Abstracts
The discovery and clinical application of biomarkers for mental disorders is faced with many challenges. In general, the current methods for discovery and validation of biomarkers have not produced the results which were first anticipated after completion of the human genome project. This is mostly due to the lack of a standardized pipeline connecting marker discovery with technologies for validation and translation to a platform that offers accuracy and ease of use in a clinical setting. As a consequence, most psychiatrists and general practitioners are still reluctant to accept that biomarker tests can supplement or replace the long standing interview-based methods for diagnosis. Despite this, the regulatory agencies now agree that improvements over the current methods are essential. Furthermore, these agencies stipulate that biomarkers are important for future drug development and have initiated efforts to modernize methods and techniques to support these efforts. Here, we review the challenges faced by this endeavour from the point of view of psychiatrists, general practitioners, the regulatory agencies and biomarker scientists. We also describe the development of a novel molecular blood-test for schizophrenia as a first promising step towards achieving this goal.
Psychiatric disorders; schizophrenia; diagnosis; biomarkers; regulatory authorities
A descoberta e a aplicação clínica de biomarcadores para desordens mentais são confrontadas com muitos desafios. Em geral, os atuais métodos de descoberta e validação de biomarcadores não produziram os resultados que foram inicialmente aguardados depois da finalização do Projeto Genoma Humano. Isso se deve principalmente à falta de processos padronizados conectando a descoberta de marcadores com tecnologias para a validação e a tradução para uma plataforma que ofereça precisão e fácil uso em clínica. Como consequência, a maior parte dos psiquiatras e praticantes em geral são relutantes em aceitar que testes de biomarcadores pode suplementar ou substituir os métodos de diagnóstico utilizados baseados em entrevista. Apesar disso, agências regulatórias concordam agora que melhoras nos correntes métodos são essenciais. Além disso, essas agências estipularam que biomarcadores são importantes para o desenvolvimento de futuras drogas e iniciaram esforços no sentido de modernizar métodos e técnicas para suportar esses esforços. Aqui revisamos os desafios encontrados por essa tentativa do ponto de vista de psiquiatras, praticantes em geral, agências reguladoras e cientistas de biomarcadores. Também descrevemos o desenvolvimento de um novo teste sanguíneo molecular para esquizofrenia como um primeiro passo a esse objetivo.
Desordens psiquiátricas; esquizofrenia; diagnóstico; biomarcadores; autoridades reguladoras
Biomarker blood tests for diagnosis and management of mental disorders: focus on schizophrenia
Sabine BahnI, II; Emanuel SchwarzI; Laura W. HarrisI; Daniel Martins-de-SouzaI, III; Hassan RahmouneI; Paul C. GuestI
IDepartment of Chemical Engineering and Biotechnology, University of Cambridge, Tennis Court Road, Cambridge, UK
IIDepartment of Neuroscience, Erasmus Medical Centre, Rotterdam, The Netherlands
IIIMax Planck Institute of Psychiatry & Ludwig Maximilians University (LMU), Munich, Germany; Laboratory of Neurosciences (LIM-27), Institute of Psychiatry, School of Medicine, University of Sao Paulo (IPq-FMUSP), Sao Paulo, SP, Brazil
Correspondence
ABSTRACT
The discovery and clinical application of biomarkers for mental disorders is faced with many challenges. In general, the current methods for discovery and validation of biomarkers have not produced the results which were first anticipated after completion of the human genome project. This is mostly due to the lack of a standardized pipeline connecting marker discovery with technologies for validation and translation to a platform that offers accuracy and ease of use in a clinical setting. As a consequence, most psychiatrists and general practitioners are still reluctant to accept that biomarker tests can supplement or replace the long standing interview-based methods for diagnosis. Despite this, the regulatory agencies now agree that improvements over the current methods are essential. Furthermore, these agencies stipulate that biomarkers are important for future drug development and have initiated efforts to modernize methods and techniques to support these efforts. Here, we review the challenges faced by this endeavour from the point of view of psychiatrists, general practitioners, the regulatory agencies and biomarker scientists. We also describe the development of a novel molecular blood-test for schizophrenia as a first promising step towards achieving this goal.
Keywords: Psychiatric disorders, schizophrenia, diagnosis, biomarkers, regulatory authorities.
Introduction
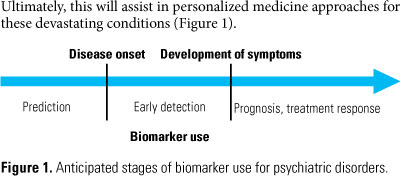
Attempts to identify molecular biomarkers for psychiatric disorders such as schizophrenia have been ongoing for many years. It has been anticipated that such biomarkers could be used as standardized tests to facilitate the diagnosis as well as the treatment and monitoring of patients. At present, these disorders are diagnosed by clinicians and psychiatrists based on subjective interviews of the individuals in question. However, the currently used diagnostic classification systems for psychiatric disorders, such as the Diagnostic and Statistical Manual of Mental Disorders IV (DSM-IV)1 and the International Classification of Disease 10 (ICD-10; http://www.who.int/classifications/icd/en/), are known to have shortcomings. It is now thought that biomarkers which are reflective of the disease pathophysiology or the mechanism of currently used therapeutics will lead to improved diagnosis and potentially pave the way for more effective treatment of patients. This may require deconstruction of existing long-standing procedures aimed at classification of broad patient categories in favour of biomarker-defined disease subtypes. Ultimately, this will assist in personalized medicine approaches for these devastating conditions (Figure 1).
