Abstract
The giant conifer aphids Cinara pinivora (Wilson, 1919) and Cinara atlantica (Wilson, 1919) (Hemiptera: Aphididae) are pests on Pinus spp. (Pinaceae) in the South and Southeast regions of Brazil. Larvae of Chrysoperla externa (Hagen, 1861) (Neuroptera, Chrysopidae) were observed feeding voraciously on these aphid colonies. In order to evaluate their potential as biological control agents, some biological parameters and their consumption capacity were studied in laboratory. Ten larvae were isolated in plastic vials and fed with aphids of small size (nymphs of 1st and 2nd instars) and 10 with aphids of medium size (nymphs of 3rd and 4th instars), maintained at 15ºC, 20ºC and 25ºC, under 12:12 h photoperiod and 70 ± 10% RH, and observed daily. The egg incubation period was nine days at 20ºC and four days at 25ºC. The mean larval development period for C. externa was 59.5 days; 22.3 days and 10.9 days, respectively at 15ºC, 20ºC and 25ºC. The pupal stage last 23.2 at 20ºC and 11.1 days at 25ºC. Unfortunately, data of egg and pupal development at 15ºC are not available because the rearing chamber overheated. The mortality rate from egg to adult was 46.2% 46.6% and 20.2% at 15ºC, 20ºC and 25ºC, respectively. The average aphid consumption of each C. externa larva to complete its development was 499.1; 341.7 and 215.1 small aphids, and 126.4; 105.6 and 67.0 medium aphids, at 15ºC, 20ºC and 25ºC, respectively. About 80% of the total food consumption was by the 3rd instar larvae. Although the development was faster and viability higher at 25ºC than at the other two temperatures, the consumption was the highest at 15ºC because the larval period was much longer. Therefore, the larvae of C. externa can be regarded as potential biological control agents of Cinara spp. throughout the year and even in cool areas of Southern Brazil during some periods o the year.
Biological control; giant conifer aphids; green lacewing; prey-predator relationship
Development and consumption capacity of Chrysoperla externa (Hagen) (Neuroptera, Chrysopidae) fed with Cinara spp. (Hemiptera, Aphididae) under three temperatures1 1 Contribution number 1356 of the Departamento de Zoologia, Universidade Federal do Paraná.
Josiane T. Cardoso; Sonia M. N. Lazzari
Departamento de Zoologia, Universidade Federal do Paraná. Caixa Postal 19020, 81531-980 Curitiba, Paraná, Brasil. E-mail: lazzari@ufpr.br; josiane@bio.ufpr.br
ABSTRACT
The giant conifer aphids Cinara pinivora (Wilson, 1919) and Cinara atlantica (Wilson, 1919) (Hemiptera: Aphididae) are pests on Pinus spp. (Pinaceae) in the South and Southeast regions of Brazil. Larvae of Chrysoperla externa (Hagen, 1861) (Neuroptera, Chrysopidae) were observed feeding voraciously on these aphid colonies. In order to evaluate their potential as biological control agents, some biological parameters and their consumption capacity were studied in laboratory. Ten larvae were isolated in plastic vials and fed with aphids of small size (nymphs of 1st and 2nd instars) and 10 with aphids of medium size (nymphs of 3rd and 4th instars), maintained at 15ºC, 20ºC and 25ºC, under 12:12 h photoperiod and 70 ± 10% RH, and observed daily. The egg incubation period was nine days at 20ºC and four days at 25ºC. The mean larval development period for C. externa was 59.5 days; 22.3 days and 10.9 days, respectively at 15ºC, 20ºC and 25ºC. The pupal stage last 23.2 at 20ºC and 11.1 days at 25ºC. Unfortunately, data of egg and pupal development at 15ºC are not available because the rearing chamber overheated. The mortality rate from egg to adult was 46.2% 46.6% and 20.2% at 15ºC, 20ºC and 25ºC, respectively. The average aphid consumption of each C. externa larva to complete its development was 499.1; 341.7 and 215.1 small aphids, and 126.4; 105.6 and 67.0 medium aphids, at 15ºC, 20ºC and 25ºC, respectively. About 80% of the total food consumption was by the 3rd instar larvae. Although the development was faster and viability higher at 25ºC than at the other two temperatures, the consumption was the highest at 15ºC because the larval period was much longer. Therefore, the larvae of C. externa can be regarded as potential biological control agents of Cinara spp. throughout the year and even in cool areas of Southern Brazil during some periods o the year.
Key words: Biological control, giant conifer aphids, green lacewing, prey-predator relationship.
