 12195001 DOI: http://dx.doi.org/10.1136/jcp.55.9.693
http://dx.doi.org/10.1136/jcp.55.9.693...
,3737 Justesen J, Stenderup K, Ebbesen EN, Mosekilde L, Steiniche T, Kassem M. Adipocyte tissue volume in bone marrow is increased with aging and in patients with osteoporosis. Biogerontology 2001;2:165-71. DOI: http://dx.doi.org/10.1023/A:1011513223894
http://dx.doi.org/10.1023/A:101151322389...
Bone histomorphometry studies demonstrated that enhanced adipogenesis in the bone marrow of ostoporotic patients was inversely correlated with trabecular bone volume.3737 Justesen J, Stenderup K, Ebbesen EN, Mosekilde L, Steiniche T, Kassem M. Adipocyte tissue volume in bone marrow is increased with aging and in patients with osteoporosis. Biogerontology 2001;2:165-71. DOI: http://dx.doi.org/10.1023/A:1011513223894
http://dx.doi.org/10.1023/A:101151322389...
Further, the increase in BMF was associated with reduced bone formation, supporting the postulated switch in differentiation of MSCs from the osteoblastic to the adipocytic pathway in osteoporosis.3636 Verma S, Rajaratnam JH, Denton J, Hoyland JA, Byers RJ. Adipocytic proportion of bone marrow is inversely related to bone formation in osteoporosis. J Clin Pathol 2002;55:693-8. PMID: 12195001 DOI: http://dx.doi.org/10.1136/jcp.55.9.693
http://dx.doi.org/10.1136/jcp.55.9.693...
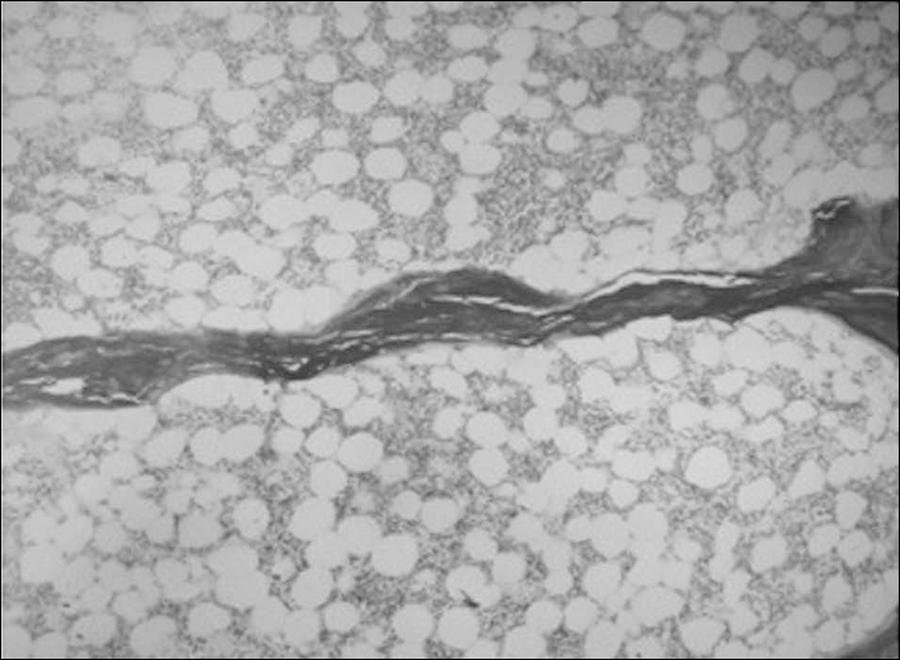
Recently, we evaluated BMF of 41 patients on peritoneal dialyses by bone histomorphometry and found an association between increased marrow adiposity and reduced osteoblast activity and bone turnover.3838 Barreto FC, Aguiar CM, Hernandes MJ. Marrow Adiposity Is Associated with Low Bone Turnover and Sclerostin levels in Peritoneal Dialysis Patients. ASBM Annual Meeting; oct 9-12, 2015. Seattle, USA. Interestingly, diabetic patients presented with higher marrow fat than non-diabetic patients (Figure 2).