The development of biomarkers and the implications of using these in diagnostics and clinical trials are moving forward, albeit at an unsteady pace. This has led to the need for establishing standard operating procedures to overcome current difficulties and, at the same time, meet the regulatory demands. Regulatory health authorities such as the US Food and Drug Administration (FDA) consider the incorporation of biomarkers into drug discovery projects as an important next phase in the pharmaceutical industry. The FDA has now called for efforts to modernize and standardize methods for the purpose of delivering more effective and safer drugs2,3. This requires that molecules achieve the status of validated biomarkers prior to regulatory decision making for use in clinical trials. To this end, the FDA has set guidelines stating that there are three classes of biomarkers which are: 1) exploratory biomarkers, 2) probable valid biomarkers and 3) known valid biomarkers4. For the exploratory biomarker class, there must be scientific evidence for proof of concept. The probable valid biomarker class requires that the biomarkers in question can be measured in analytical test systems with strict performance characteristics and that there are established scientific findings which explain the relevance and significance of the results. The known valid biomarker class requires that the results can be replicated in different laboratories and in different sites.
In the case of psychiatric disorders, it is anticipated that this will be difficult to achieve. One reason for this is that these conditions are poorly understood, there is an overlap of symptoms across different disorders and there is considerable heterogeneity in how these conditions are manifested in different individuals. However, emerging molecular profiling platforms have facilitated the identification of biomarkers through the simultaneous measurement of hundreds or thousands of molecules. This has served to increase the accuracy of the findings and minimize the amount of sample required and the running costs. We have recently employed a multiplexed immunoassay profiling platform to analyze serum samples from schizophrenia patients which led identification of a panel of molecules that could classify schizophrenia subjects compared to controls with an accuracy of greater than 80%5.
In this review, we will discuss the challenges of developing and implementing a molecular biomarker test for psychiatric disorders. We will address the general problem of introducing the new paradigm of utilizing molecular biomarkers in the field of psychiatry which has, to date, relied on non-molecular approaches. We will also discuss the associated challenges and methods for the identification of biomarkers. Finally, we will elaborate on the potential uses of molecular biomarkers in the field of psychiatric disorders, particularly for improved clinical classification and management of patients and as a means of facilitating drug discovery within the pharmaceutical industry.
Current difficulties in psychiatric diagnosis
Most psychiatrists agree that schizophrenia is an umbrella term for a mixture of aetiologies that present with similar symptoms, in the same way that most of the acute infectious disorders present with fever6. Therefore, misdiagnosis is a common problem in psychiatric practice. For example, a study in the late 1990s found that 31% of bipolar disorder patients were initially diagnosed with schizophrenia7. Another study challenged the fundamental assumptions of the current classifications systems8. This study suggested that there are no methods to validate the current diagnostic concepts which are independent of the concept itself.
A further complication is that clinicians do not usually use even use these classification systems to establish a diagnosis. Instead, most apply a heuristic unstructured interview approach. In this case, the diagnoses may be based on experience and personal views, rather than through matching to guidelines or criteria of a diagnostic system. This can result in systematic errors in judgement based on misconceptions, or it could rely on selective memory. To complicate matters, there have been few or no attempts to address the problem of false positives in diagnoses of mental disorders9. One study which investigated the influence of ethnicity on patient diagnosis found that clinicians tended to over-diagnose schizophrenia in African Americans10. This bias was removed when examiners were provided with ethnicity-blinded transcripts of the patient interviews in question.
Interestingly, there is an apparent increase in schizophrenia prevalence using ICD-10 compared to DSM-IV criteria for diagnosis11. Both of these systems conceptualise that mental disorders are distinct disease entities with common pathologies and can be defined by operational sets of criteria based on signs and symptoms. However, it is unlikely that specific symptoms are linked to defined disease entities. For example, patients with neurological, traumatic, infectious and metabolic disorders can present with symptoms similar to those in schizophrenia12,13. In addition, some individuals have been known to fake symptoms of schizophrenia and other mental disorders14 for various reasons.
A study from 1970 found that time-dependent changes in diagnosis resulted frequently in misjudgement of prognosis and sub-optimal treatment15. Prospective studies of patients who presented initially with first-episode psychosis showed that the initial diagnosis of schizophrenia was mostly stable over a five year follow up period. However, diagnoses of other conditions such as major depressive disorder with psychosis, drug-induced psychosis and schizophreniform psychosis had to be revised more frequently over time16. In particular, the ICD-10 categories listed as "acute and transient psychotic disorders" and "brief psychotic disorder" re-present more difficult diagnostic challenges. In a follow-up study of 503 cases, the diagnosis of 60% of the patients was changed at least once17. Similarly, long-term studies of mood disorders have described substantial changes in diagnosis from major depressive disorder to bipolar disorder and schizophrenia18. A recent study which investigated psychiatric patients at four time points found that only 50% of patients retained their initial diagnosis19. Schizophrenia patients had the most stable diagnosis as 78% retained their initial diagnosis. This was followed by bipolar disorder patients as 69% retained the initial diagnosis, while for major depressive disorder patients only 42% of the diagnoses remained stable. The largest diagnostic shift was from non-schizophrenia to schizophrenia. Of 306 patients with a non-schizophrenia diagnosis at baseline, 32% were eventually diagnosed with this condition.
The importance of early diagnosis
The concordance rate for the development of schizophrenia in identical twins has ranged from 10%-70% in different studies20-22. Such studies have provided indisputable evidence that there is a genetic component and predisposition for schizophrenia. However, these findings also suggest that an individual will not necessarily develop schizophrenia even when such a genetic predisposition exists. In fact, environmental and other non-genetic factors appear to play a more important role in most patients. Factors which could affect brain function include pregnancy and delivery complications, such as intrauterine hypoxia, infections and malnutrition23,24. There are also non-biological factors which could precipitate the onset of psychiatric diseases, including psychosocial stresses such as the experience of a natural disaster, loss of a family member, or the chronic experience of a bad environment or a dysfunctional family life25.
This suggests that disease prevention or minimization might be possible if environmental risk-factors can be determined and avoided. We and other researchers have indicated that the occurrence of metabolic abnormalities such as insulin resistance occurs in 20%-50% of first onset subjects with schizophrenia26-28. In addition, several researchers have found alterations in circulating inflammatory and immune response factors in first onset schizophrenia patients29,30. In a preliminary study, we have shown that various markers relating to these subgroups can be identified in patients even prior to disease onset31. This study analyzed sera obtained from USA military personnel approximately 30 days before the onset of symptoms.