The aphids Cinara pinivora (Wilson, 1919) and Cinara atlantica (Wilson, 1919) (Hemiptera, Aphididae) were recorded into reforestation areas of Pinus spp. (Pinaceae) of Brazil in mid 1990's, when the initial outbreaks caused considerable damages and concern in the mill and lumber industry (E.T. IEDE and S.M.N. LAZZARI, pers. comm.). Plant response to large Cinara Curtis, 1835 populations are premature needle shedding, stem twisting, oversprouting, and plant stunting (PENTEADO et al. 2000). A few predators such as coccinellids, syrphids, and chrysopids, have been observed attacking Cinara populations, but no parasitoids have been found so far. In face of the problems resulting from chemical control of forest pests (environment contamination, resistance, and application difficulties), biological control represents an efficient alternative for suppression of Cinara spp. populations.
According to FRAZER (1988), the main factors that affect feeding and efficiency of predators as biological control agents are: voracity (maximum number of preys consumed by the predator), functional response (relationship between number of prey captured and number of prey eaten), numeric response (increasing of predator number with increasing density of the prey); host preference; and predator ability of capturing prey.
The green lacewing Chrysoperla externa (Hagen, 1861) (Neuroptera, Chrysopidae) is commonly found in Brazil predating several homopterous and insect eggs. According to ADAMS & PENNY (1985), its distribution is from the South of the United States to the South of South America.
CANARD & PRINCIPI (1984), FRAZER (1988) VENZON & CARVALHO (1993) and CANARD (1997) state that predator efficiency is affected by diet and temperature. Thus, the evaluation of the efficiency of a predator for biological control programs should include the effect of different temperatures on its performance in the laboratory, before its manipulation in the field. This research had the objective of determining the potential impact of C. externa on Cinara spp. populations by determining the predator's development rate, viability of eggs and larvae under different temperatures, as well as the consumption by the larvae fed with these pine aphids.
MATERIAL AND METHODS
Specimens of C. pinivora and C. atlantica were collected directly from pine trees in the cities of Curitiba and Rio Negro, Paraná, in southern Brazil. The eggs and pupae of C. externa were obtained from the Departamento de Fitossanidade of the Universidade Estadual Paulista, in Jaboticabal, São Paulo. All the experiments of consume and biology were carried out at the Department of Zoology of the Universidade Federal do Paraná, Curitiba, Paraná.
In order to provide suitable size prey for each larval instar of the predator the aphids were separated in two groups by size. The small aphids included 1st and 2nd instar nymphs and the medium aphids corresponded to the 3rd and 4th instar nymphs. Adult aphids were not offered because it is difficult to precise the number of nymphs they produce. One hundred aphids of each size were weighed to compare the aphid mass consumed; a small aphid weighed an average of 0.00043 g, and a medium 0.00087 g/aphid. The C. externa eggs were kept in growth chamber at 25ºC until the eclosion and the newly emerged larvae were separated for the tests.
For each temperature, twenty 1st instar larvae of C. externa were placed individually in transparent plastic pots of 120 mL, with filter paper on the bottom, and covered with plastic film. In ten pots the larvae were fed with aphids of small size and in the other ten, with medium size aphids. The pots were placed in growth chambers under 15ºC, 20ºC and 25ºC, with photoperiod L:D 12:12 h and RH 70 ± 10%. There was not a precise number of aphids offered, but they were counted every 24 hours and replaced, so that there was always plenty of food until the predators completed their larval development. After pupation, the chrysopids cocoons were maintained at 20ºC and 25ºC and observed daily until the adult emergence. The larval mortality of C. externa rate was evaluated under the three temperatures, while pupal mortality and egg viability were estimated only at 20ºC and 25ºC because the experiments at 15ºC were lost due to the overheating of the chambers and there was no material to replace them.
The data were analysed by the Tukey test at 5% probability and regression analysis to assess the influence of temperature on consumption of aphids by C. externa larvae.
RESULTS AND DISCUSSION
Development of Chrysoperla externa
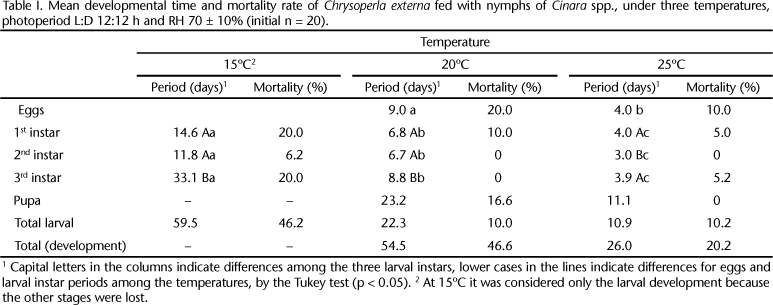
The average time for the egg incubation at 20ºC was nine days, while at 25ºC it was significantly shorter, four days only. Egg viability was 80% at 20ºC and 90% at 25ºC (Tab. I).