Adynamic bone disease in an ESRD patient. An isolated trabecula among a great number of adipocytes in the bone marrow. (Bone section stained with hematoxylin and eosyn; magnification x 20.)
In summary, it is clear that there are complex interactions among the skeleton, obesity and BMF and these interactions have important implications for the development of skeletal fragility in T2D.
Dm and renal osteodystrophy
Diabetes mellitus predisposes chronic kidney disease (CKD) patients to a low bone turnover state, called adynamic bone disease (ABD). Importantly, the prevalence of ABD has increased during the last decades and it has been found as the most prevalent bone disorder among pre dialysis and peritoneal dialysis (PD) patients.3939 Barreto FC, Barreto DV, Canziani ME, Tomiyama C, Higa A, Mozar A, et al. Association between indoxyl sulfate and bone histomorphometry in pre-dialysis chronic kidney disease patients. J Bras Nefrol 2014;36:289-96. DOI: http://dx.doi.org/10.5935/0101-2800.20140042
http://dx.doi.org/10.5935/0101-2800.2014...
,4040 de Oliveira RA, Barreto FC, Mendes M, dos Reis LM, Castro JH, Britto ZM, et al. Peritoneal dialysis per se is a risk factor for sclerostin-associated adynamic bone disease. Kidney Int 2015;87:1039-45. DOI: http://dx.doi.org/10.1038/ki.2014.372
http://dx.doi.org/10.1038/ki.2014.372...
The growing prevalence of DM among the CKD population has been suggested as one of the possible explanation for these findings. Furthermore, the pathological significance of ABD in CKD has been demonstrated through its association with vascular calcification, bone fragility and mortality.4141 Brandenburg VM, Floege J. Adynamic bone disease-bone and beyond. NDT Plus 2008;1:135-47.
A recent study in which renal osteodystrophy was evaluated in 41 PD patients, de Oliveira et al. have further confirmed the association between DM and ABD, which could be, at least partially, explained by the presence of higher bone and serum levels of sclerostin in diabetic patients.4040 de Oliveira RA, Barreto FC, Mendes M, dos Reis LM, Castro JH, Britto ZM, et al. Peritoneal dialysis per se is a risk factor for sclerostin-associated adynamic bone disease. Kidney Int 2015;87:1039-45. DOI: http://dx.doi.org/10.1038/ki.2014.372
http://dx.doi.org/10.1038/ki.2014.372...
This hypothesis still needs to be tested in pre dialysis and hemodialysis populations. Notably, accumulation of uremic toxins may potentially exacerbate the suppressive effect of diabetes on bone turnover through their deleterious effects on bone cell function.4242 Barreto FC, Barreto DV, Liabeuf S, Drüeke TB, Massy ZA. Effects of uremic toxins on vascular and bone remodeling. Semin Dial 2009;22:433-7. DOI: http://dx.doi.org/10.1111/j.1525-139X.2009.00595.x
http://dx.doi.org/10.1111/j.1525-139X.20...
Hyperglycemia and insulin deficiency both inhibit parathyroid hormone secretion and may act, synergistically, with the direct effects of DM and uremic toxins on bone cells to further suppress bone turnover in CKD. Finally, despite of being considered a classical risk factor for ABD, it remains to be demonstrated whether a better glycemic control would ameliorate bone turnover in diabetic CKD patients.
Conclusion
There is an interesting relationship between T2D as well as glucose metabolism and the skeleton. Further, an interaction among fat and bone has been demonstrated. In this regard, a great number of features, discussed above, play a role in the impairment of bone quality seen in T2D. An adequate glycaemic control is important and might help reduce the risk of bone fragility, since it may decrease the accumulation of AGEs in bone matrix. In addition, a delay in the complications of DM, such as neuropathy and retinopathy, achieved with improved glucose control may help decrease the risk of falls.