It will be important to determine whether or not disease conversion can be prevented or even minimized in at-risk individuals. There have been extensive reports outlining the importance of early intervention in individuals with a high-risk of developing schizophrenia32-34. A delay in diagnosis can have deleterious effects on patient lives, including substance abuse, social alienation from family and friends, increased accidents, self-harm and harm to o-thers. In addition, misdiagnosis can lead to ineffective treatment or treatment which produces even more harmful effects. For example, misdiagnosis of bipolar disorder as schizophrenia has been found to be associated with increased risk of attempted suicide35, longer hospitalisation7 and serious psychological, legal and financial problems36. In addition, misdiagnosis has a number of socioeconomic consequences including high medical costs, absence from work and negative effects on family and relationships37.
Potential for the use of biomarkers in psychiatry
The FDA, pharmaceutical companies and biotechnology industry now accept that adoption of biomarker-based platforms will be beneficial in the development of better and more efficacious diagnostics and surrogate markers for drug discovery. A biomarker is defined as "measurable characteristics that reflect physiological, pharmacological, or disease processes" (European Medicines Agency; http://www.emea.europa.eu). In the case of bio-monitoring, a biomarker is the presence of any substance or a change in any biological structure or process that can be measured as a result of exposure (Biomonitoringinfo.org/glossary). In the case of psychiatric conditions, there are many anticipated benefits (Table 1).
Currently, only a few biomarker tests have been used in the field of psychiatric disorders. For example, the niacin skin flush response test38,39 has been used sporadically for several years for diagnosis of schizophrenia40. This works due to the fact that some schizophrenia patients exhibit a reduced skin flush in response to topical application of niacin. More recently, genomic biomarkers including variants of hepatic cytochrome p450 enzymes have been used for prediction of toxicities in specific subpopulations in response to antipsychotic treatments41. Also various polymorphisms in serotonergic transporter and receptor genes have been associated with response to selective serotonin reuptake inhibitor-based antidepressants42.
There are only a few molecular tests that predict pharmacodynamic response and these are mainly restricted to the oncology field. A good example of this is the test for Her2 gene expression in breast cancer cells. This is a cell surface receptor which can be blocked by an antibody-based therapeutic called HerceptinTM43 (Table 2).
It is clear that the next phase for psychiatry should include development of a diagnostic system which is based on the underlying pathophysiolology as has been the case for most other areas of medicine. To this end, we recently developed a blood-based biomarker test (VeriPsychTM) based on an algorithm of 51 molecular biomarkers which has now been launched in the USA5,48. This is the first biomarker blood test with a diagnostic application that has entered clinical practice.
One problem that we have faced in the development of this test relates to the fact that the targeted patient populations were selected and classified on the basis of DSM-IV criteria which is sometimes questionable, as discussed above. Therefore, our initial studies involved systematic selection of patients with regards to psychopathology and disease stage with a focus on paranoid schizophrenia patients who were mostly in the first episode of illness and drug naive. Furthermore, subjects with any co-morbidities were excluded and patients were matched to control subjects with similar socio-economical backgrounds and education status. In addition, patients were monitored over several years to confirm disease and symptom stability over time. At the time of sample collection, patients were assessed using the Positive and Negative Syndrome Scale (PANSS), DSM-IV and other rating systems. Control subjects were also assessed using DSM-IV. In the initial testing phase, we analyzed samples from three independent clinics in Germany, since these used identical standard operating procedures for sample acquisition and storage, and we also established the inter-rater reliability across the different sites.
This approach allowed us to establish the expression levels of approximately 200 proteins and small molecules in serum samples from all subjects. These were then used to select molecules which showed the most significant association with the diagnosis of paranoid schizophrenia. We also tested serum samples from patients with conformed bipolar disorder, major depressive disorder and Asperger syndrome which allowed us to establish a schizophrenia-specific signature of 34 molecules48. A further 17 molecules were added which allowed discrimination against bipolar disorder and major depressive disorder for differential diagnostic purposes. After this, we found that the same test worked even when using less well-characterised samples from drug-treated, chronic and more broadly diagnosed schizophrenia patients.
The specificity of the test was achieved using an algorithm comprised of multiple molecules. This algorithm was trained to identify molecules associated with schizophrenia and then we were able to identify stable molecular differences in the patient populations. Through studies of first onset patients we have discovered that most patients with schizophrenia have differences in the levels of insulin-related molecules28, other hormones of the diffuse neuroendocrine system49, inflammatory factors5,50 and molecules associated with endothelial cell function48. It is anticipated that determination of which of these factors are altered in each subject will be useful for patient stratification prior to antipsychotic treatment.
The history of blood-based biomarkers for psychiatric disorders
Blood has been regarded as a source of information on illness and health since ancient times. With the emergence of experimental medical techniques in the mid-1800s, studies of blood from persons with psychiatric illnesses were carried out to identify any physical characteristics that could be used to distinguish the sick from the healthy or if it could be used to confirm an array of discrete natural disease entities that could be sorted into clinical categories. The history of these studies in psychiatry over the past 150 years comprised four main phases (see Noll51 for a fuller account).
The first phase was described as the corpuscular richness paradigm (1854). Microscopic investigations of blood cell morphology in psychiatric patients were conducted in a Scottish insane asylum by W. Lauder Lindsey. Through the use of a low-powered microscope, he examined and counted the numbers of different blood cells in samples from his patients and staff. However, this work came to the conclusion that "insanity and the different types and phases thereof are not characterized by a particular morbid state of the blood." S. Rutherford Macphail (1884)52 reviewed subsequent studies and concluded that there was an overall "deficiency of corpuscular richness of the blood in the first stages of insanity". Similar studies were carried out through to the first two decades of the twentieth century.