Several studies demonstrate a tendency of increasing egg incubation period with decreasing temperatures, as observed here. HONEK & KOCOUREK (1988), studying egg incubation of Chrysoperla carnea (Stephens, 1836) (Neuroptera, Chrysopidae), found 10.9 days at 15ºC and 4.9 days at 24ºC; BUTLER & RITCHIE (1970) obtained a period of 13.1 days at 15ºC and 4.2 days at 25ºC for C. carnea.
Analysing the data on table I, it can be observed statistical difference for the duration of each instar and for the total period of larval development (59.5; 22.3 and 10.9 days), inversely to temperature. It can be observed, also, that the 3rd instar presented a developmental period statistically longer at 15ºC and 20ºC compared to the first two instars. At 25ºC, however, it was not statistically different from the 1st instar, but the 2nd instar was significantly different from the others.
The total larval period found for C. externa at 25ºC (10.9 days) showed a similar pattern observed when this species was fed with the greenbug, Schizaphis graminum (Rondani, 1852) (Hemiptera, Aphididae), it took 10.8 to 12 days to complete its larval development (FONSECA et al. 2000). It was also observed by VENZON & CARVALHO (1993) for the green lacewing Ceraeochrysa cubana (Hagen, 1861) (Neuroptera, Chrysopidae) that the larval development time decreases with temperature elevation. BUTLER & RITCHIE (1970) also observed the same tendency, studying the development of C. carnea in constant and variable temperatures. According to LIAO et al. (1985) the duration of the third instar of the Chrysopa quadripunctata (Burmeinster, 1839), Chrysoperla rufilabris (Burmeinster, 1839), Micromus posticus (Walker, 1853) (Neuroptera, Chrysopidae), was also longer than the others, when reared at 20ºC.
The tendency of shorter biological cycle of C. externa inversely to temperature registered in this research was also observed for C. carnea for which the period from egg to adult lasted 55.4 to 31.2 days, respectively, at 15ºC and 25ºC (BUTLER & RITCHIE 1970). For C. cubana, the cycle was 43.9; 28.5 and 19.2 days, at 20ºC, 25ºC and 30ºC, respectively (VENZON & CARVALHO 1993).
The pupal stage last 23.2 days at 20ºC and only 11.1 days at 25ºC. At 15ºC, the periods of egg and pupal development were not evaluated. These periods were a little shorter than those presented by VENZON & CARVALHO (1993) who found 20.1 to 22.5 days at 20ºC, and from 12.7 to 14.3 days at 25ºC for C. cubana larvae fed on six different diets, including Anagasta kuehniella (Zeller, 1879) (Lepidoptera: Pyralidae) eggs, the aphid Toxoptera spp. Koch, 1856 (Hemiptera, Aphididae) and artificial diet.
The total mortality rate of the larvae was 46,2% when these were reared at 15ºC, particularly in the 1st and 3rd instars (Tab. I). Under the other two temperatures, larval mortality did not surpass 10,2%, and was concentrated in the 1st instar at 20ºC and in the 1st and 3rd at 25ºC. The pupal mortality rate was 16.6% at 20ºC; the individuals died as pharate adults, but it was not possible to establish the influence of temperature on this matter.
Consumption of nymphs of Cinara by Chrysoperla externa larvae
The total consumption of small aphids by the larvae of C. externa decreased with the increase of temperature: 499.1; 341.7 and 215.1 aphids/larva, respectively for the temperatures of 15ºC, 20ºC and 25ºC. For the medium aphids, the consumption was 126.4; 105.6 and 67.0 aphids/larva at 15ºC, 20ºC and 25ºC, respectively (Tab. II). It can be observed that the 2nd instar larvae consumed more medium aphids at 20ºC than at the other temperatures; but it did not affect the final consumption. The consumption at this temperature was significantly different from the 25ºC, but not from 15ºC.
Considering that the body mass of an individual medium aphid is approximately the double of the mass of a small one, it is observed that C. externa larvae consumed more small than medium aphids, also due to the easiness for manipulating smaller preys. The total consumption at 15ºC was significantly higher than at 20ºC and 25ºC in function of longer development period at this temperature, as showed on table I.
The consumption, as it can be observed on table II, increased significantly from the first to the last instar under the three temperatures, as expected. The table also shows that no temperature stood out significantly, but the amount of consumed aphids overlapped in some instars, mainly for the medium aphids.
CANARD & PRINCIPI (1984) mention that chrysopids in general, consume 80% of the total food intake during the third instar, increasing the consumption with the decrease of temperature. That tendency was also confirmed by FONSECA et al. (2000) for this same species fed with the greenbug S. graminum and by LIAO et al. (1985) for the green lacewings C. quadrinpunctata and C. rufilabris fed with the aphid Monellia caryella (Fitch, 1855) (Hemiptera: Aphididae).