However, besides the glucose control, all patients with DM should be encouraged to prevent osteoporosis and falls, by reducing all of the other risk factors, such as smoking, sedentary lifestyle and vitamin D deficiency.
It is interesting to point out, that the bone disease in diabetes has some similarities with ABD, where along with a low bone remodeling rate there are also higher levels of circulating sclerostin and lower levels of circulating PTH. All these findings may indicate that a new class of drug, sclerostin inhibitors, might be a promising therapeutic option in diabetic patients with bone fragility.
References
-
1Liao CC, Lin CS, Shih CC, Yeh CC, Chang YC, Lee YW, et al. Increased risk of fracture and postfracture adverse events in patients with diabetes: two nationwide population-based retrospective cohort studies. Diabetes Care 2014;37:2246-52. PMID: 24804698 DOI: http://dx.doi.org/10.2337/dc13-2957
» http://dx.doi.org/10.2337/dc13-2957 -
2Jackuliak P, Payer J. Osteoporosis, fractures, and diabetes. Int J Endocrinol 2014;2014:820615. DOI: http://dx.doi.org/10.1155/2014/820615
» http://dx.doi.org/10.1155/2014/820615 -
3Farr JN, Khosla S. Determinants of bone strength and quality in diabetes mellitus in humans. Bone 2015; pii: S87563282(15)00301-4. [Epub ahead of print] DOI: http://dx.doi.org/10.1016/j.bone.2015.07.027
» http://dx.doi.org/10.1016/j.bone.2015.07.027 -
4Farr JN, Drake MT, Amin S, Melton LJ 3rd, McCready LK, Khosla S. In vivo assessment of bone quality in postmenopausal women with type 2 diabetes. J Bone Miner Res 2014;29:787-95. DOI: http://dx.doi.org/10.1002/jbmr.2106
» http://dx.doi.org/10.1002/jbmr.2106 -
5Leslie WD, Aubry-Rozier B, Lamy O, Hans D; Manitoba Bone Density Program. TBS (trabecular bone score) and diabetes-related fracture risk. J Clin Endocrinol Metab 2013;98:602-9. DOI: http://dx.doi.org/10.1210/jc.2012-3118
» http://dx.doi.org/10.1210/jc.2012-3118 -
6Moreira CA, Dempster DW. Bone histomorphometry in diabetes mellitus. Osteoporosis Int 2015 Aug 5. [Epub ahead of print] DOI:http://dx.doi.org/10.1007/s00198-015-3258-z
» http://dx.doi.org/10.1007/s00198-015-3258-z -
7Janghorbani M, Van Dam RM, Willett WC, Hu FB. Systematic review of type 1 and type 2 diabetes mellitus and risk of fracture. Am J Epidemiol 2007;166:495-505. PMID: 17575306 DOI: http://dx.doi.org/10.1093/aje/kwm106
» http://dx.doi.org/10.1093/aje/kwm106 -
8Larsen KI, Falany ML, Ponomareva LV, Wang W, Williams JP. Glucose-dependent regulation of osteoclast H(+)-ATPase expression: potential role of p38 MAP-kinase. J Cell Biochem 2002;87:75-84. PMID: 12210724 DOI: http://dx.doi.org/10.1002/jcb.10252
» http://dx.doi.org/10.1002/jcb.10252 -
9Hernandez CJ, Tang SY, Baumbach BM, Hwu PB, Sakkee AN, van der Ham F, et al. Trabecular microfracture and the influence of pyridinium and non-enzymatic glycation-mediated collagen cross-links. Bone 2005;37:825-32. PMID: 16140600 DOI: http://dx.doi.org/10.1016/j.bone.2005.07.019
» http://dx.doi.org/10.1016/j.bone.2005.07.019 -
10Ahmed N. Advanced glycation endproducts-role in pathology of diabetic complications. Diabetes Res Clin Pract 2005;67:3-21. PMID: 15620429 DOI:http://dx.doi.org/10.1016/j.diabres.2004.09.004
» http://dx.doi.org/10.1016/j.diabres.2004.09.004 -
11Huebschmann AG, Regensteiner JG, Vlassara H, Reusch JE. Diabetes and advanced glycoxidation end products. Diabetes Care 2006;29:1420-32. PMID: 16732039 DOI: http://dx.doi.org/10.2337/dc05-2096
» http://dx.doi.org/10.2337/dc05-2096 -
12Lin L. RAGE on the Toll Road? Cell Mol Immunol 2006;3:351-8.