The next phase was called the metabolic paradigm (circa 1895). Over this phase, blood was used to detect and measure "inner secretions" from "secreting organs", particularly from ductless glands such as thyroid, adrenals and pituitary. After 1905, these inner secretions became known as hormones from the Greek word ὁρή, meaning "impetus". This emerging new paradigm was immediately seized upon by "modern" psychiatrists who were seeking a new direction for studies of subjects with psychiatric illnesses. It was thought that an over- or under production of inner secretions could psychiatric disorders in the same way that diabetes was caused by the lack of an "internal secretion", as suspected in the 1890s (identified as insulin in 1921). This led to the claims of German psychiatrist Emil Kraepelin (1856-1926) that the severe psychotic disorder he described as "dementia praecox" (in 1896) was the result of an ongoing systemic metabolic disease, affecting the cerebral cortex resulting in a chronic mental deterioration53. In 1908, Swiss psychiatrist Eugen Bleuler (1857-1939) proposed that schizophrenia was an expansion of this disorder, and the term "schizophrenia" became more in use and not Kraepelin's original disease concept. For more than a century changes in pituitary function and related hormonal abnormalities in various psychiatric disorders have been established and remain a strong biological finding today.
The third phase was termed the immunoserodiagnostic paradigm (1906). The development of the Wasserman reaction test for neurosyphillis was the first breakthrough in biological psychiatry. This was the first diagnostic blood test for a discrete form of insanity observed in asylums. In 1909 two German psychiatrists injected cobra venom into patients with dementia praecox and manic-depressive insanity and reported that all of the former, and some of the latter, had reactions to the toxin, and no reaction was observed in healthy people. However, these findings could not be replicated and were eventually refuted. A much more influential test was developed by the prominent Swiss biochemist Emil Abderhalden (1877-1950). Also, a "defensive ferments reaction test", was developed by the German psychiatrist August Fauser (1856-1938) which diagnosed dementia praecox compared to manic-depressive insanity and healthy subjects over a series of studies. From 1912-1920 many scientists believed that a blood test for madness had been found. However, a series of studies were published which did not verify the existence of defensive ferments and the test was cast into doubt. Throughout the 1900s, changes in immune function and inflammation have been linked to various psychiatric disorders through several lines of evidence.
The final stage was referred to as the medical genomics (2005)/post-genomics (2010) paradigm. In this stage, blood tests have been used which target the genome and the proteome. However, attempts to specify most medical diseases including psychiatric disorders at the genome level have proved to be ineffective. This could be related to the lack of certainty as to how strong the genetic and environmental contributions are, and how they interact to precipitate the onset of illness. Despite 20 years of intensive investigations, no single gene or combination of genes have been found that significantly increase the probability of developing schizophrenia. In 2009 results from the largest Genome Wide Association Studies (GWAS) were published as three papers in Nature54-56. None of these studies identified any genetic marker that was significantly associated with schizophrenia. However, three chromosomal regions were implicated. The most significant of these was the short arm of chromosome 6, which is the location of the major histocompati-bility complex (MHC) genes.
The need for biomarker standardization
The field of clinical proteomics has raised high hopes through a number of early reports on potential biomarkers. However, in most cases these could not be validated in subsequent studies or in clinical trials. Potential reasons for the failure to incorporate biomarkers into the clinic include problems in design, the possibility that biomarkers may not be causal but rather a result of the disease process, a lack of congruence in animal models of the disease and the corresponding human condition, or the practice of enrolling patients in trials who are at different stages of the disease process57.
The idea that biomarker research has not lived up to the initial hype has been shown by the number of so-called "breakthrough" biomarkers which have yet to reach the market. Apart from a few biomarkers used in cancer research, most have not been validated. However, most of these are used only for monitoring treatment response and not suitable for early diagnosis apart from the example of prostate-specific antigen58.
The Human Proteome Organization (HUPO) which emerged from the Human Genome Project has developed several initiatives aimed at overcoming the issues of irreproducibility. These initiatives are focussed on plasma, liver, brain, disease glycomics/proteomics, disease biomarkers, mouse disease models, model organisms, kidney/urine, cardiovascular disease, stem cells, the Human Antibody Initiative and the Proteomics Standards Initiative (http://hupo.org/). Each individual program is based in one country and includes subprojects involving international laboratories as partners. This was carried out since most of the irreproducibility problem is thought to result from sources of variability at the level of the sample, sample handling, study design, technical issues and user-related differences. However, a proteomics-based test for ovarian cancer has now been approved by the FDA, demonstrating that success in this area is possible59.
The development and use of biomarkers has triggered the need to establish standard operating procedures to meet the regulatory demands. The regulatory authorities, in particular the FDA, now consider that biomarkers are important for the development of future drugs. In the Critical Path Initiative, the FDA produced a white paper for modernizing methods, tools and techniques to facilitate development of more efficient and safer drugs2,3. The regulatory aspects of biomarkers were first described in a guidance associated with the Pharmacogenomic Data Submission by the FDA (http://www.fda.gov/OHRMS/DOCKETS/98fr/2003d-0497-gdl0002.pdf)60. This outlined that molecules require the status of validated biomar-kers (described above) to be used in regulatory decision-making, including decisions on clinical trials dose regimens and for patient selection. Currently, only well-established tests have been used in regulatory decisions such as the determination of serum creatinine levels to monitor kidney function and carrying out fasting glucose tolerance tests combined with insulin and glucose measurements to establish insulin sensitivity61,62.
An example of test which was developed in accordance with the biomarker qualification process was on rat kidney safety biomar-kers. These biomarkers became part of a cross-validation study and achieved the status of known valid biomarkers as part of the Predictive Safety Testing Consortium (PSTC; 2, 3). The PSTC was founded by the FDA to act as a liaison between the FDA, pharmaceutical companies and academia in biomarker qualification for preclinical and clinical use. The nephrotoxicity subgroup of the PSTC identified seven kidney safety biomarkers for limited use in preclinical and clinical drug development60,63. A Rat KidneyMAPTM has now been developed as a multiplexed immunoassay by Rules Based Medicine in collaboration with the PSTC for early detection of renal damage, which is common in drug development programs (http://www.rulesbasedmedicine.com/products-services/rodentmap-services/rat-kidneymap/)64.