The regression curves (Fig. 1) explain the aphid consumption by the larvae to complete their development at the three temperatures. The partial regression analysis showed that the variation presented in consumption by the 3rd larval instar fed with small and medium size aphids and the 2nd instar fed with small aphids can be explained by the influence of temperature on 80% of the cases. The determination values and the equations obtained were: R2 = 0,5575 (y = 0,1041x2 4,9447x + 75,383) for the 1st instar larvae; R2 = 0,9264 (y = 0,4386x2 21,582x + 296,42) for the 2nd and R2 = 0,8991 (y = 0,0647x2 26,201x + 781,58) for the 3rd, all fed with small size aphids. For the medium size aphids, the values were: R2 = 0,2916 (y = 0,329x2 1,5956x + 24,533); R2 = 0,3209 (y = 0,2003x2 + 7,9868x 62,152), and R2 = 0,7809 (y = 0,1951x2 +2,1675 x + 117,24), respectively for the 1st, 2nd and 3rd larval instars of C. externa.
In conclusion, the development of C. externa was better and the viability higher at 25ºC. However, C. externa consumed significantly more aphids at 15ºC because the development period was longer at lower temperatures. Thus, C. externa can be considered as a potential functional predator on Cinara populations throughout the year in Southern and South-eastern Brazil where these aphid species represent serious pests.
ACKNOWLEDGEMENTS
This study was partially supported by a grant to the authors from CNPq-Brazil. We are grateful to Dr. David Voegtlin, Illinois Natural History Survey, Champaign, Illinois, USA for the valuable suggestions and the manuscript review and to Dr. Sérgio de Freitas, Universidade Estadual Paulista, São Paulo, Brazil for providing the chrysopid eggs and pupae.
Received in 25.III.2003; acepted in 03.X.2003.
- ADAMS, P.A. & N.D. PENNY. 1985. Neuroptera of the Amazon Basin Introduction and Chrysopini. Acta Amazonica, Manaus, 15: 413-479.
- BUTLER, G.D. & P.L. RITCHIE. 1970. Development of Chrysopa carnea at constant and fluctuating temperatures. Journal of Economic Entomology, Maryland, 63: 1028-1030.
- CANARD, M. 1997. Can lacewings feed on pests in winter? (Neuroptera: Chrysopidae and Hemerobiidae). Entomophaga, Paris, 42: 113-117.
- CANARD, M. & M.M. PRINCIPI. 1984. Development of Chrysopidae, p. 5775. In: M. CANARD; Y. SÉMÉRIA & T.R. NEW (Eds). Biology of Chrysopidae. The Hague, W. Junk Publishers, 294p.
- FONSECA, A.R.; C.F. CARVALHO & B. SOUZA. 2000. Resposta funcional de Chrysoperla externa (Hagen) (Neuroptera: Chrysopidae) alimentada com Schizaphis graminum (Rondani) (Hemiptera: Aphididae). Anais da Sociedade Entomológica do Brasil, Londrina, 29: 309-317.
- FRAZER, B.D. 1988. Predators, p. 217-230. In: A.K. MINKS & P. HARREWIJN (Eds). Aphids, their biology, natural enemies and control. Amsterdam, Elsevier, vol. 2B, 364p.
- HONEK, A. & F. KOCOUREK. 1988. Thermal requirements for development of aphidophagous Coccinellidae (Coleoptera), Chrysopidae, Hemerobiidae (Neuroptera), and Syrphidae (Diptera): some general trends. Oecologia, Berlim, 76: 455-460.
- LIAO, H.T.; M.K. HARRIS & F. MANSOUR. 1985. Impact of natural enemies on the blackmargined pecan aphid, Monellia caryella (Homoptera: Aphididae). Environmental Entomology, College Park, 14: 122-126.
- PENTEADO S.R.C.; R.F. TRETINI; E.T. IEDE & W. REIS FILHO. 2000. Pulgão do Pinus: nova praga florestal. Série Técnica Instituto de Pesquisas e Estudos Florestais, Piracicaba, 13: 97-102.
- VENZON, M. & C.F. CARVALHO. 1993. Desenvolvimento larval, pré-pupal e pupal de Ceraeochrysa cubana (Hagen) (Neuroptera: Chrysopidae) em diferentes dietas e temperaturas. Anais da Sociedade Entomológica do Brasil, Londrina, 22: 477-483.
Publication Dates
-
Publication in this collection
07 Apr 2004 -
Date of issue
Dec 2003
History
-
Accepted
03 Oct 2003 -
Received
25 Mar 2003