-
13Shoji T, Koyama H, Morioka T, Tanaka S, Kizu A, Motoyama K, et al. Receptor for advanced glycation end products is involved in impaired angiogenic response in diabetes. Diabetes 2006;55:2245-55. PMID: 16873687 DOI: http://dx.doi.org/10.2337/db05-1375
» http://dx.doi.org/10.2337/db05-1375 -
14Saito M, Fujii K, Soshi S, Tanaka T. Reductions in degree of mineralization and enzymatic collagen cross-links and increases in glycation-induced pentosidine in the femoral neck cortex in cases of femoral neck fracture. Osteoporos Int 2006;17:986-95. DOI: http://dx.doi.org/10.1007/s00198-006-0087-0
» http://dx.doi.org/10.1007/s00198-006-0087-0 -
15Yamamoto M, Yamaguchi T, Yamauchi M, Yano S, Sugimoto T. Serum pentosidine levels are positively associated with the presence of vertebral fractures in postmenopausal women with type 2 diabetes. J Clin Endocrinol Metab 2008;93:1013-9. PMID: 18160470 DOI:http://dx.doi.org/10.1210/jc.20071270
» http://dx.doi.org/10.1210/jc.20071270 -
16Sanguineti R, Storace D, Monacelli F, Federici A, Odetti P. Pentosidine effects on human osteoblasts in vitro. Ann N Y Acad Sci 2008;1126:166-72. PMID: 18448811 DOI: http://dx.doi.org/10.1196/annals.1433.044
» http://dx.doi.org/10.1196/annals.1433.044 -
17Neumann T, Lodes S, Kästner B, Franke S, Kiehntopf M, Lehmann T, et al. High serum pentosidine but not esRAGE is associated with prevalent fractures in type 1 diabetes independent of bone mineral density and glycaemic control. Osteoporos Int 2014;25:1527-33. PMID: 24599273 DOI:http://dx.doi.org/10.1007/s00198-014-2631-7
» http://dx.doi.org/10.1007/s00198-014-2631-7 -
18Farlay D, Armas LA, Gineyts E, Akhter MP, Recker RR, Boivin G. Nonenzymatic Glycation and Degree of Mineralization Are Higher in Bone From Fractured Patients With Type 1 Diabetes Mellitus. J Bone Miner Res 2015 Aug 1. [Epub ahead of print] DOI: http://dx.doi.org/10.1002/jbmr.2607
» http://dx.doi.org/10.1002/jbmr.2607 -
19Niu T, Rosen CJ. The insulin-like growth factor-I gene and osteoporosis: a critical appraisal. Gene 2005;361:38-56. PMID: 16183214 DOI:http://dx.doi.org/10.1016/j.gene.2005.07.016
» http://dx.doi.org/10.1016/j.gene.2005.07.016 -
20Garnero P, Sornay-Rendu E, Delmas PD. Low serum IGF-1 and occurrence of osteoporotic fractures in postmenopausal women. Lancet 2000;355:898-9. PMID: 10752709 DOI: http://dx.doi.org/10.1016/S0140-6736(99)05463-X
» http://dx.doi.org/10.1016/S0140-6736(99)05463-X -
21Rubin MR. Bone cells and bone turnover in diabetes mellitus. Curr Osteoporos Rep 2015;13:186-91. DOI: http://dx.doi.org/10.1007/s11914-015-0265-0
» http://dx.doi.org/10.1007/s11914-015-0265-0 -
22Dobnig H, Piswanger-Sölkner JC, Roth M, Obermayer-Pietsch B, Tiran A, Strele A, et al. Type 2 diabetes mellitus in nursing home patients: effects on bone turnover, ß, and fracture risk. J Clin Endocrinol Metab 2006;91:3355-63. DOI: http://dx.doi.org/10.1210/jc.2006-0460