Perhaps the most useful strategy for biomarker qualification is through their co-development with drugs65. This strategy was first described in a draft guidance issued by the FDA [US Department of Health and Human Services, FDA (2005) Drug-Diagnostic Co-Development Concept Paper (http://www.fda.gov/cder/genomics/pharmacoconceptfn.pdf)]. The idea behind this draft is that increased knowledge of the biology surrounding a particular biomarker and a strong association between the biomarker and clinical outcome, will lead to a more efficient development process with fewer risks. Also, early interaction with the relevant regulatory agencies is essential so that studies are designed and biomarker tests carried out correctly.
The development of biomarker assays for psychiatric disorders
The European health authorities have lent support to the development and implementation of biomarkers through agencies such as the Innovative Medicines Initiative66,67. This initiative is a partnership between the European Commission and pharmaceutical companies with the aim of promoting more efficient identification and development of medicines. One of the main objectives is the discovery of translational biomarkers, including those for psychiatric conditions like schizophrenia and autism spectrum disorders. The European Commission has contributed one billion Euros to this programme and this amount has been matched by in kind contributions from member companies of the European Federation of Pharmaceutical Industries and Associations (EFPIA).
Diagnostic assays in the USA are regulated by Clinical Laboratory Improved Amendments (CLIA; http://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/IVDRegulatoryAssistance/ucm124105.ht). These federal regulatory standards govern any tests performed in a clinical laboratory on human samples for the purpose of diagnosis, disease prevention, treatment or assessment of health. Commercially available tests marketed under CLIA are categorized by the FDA into three groups depending on potential risks for health. This categorization considers the necessary knowledge, training, materials and judgement to carry out such tests and other factors including operational, maintenance and quality control procedures. On the other hand, tests can be given a waiver if they are accurate, basic, exclude erroneous interpretation and pose no risk to human health if interpreted incorrectly.
The development of diagnostic assays for all diseases inclu-ding psychiatric disorders requires the repeated demonstration of specific performance characteristics including accuracy, precision, sensitivity and specificity. These factors are now considered absolute requirements since variability in biomarker measurements can be affected by biological, environmental, sample collection and analytical factors. Development of multiplexed immunoassays for example requires validation of the assay structure and analytical performance to maximize precision and accuracy. In this case, the associated challenges include selection and immobilization of capture ligands, calibration, reagent-antibody compatibility, dynamic range and limits of detection68.
It is now accepted that single biomarkers are unlikely to be effective given the complexity of most diseases, particularly psychiatric disorders69. Also, most psychiatric conditions appear to be the result of a complex interaction between environmental and genetic factors70. Therefore, a panel of biomarkers must be employed to reflect the complexity and increase specificity of the measurements. Such biomarker panels must consist of rigorously-validated molecules in multiple centres and across different time points in order to provide a reproducible and accurate test.
Biomarker panels must also be disease-specific, at least relative to other diseases which have similar symptoms. Again, this is particularly difficult for psychiatric disorders as these have many areas of overlap of subjectively-assessed behavioural symptoms. Examples for this are the overlap of negative symptoms between schizophrenia and major depressive disorder71, the similarity in psychotic symptoms between manic bipolar disorder and schizophrenia72, and the shared cognitive deficits across all of these conditions73. In addition, conditions such as bipolar disorder can consist of multiple stages through the cycling of mania and depression72. Identification of valid biomarkers which could predict the switch between stages would be invaluable.
Finally, biomarker tests must be in a format that is high throughput, accurate and user friendly to allow use by clinicians, hospital staff and scientists. Mass spectrometry and two-dimensional gel electrophoresis techniques would be too cumbersome and require too much expertise to be considered as realistic possibilities. Instead, automated platforms such as the multiplexed immunoassay system and multiple reaction monitoring74 are more likely candidates as clinically-friendly platforms which have already shown some pro-mise. Also holographic sensors have already been employed for detection of biological materials75 and molecules76-78 and could therefore be adapted as a robust and comprehensive readout of a biomarker signature in clinical applications.
Development of a molecular blood test for schizophrenia
Over the last decade, most of the proteome based-biomarkers studies linked to schizophrenia have been carried out targeting only a few or single molecules. However, over the last two to three years a more comprehensive approach has been applied using liquid chromatography mass spectrometry and multiplex immunoassay profiling platforms which target tens to hundreds of proteins simultaneously. We have recently developed the first multiplex immunoassay biomarker test (VeripsychTM) for psychiatric disorders in collaboration with Rules Based Medicine and Psynova Neurotech5,48. This 51-plex assay system was launched in 2010 as a CLIA-approved test to aid diagnosis of schizophrenia. This assay employs a proprietary algorithm to achieve a sensitivity of 83% and specificity of 83%. It is anticipated that this biomarker test may be employed at multiple stages of the schizophrenia disease process to improve patient lives (Figure 2).
Decision-modelling analyses were carried out to construct a socio-economic case for a biomarker-based test such as VeriPsych for early diagnosis of schizophrenia and to determine the prospective market79. This showed that the cost of each patient in the United Kingdom diagnosed after the first psychotic episode, would be approximately £ 182,000 over a five year period. However, the cost for a patient diagnosed early would be only around £ 27,000 indicating that this could potentially save 6.7-fold in costs. This suggests that there is a good socio-economic case for introducing better diagnostic tools for detection of schizophrenia during the prodromal phase.
Psychiatrists and healthcare professionals have, so far, met this test with mixed reactions. Most agreed that a sensitive and specific blood test for psychiatric disorders would be a welcome and major advance in the field although many are resistant to actually using such the test in clinical practice. A specific and justified criticism of the current blood test is that it was developed to distinguish schizophrenia patients from healthy controls and not as a differential diagnostic of schizophrenia from other psychiatric disorders. However, the next version of the test will attempt to address this short coming by including a differential diagnostic capability for schizophrenia compared to major depressive disorder and bipolar disorder. Our market research showed that most psychiatrists believe they are good at diagnosing schizophrenia patients using a basic clinical interview. However, they routinely felt that colleagues were less expert at achieving the correct diagnosis. In addition to a diagnostic aid for psychiatrists, a further application of the test may be to improve insight into and acceptance of the illness by the patients themselves, as described in a recent anecdotal report80. Currently lack of objective evidence leads many patients to have poor confidence in their diagnosis. This application may broaden the clinical utility of the test.