» http://dx.doi.org/10.1210/jc.2006-0460 -
23Krakauer JC, McKenna MJ, Buderer NF, Rao DS, Whitehouse FW, Parfitt AM. Bone loss and bone turnover in diabetes. Diabetes 1995;44:775-82. PMID: 7789645 DOI: http://dx.doi.org/10.2337/diab.44.7.775
» http://dx.doi.org/10.2337/diab.44.7.775 -
24Manavalan JS, Cremers S, Dempster DW, Zhou H, Dworakowski E, Kode A, et al. Circulating osteogenic precursor cells in type 2 diabetes mellitus. J Clin Endocrinol Metab 2012;97:3240-50. PMID: 22740707 DOI: http://dx.doi.org/10.1210/jc.2012-1546
» http://dx.doi.org/10.1210/jc.2012-1546 -
25Canalis E. Wnt signalling in osteoporosis: mechanisms and novel therapeutic approaches. Nat Rev Endocrinol 2013;9:575-83. DOI:http://dx.doi.org/10.1038/nrendo.2013.154
» http://dx.doi.org/10.1038/nrendo.2013.154 -
26Gennari L, Merlotti D, Valenti R, Ceccarelli E, Ruvio M, Pietrini MG, et al. Circulating sclerostin levels and bone turnover in type 1 and type 2 diabetes. J Clin Endocrinol Metab 2012;97:1737-44. PMID: 22399511 DOI: http://dx.doi.org/10.1210/jc.2011-2958
» http://dx.doi.org/10.1210/jc.2011-2958 -
27García-Martín A, Rozas-Moreno P, Reyes-García R, Morales-Santana S, García-Fontana B, García-Salcedo JA, et al. Circulating levels of sclerostin are increased in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab 2012;97:234-41. PMID: 22031520 DOI: http://dx.doi.org/10.1210/jc.2011-2186
» http://dx.doi.org/10.1210/jc.2011-2186 -
28Zhou YJ, Li A, Song YL, Zhou H, Li Y, Tang YS. Role of sclerostin in the bone loss of postmenopausal chinese women with type 2 diabetes. Chin Med Sci J 2013;28:135-9. DOI: http://dx.doi.org/10.1016/S1001-9294(13)60038-3
» http://dx.doi.org/10.1016/S1001-9294(13)60038-3 -
29Ardawi MS, Akhbar DH, Alshaikh A, Ahmed MM, Qari MH, Rouzi AA, et al. Increased serum sclerostin and decreased serum IGF-1 are associated with vertebral fractures among postmenopausal women with type-2 diabetes. Bone 2013;56:355-62. DOI: http://dx.doi.org/10.1016/j.bone.2013.06.029
» http://dx.doi.org/10.1016/j.bone.2013.06.029 -
30Zanatta LC, Boguszewski CL, Borba VZ, Kulak CA. Osteocalcin, energy and glucose metabolism. Arq Bras Endocrinol Metabol 2014;58:444-51. PMID: 25166034 DOI: http://dx.doi.org/10.1590/0004-2730000003333
» http://dx.doi.org/10.1590/0004-2730000003333 -
31Pittas AG, Harris SS, Eliades M, Stark P, Dawson-Hughes B. Association between serum osteocalcin and markers of metabolic phenotype. J Clin Endocrinol Metab 2009;94:827-32. PMID: 19088165 DOI: http://dx.doi.org/10.1210/jc.20081422
» http://dx.doi.org/10.1210/jc.20081422 -
32Tanaka S, Kuroda T, Saito M, Shiraki M. Overweight/obesity and underweight are both risk factors for osteoporotic fractures at different sites in Japanese postmenopausal women. Osteoporosis Int 2013;24:69-76. DOI: http://dx.doi.org/10.1007/s00198-012-2209-1