Conclusions
This chapter has described the challenges and recent successes associated with the discovery and development of blood-based biomarkers for psychiatric disorders. The current diagnostic process and strategies for developing novel pharmaceutical compounds are in need of a paradigm change. Despite this, there is a reluctance to accept the idea that biomarkers will be of any help at all in this endeavour81. It is true that only a handful of the thousands of promising biomarkers identified have lived up to the initial hype. However, the regulatory health authorities now consider the identification, validation and implementation of biomarkers to be of critical importance for the future drug discovery. As a result, they have now called for efforts to modernize methods, tools and techniques to achieve this goal. Given the complex nature and low abundance of many proteomic biomarkers, this will most likely require the development of more reproducible and sensitive methods and a massive integration of technologies. However, there is now reason for optimism that further technological advancements and interdisciplinary approaches will overcome these current limitations in the field of biomarkers to help usher the study of psychiatric disorders fully into the 21st century.
Acknowledgments
This research was supported by the Stanley Medical Research Institute (SMRI), the European Union FP7 SchizDX research programme (grant reference 223427) and the NEWMEDS Innovative Medicines Initiative. We also thank Enrique Millan for research gathered through his MBA individual project entitled The Value of the Schizophrenia Diagnostic Market, carried out with the Judge Business School at Cambridge University and Psynova Neurotech in Cambridge UK.
References
- 1. Schaffer D. A participant's observations: preparing DSM-IV. Can J Psychiatry. 1996;41:325-9.
- 2. Ovens J. Funding for accelerating drug development initiative critical. Nat Rev Drug Discov. 2006;5:271.
- 3. Marson B. "Critical Path" is on the road forward; FDA reports industry activity is high. The Pink Sheet. 2007;69:29.
- 4. Goodsaid F, Frueh FW. Implementing the U.S. FDA guidance on pharmacogenomic data submissions. Environ Mol Mutagen. 2007;48:354-8.
- 5. Schwarz E, Izmailov R, Spain M, Barnes A, Mapes JP, Guest PC, et al. Validation of a blood-based laboratory test to aid in the confirmation of a diagnosis of schizophrenia. Biomark Insights. 2010;5:39-47.
- 6. Tsuang MT. Heterogeneity of schizophrenia. Biol Psychiatry. 1975;10:465-74.
- 7. Gonzalez-Pinto A, Gutierrez M, Mosquera F, Ballesteros J, Lopez P, Ezcurra J, et al. First episode in bipolar disorder: misdiagnosis and psychotic symptoms. J Affect Disord. 1998;50:41-4.
- 8. Follette WC, Houts AC. Models of scientific progress and the role of theory in taxonomy development: a case study of the DSM. J Consult Clin Psychol. 1996;64:1120-32.
- 9. Wakefield JC. Misdiagnosing normality: psychiatry's failure to address the problem of false positive diagnoses of mental disorder in a changing professional environment. J Ment Health. 2010;19:337-51.
- 10. Strakowski SM, Keck PE Jr, Arnold LM, Collins J, Wilson RM, Fleck DE, et al. Ethnicity and diagnosis in patients with affective disorders. J Clin Psychiatry. 2003;64:747-54.
- 11. Cheniaux E, Landeira-Fernandez J, Versiani M. The diagnoses of schizophrenia, schizoaffective disorder, bipolar disorder and unipolar depression: interrater reliability and congruence between DSM-IV and ICD-10. Psychopathology. 2009;42:293-8.
- 12. Yolken RH, Dickerson FB, Fuller Torrey E. Toxoplasma and schizophrenia. Parasite Immunol. 2009;31:706-15.
- 13. Lovatt A, Mason O, Brett C, Peters E. Psychotic-like experiences, appraisals, and trauma. J Nerv Ment Dis. 2010;198:813-9.
- 14. Bagby RM, Rogers R, Buis T, Nicholson RA, Cameron SL, Rector NA, et al. Detecting feigned depression and schizophrenia on the MMPI-2. J Pers Assess. 1997;68:650-64.
- 15. Robins E, Guze SB. Establishment of diagnostic validity in psychiatric illness: its application to schizophrenia. Am J Psychiatry. 1970;126:983-7.
- 16. Schwartz JE, Fennig S, Tanenberg-Karant M, Carlson G, Craig T, Galambos N, et al. Congruence of diagnoses 2 years after a first-admission diagnosis of psychosis. Arch Gen Psychiatry. 2000;57:593-600.
- 17. Castagnini A, Bertelsen A, Berrios GE. Incidence and diagnostic stability of ICD-10 acute and transient psychotic disorders. Compr Psychiatry. 2008;49(3):255-61.
- 18. Clayton PJ, Guze SB, Cloninger CR, Martin RL. Unipolar depression: diag-nostic inconsistency and its implications. J Affect Disord. 1992;26:111-6.
- 19. Bromet EJ, Kotov R, Fochtmann LJ, Carlson GA, Tanenberg-Karant M, Ruggero C, et al. Diagnostic shifts during the decade following first admission for psychosis. Am J Psychiatry. 2011;168(11):1186-94.
- 20. Torrey EF. Are we overestimating the genetic contribution to schizophrenia? Schizophr Bull. 1992;18:159-70.
- 21. McGue M. When assessing twin concordance, use the probandwise not the pairwise rate. Schizophr Bull. 1992;18:171-6.
- 22. Tsuang M. Schizophrenia: genes and environment. Biol Psychiatry. 2000;47:210-20.
- 23. Dauncey MJ, Bicknell RJ. Nutrition and neurodevelopment: mechanisms of developmental dysfunction and disease in later life. Nutr Res Rev. 1999;12:231-53.
- 24. Schlotz W, Phillips DI. Fetal origins of mental health: evidence and mechanisms. Brain Behav Immun. 2009;23:905-16.
- 25. Koenig JI, Kirkpatrick B, Lee P. Glucocorticoid hormones and early brain development in schizophrenia. Neuropsychopharmacology. 2002;27:309-18.
- 26. Ryan MC, Collins P, Thakore JH. Impaired fasting glucose tolerance in first-episode, drug-naive patients with schizophrenia. Am J Psychiatry. 2003;160:284-9.
- 27. Spelman LM, Walsh PI, Sharifi N, Collins P, Thakore JH. Impaired glucose tolerance in first-episode drug-naive patients with schizophrenia. Diabet Med. 2007;24:481-45.
- 28. Guest PC, Wang L, Harris LW, Burling K, Levin Y, Ernst A, et al. Increased levels of circulating insulin-related peptides in first-onset, antipsychotic naive schizophrenia patients. Mol Psychiatry. 2010;15:118-9.
- 29. Szulc A, Galińska B, Konarzewska B, Gudel-Trochimowicz I, Popławska R. Immunological marker activity in first episode schizophrenic patients. Pol Merkur Lekarski. 2001;10:450-2.
- 30. Van Venrooij JA, Fluitman SB, Lijmer JG, Kavelaars A, Heijnen CJ, Westenberg HG, et al. Impaired neuroendocrine and immune response to acute stress in medication-naive patients with a first episode of psychosis. Schizophr Bull. 2012;38(2):272-9.
- 31. Schwarz E, Guest PC, Rahmoune H, Martins-de-Souza D, Niebuhr DW, Weber NS, et al. Identification of a blood-based biological signature in subjects with psychiatric disorders prior to clinical manifestation. World J Biol Psychiatry. 2011. (in press)
- 32. Agius M, Shah S, Ramkisson R, Murphy S, Zaman R. Three year outcomes of an early intervention for psychosis service as compared with treatment as usual for first psychotic episodes in a standard community mental health team. Preliminary results. Psychiatr Danub. 2007;19:10-9.
- 33. Salokangas RK, McGlashan TH. Early detection and intervention of psychosis. A review. Nord J Psychiatry. 2008;62:92-105.
- 34. Yap HL. Early psychosis intervention. Singapore Med J. 2010;51;689-93.
- 35. Thomas P. The many forms of bipolar disorder: a modern look at an old illness. J Affect Disord. 2004;79(Suppl 1):S3-8.
- 36. Hirschfeld RM. Bipolar spectrum disorder: improving its recognition and diagnosis. J Clin Psychiatry. 2001;62(Suppl 14):5-9.
- 37. Post RM. The impact of bipolar depression. J Clin Psychiatry. 2005;66(Suppl 5):5-10.
- 38. Kashshai D, Mate B. The effect of nicotinic acid on the temperature of the skin of patients with schizophrenia in different states. Zh Nevropatol Psikhiatr Im S S Korsakova. 1961;61:1688-98.
- 39. Vaddadi KS. Niacin flushing and schizophrenia. Med Hypotheses. 1981;7:599-600.
- 40. Wilson DW, Douglass AB. Niacin skin flush is not diagnostic of schizophrenia. Biol Psychiatry. 1986;21:974-7.
- 41. Fleeman N, Dundar Y, Dickson R, Jorgensen A, Pushpakom S, McLeod C, et al. Cytochrome P450 testing for prescribing antipsychotics in adults with schizophrenia: systematic review and meta-analyses. Pharmacogenomics J. 2010;11:1-14.
- 42. Kato M, Serretti A. Review and meta-analysis of antidepressant pharmacogenetic findings in major depressive disorder. Mol Psychiatry. 2010;15:473-500.
- 43. Desmedt C, Sperinde J, Piette F, Huang W, Jin X, Tan Y, et al. Quantitation of HER2 expression or HER2:HER2 dimers and differential survival in a cohort of metastatic breast cancer patients carefully selected for trastuzumab treatment primarily by FISH. Diagn Mol Pathol. 2009;18:22-9.
- 44. Spadoni LR, Mclean RB, Herrmann WL. A rapid immunological test for the detection of early pregnancy. West J Surg Obstet Gynecol. 1964;72:92-7.
- 45. Ball RH, Caughey AB, Malone FD, Nyberg DA, Comstock CH, Saade GR, et al.; First and Second Trimester Evaluation of Risk (FASTER) Research Consortium. First- and second-trimester evaluation of risk for Down syndrome. Obstet Gynecol. 2007;110(1):10-7.
- 46. Cronin M, Sangli C, Liu ML, Pho M, Dutta D, Nguyen A, et al. Analytical validation of the Oncotype DX genomic diagnostic test for recurrence prognosis and therapeutic response prediction in node-negative, estrogen receptor-positive breast cancer. Clin Chem. 2007;53(6):1084-91.
- 47. Howey JE, Bennet WM, Browning MC, Jung RT, Fraser CG. Clinical utili-ty of assays of glycosylated haemoglobin and serum fructosamine compared: use of data on biological variation. Diabet Med. 1989;6(9):793-6.
- 48. Schwarz E, Guest PC, Rahmoune H, Harris LW, Wang L, Leweke FM, et al. Identification of a biological signature for schizophrenia in serum. Mol Psychiatry. 2012;17(5):494-502.
- 49. Guest PC, Schwarz E, Krishnamurthy D, Harris LW, Leweke FM, Rothermundt M, et al. Altered levels of circulating insulin and other neuroendocrine hormones associated with the onset of schizophrenia. Psychoneuroendocrinology. 2011;36(7):1092-6.
- 50. Steiner J, Jacobs R, Panteli B, Brauner M, Schiltz K, Bahn S, et al. Acute schizophrenia is accompanied by reduced T cell and increased B cell immunity. Eur Arch Psychiatry Clin Neurosci. 2010;260;509-18.