» http://dx.doi.org/10.1007/s00198-012-2209-1 -
33Compston JE, Watts NB, Chapurlat R, Cooper C, Boonen S, Greenspan S, et al. Obesity is not protective against fracture in postmenopausal women: GLOW. Am J Med 2011;124:1043-50. DOI: http://dx.doi.org/10.1016/j.amjmed.2011.06.013
» http://dx.doi.org/10.1016/j.amjmed.2011.06.013 -
34Paccou J, Hardouin P, Cotten A, Penel G, Cortet B. The Role of Bone Marrow Fat in Skeletal Health: Usefulness and perspectives for clinicians. J Clin Endocrinol Metab 2015. 100:3613-21. PMID: 26244490 DOI: http://dx.doi.org/10.1210/jc.20152338
» http://dx.doi.org/10.1210/jc.20152338 -
35Shen W, Chen J, Punyanitya M, Shapses S, Heshka S, Heymsfield SB. MRI-measured bone marrow adipose tissue is inversely related to DXA-measured bone mineral in Caucasian women. Osteoporosis Int 2007;18:641-7. DOI: http://dx.doi.org/10.1007/s00198-006-0285-9
» http://dx.doi.org/10.1007/s00198-006-0285-9 -
36Verma S, Rajaratnam JH, Denton J, Hoyland JA, Byers RJ. Adipocytic proportion of bone marrow is inversely related to bone formation in osteoporosis. J Clin Pathol 2002;55:693-8. PMID: 12195001 DOI: http://dx.doi.org/10.1136/jcp.55.9.693
» http://dx.doi.org/10.1136/jcp.55.9.693 -
37Justesen J, Stenderup K, Ebbesen EN, Mosekilde L, Steiniche T, Kassem M. Adipocyte tissue volume in bone marrow is increased with aging and in patients with osteoporosis. Biogerontology 2001;2:165-71. DOI: http://dx.doi.org/10.1023/A:1011513223894
» http://dx.doi.org/10.1023/A:1011513223894 -
38Barreto FC, Aguiar CM, Hernandes MJ. Marrow Adiposity Is Associated with Low Bone Turnover and Sclerostin levels in Peritoneal Dialysis Patients. ASBM Annual Meeting; oct 9-12, 2015. Seattle, USA.
-
39Barreto FC, Barreto DV, Canziani ME, Tomiyama C, Higa A, Mozar A, et al. Association between indoxyl sulfate and bone histomorphometry in pre-dialysis chronic kidney disease patients. J Bras Nefrol 2014;36:289-96. DOI: http://dx.doi.org/10.5935/0101-2800.20140042
» http://dx.doi.org/10.5935/0101-2800.20140042 -
40de Oliveira RA, Barreto FC, Mendes M, dos Reis LM, Castro JH, Britto ZM, et al. Peritoneal dialysis per se is a risk factor for sclerostin-associated adynamic bone disease. Kidney Int 2015;87:1039-45. DOI: http://dx.doi.org/10.1038/ki.2014.372
» http://dx.doi.org/10.1038/ki.2014.372 -
41Brandenburg VM, Floege J. Adynamic bone disease-bone and beyond. NDT Plus 2008;1:135-47.
-
42Barreto FC, Barreto DV, Liabeuf S, Drüeke TB, Massy ZA. Effects of uremic toxins on vascular and bone remodeling. Semin Dial 2009;22:433-7. DOI: http://dx.doi.org/10.1111/j.1525-139X.2009.00595.x
» http://dx.doi.org/10.1111/j.1525-139X.2009.00595.x
Publication Dates
-
Publication in this collection
Oct-Dec 2015
History
-
Received
05 Oct 2015 -
Accepted
05 Oct 2015