- 51. Noll R. The blood of the insane. Hist Psychiatry. 2006;17:395-418.
- 52. Macphail SR. Clinical observations on the blood of the insane. Br J Psychiatry. 1884;30:378-89.
- 53. Noll R. American madness: the rise and fall of dementia praecox. Cambridge: Harvard University Press; 2011.
- 54. Purcell SM, Wray NR, Stone JL, Visscher PM, O'Donovan MC, Sullivan PF, et al.; International Schizophrenia Consortium (2009) Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature. 2009;460:748-52.
- 55. Shi J, Levinson DF, Duan J, Sanders AR, Zheng Y, Pe'er I, et al. Common variants on chromosome 6p22.1 are associated with schizophrenia. Nature. 2009;460:753-7.
- 56. Stefansson H, Ophoff RA, Steinberg S, Andreassen OA, Cichon S, Rujescu D, et al. Common variants conferring risk of schizophrenia. Nature. 2009;460:744-7.
- 57. Flood DG, Marek GJ, Williams M. Developing predictive CSF biomar-kers-A challenge critical to success in Alzheimer's disease and neuropsychiatric translational medicine. Biochem Pharmacol. 2011;81(12):1422-34.
- 58. Gjertson CK, Albertsen PC. Use and assessment of PSA in prostate cancer. Med Clin North Am. 2011;95:191-200.
- 59. Zhang Z, Chan DW. The road from discovery to clinical diagnostics: lessons learned from the first FDA-cleared in vitro diagnostic multivariate index assay of proteomic biomarkers. Cancer Epidemiol Biomarkers Prev. 2010;19:2995-9.
- 60. Dieterle F, Sistare F, Goodsaid F, Papaluca M, Ozer JS, Webb CP, et al. Renal biomarker qualification submission: a dialog between the FDA-EMEA and Predictive Safety Testing Consortium. Nat Biotechnol. 2010;28:455-62.
- 61. Truglia JA, Livingston JN, Lockwood DH. Insulin resistance: receptor and post-binding defects in human obesity and non-insulin-dependent diabetes mellitus. Am J Med. 1985;79:13-22.
- 62. Khristov V. Glucose clamping: a modern method for research on insulin secretion and resistance. Vutr Boles. 1986;25:32-9.
- 63. Ozer JS, Dieterle F, Troth S, Perentes E, Cordier A, Verdes P, et al. A panel of urinary biomarkers to monitor reversibility of renal injury and a serum marker with improved potential to assess renal function. Nat Biotechnol. 2010;28:486-94.
- 64. Swain A, Turton J, Scudamore CL, Pereira I, Viswanathan N, Smyth R, et al. Urinary biomarkers in hexachloro-1:3-butadiene-induced acute kidney injury in the female Hanover Wistar rat; correlation of α-glutathione S-transferase, albumin and kidney injury molecule-1 with histopathology and gene expression. J Appl Toxicol. 2011;31(4):366-77.
- 65. Goodsaid F, Frueh F. Process map proposal for the validation of genomic biomarkers. Pharmacogenomics. 2006;7:773-82.
- 66. Kamel N, Compton C, Middelveld R, Higenbottam T, Dahlén SE. The Innovative Medicines Initiative (IMI): a new opportunity for scientific collaboration between academia and industry at the European level. Eur Respir J. 2008;31:924-6.
- 67. Hunter AJ. The Innovative Medicines Initiative: a pre-competitive initiative to enhance the biomedical science base of Europe to expedite the development of new medicines for patients. Drug Discov Today. 2008;13:371-3.
- 68. Ellington AA, Kullo IJ, Bailey KR, Klee GG. Antibody-based protein multiplex platforms: technical and operational challenges. Clin Chem. 2010;56(2):186-93.
- 69. Boja ES, Jortani SA, Ritchie J, Hoofnagle AN, Tezak Z, Mansfield E, et al. The journey to regulation of protein-based multiplex quantitative assays. Clin Chem. 2011;57:560-7.
- 70. Dick DM. Gene-environment interaction in psychological traits and disorders. Annu Rev Clin Psychol. 2011;7:383-409.
- 71. Fleischhacker W. Negative symptoms in patients with schizophrenia with special reference to the primary versus secondary distinction. Encephale. 2000;26 Spec No 1:12-4.
- 72. Dunayevich E, Keck PE Jr. Prevalence and description of psychotic features in bipolar mania. Curr Psychiatry Rep. 2000;2:286-90.
- 73. Ferrier IN, Stanton BR, Kelly TP, Scott J. Neuropsychological function in euthymic patients with bipolar disorder. Br J Psychiatry. 1999;175:246-51.
- 74. Gallien S, Duriez E, Domon B. Selected reaction monitoring applied to proteomics. J Mass Spectrom. 2011;46:298-312.
- 75. Bhatta D, Christie G, Madrigal-González B, Blyth J, Lowe CR. Holographic sensors for the detection of bacterial spores. Biosens Bioelectron. 2007;23:520-7.
- 76. Kabilan S, Marshall AJ, Sartain FK, Lee MC, Hussain A, Yang X, et al. Holographic glucose sensors. Biosens Bioelectron. 2005;20:1602-10.
- 77. Sartain FK, Yang X, Lowe CR. Holographic lactate sensor. Anal Chem. 2006;78:5664-70.
- 78. Tan EV, Lowe CR. Holographic enzyme inhibition assays for drug discovery. Anal Chem. 2009;81:7579-89.
- 79. Millan E. The value of the schizophrenia diagnostic market. Masters of Business Administration Individual Project (Judge Business School, Cambridge University). MBA thesis; 2007.
- 80. Kelly D, Thedford S, Vyas G. A new blood-based diagnostic aid for schizophrenia. Psychiatr Serv. 2011;62:1107.
- 81. Poste G. Bring on the biomarkers. Nature. 2011;469:156-7.
Endereço para correspondência:
Publication Dates
-
Publication in this collection
11 Dec 2012 -
Date of issue
2013
History
-
Received
23 Sept 2012 -
Accepted
07 Nov 2012