Abstracts
The aim of this work was to study the physical, chemical, physiological and microbiological changes during the flow chart of fresh-cut strawberry. Strawberry cvs. Camarosa, Dover and Tudla, derived from experimental area of the Universidade Federal de Viçosa, were selected by color (red ¾) and absence of wound. Afterwards, the minimal processing was evaluated, as follows: fast cooling, water and ice, removal of the calyx followed by conservation at 5±0.5°C and 90-95% RH for 13 days, sanitation, drainage, cut in halves. Removal of the calyx did not result significant difference for fresh weight, total soluble solids, total titratable acidity, pH and ratio TSS/TTA. The rapid cooling resulted in lower electrolyte leakage and respiratory rate, especially sanitized fruits. Sanitization by immersion in chlorine solution slowed the growth of fungi and yeast. Drainage for 20 minutes eliminated practically all water on the surface of the fruits. The fresh-cutting did not affect the visual and nutritional quality of strawberries like appearance, microbiot, vitamin C, anthocyanins and phenolic compounds, consisting of alternative market potential economically viable.
Fragaria x ananassa Duch; sanitation; drainage; anthocyanins
O objetivo deste trabalho foi estudar mudanças físicas, químicas, fisiológicas e microbiológicas durante o fluxograma de processamento mínimo de morango. Morangos das cultivares Camarosa, Dover e Tudla oriundos de área experimental da Universidade Federal de Viçosa, foram selecionados por cor (¾ vermelho) e ausência de danos. Posteriormente, foi avaliado o fluxograma de processamento mínimo: resfriamento rápido, água e gelo; remoção do cálice acompanhado de conservação a 5±0,5ºC e 90-95% UR, por 13 dias; sanitização; drenagem; corte, em metades. A remoção do cálice não resultou diferença significativa para massa fresca, sólidos solúveis totais, acidez total titulável, pH e razão SST/ATT. O resfriamento rápido ocasionou menores extravasamentos de eletrólitos e taxa respiratória, sobretudo nos frutos sanitizados. A sanitização por imersão em solução de cloro retardou o crescimento de fungos e levedura. A drenagem por 20 minutos eliminou praticamente toda a água na superfície dos frutos. O processamento mínimo não afetou a qualidade visual e nutricional dos morangos como aparência, microbiota, vitamina C, antocianinas e compostos fenólicos, consistindo em alternativa de mercado potencial economicamente viável.
Fragaria x ananassa Duch; sanitização; drenagem; antocianinas
RESEARCH PESQUISA
Quality of fresh-cut strawberry
Qualidade de morango minimamente processado
Franciscleudo B CostaI; Priscila S DuarteII; Rolf PuschmannII; Fernando L FingerIII
IUFCG-Unid. Acad. Tecnol. Alimentos, 58840-000 Pombal-PB; franciscleudo@ccta.ufcg.edu.br
IIUFV-Depto Biol. Vegetal, 36570-000 Viçosa-MG
III UFV-Depto Fitotecnia
ABSTRACT
The aim of this work was to study the physical, chemical, physiological and microbiological changes during the flow chart of fresh-cut strawberry. Strawberry cvs. Camarosa, Dover and Tudla, derived from experimental area of the Universidade Federal de Viçosa, were selected by color (red ¾) and absence of wound. Afterwards, the minimal processing was evaluated, as follows: fast cooling, water and ice, removal of the calyx followed by conservation at 5±0.5°C and 90-95% RH for 13 days, sanitation, drainage, cut in halves. Removal of the calyx did not result significant difference for fresh weight, total soluble solids, total titratable acidity, pH and ratio TSS/TTA. The rapid cooling resulted in lower electrolyte leakage and respiratory rate, especially sanitized fruits. Sanitization by immersion in chlorine solution slowed the growth of fungi and yeast. Drainage for 20 minutes eliminated practically all water on the surface of the fruits. The fresh-cutting did not affect the visual and nutritional quality of strawberries like appearance, microbiot, vitamin C, anthocyanins and phenolic compounds, consisting of alternative market potential economically viable.
Key words: Fragaria x ananassa Duch., sanitation, drainage, anthocyanins.
RESUMO
O objetivo deste trabalho foi estudar mudanças físicas, químicas, fisiológicas e microbiológicas durante o fluxograma de processamento mínimo de morango. Morangos das cultivares Camarosa, Dover e Tudla oriundos de área experimental da Universidade Federal de Viçosa, foram selecionados por cor (¾ vermelho) e ausência de danos. Posteriormente, foi avaliado o fluxograma de processamento mínimo: resfriamento rápido, água e gelo; remoção do cálice acompanhado de conservação a 5±0,5ºC e 90-95% UR, por 13 dias; sanitização; drenagem; corte, em metades. A remoção do cálice não resultou diferença significativa para massa fresca, sólidos solúveis totais, acidez total titulável, pH e razão SST/ATT. O resfriamento rápido ocasionou menores extravasamentos de eletrólitos e taxa respiratória, sobretudo nos frutos sanitizados. A sanitização por imersão em solução de cloro retardou o crescimento de fungos e levedura. A drenagem por 20 minutos eliminou praticamente toda a água na superfície dos frutos. O processamento mínimo não afetou a qualidade visual e nutricional dos morangos como aparência, microbiota, vitamina C, antocianinas e compostos fenólicos, consistindo em alternativa de mercado potencial economicamente viável.
Palavras-chave:Fragaria x ananassa Duch., sanitização, drenagem, antocianinas.
Minimal processing results in the physical alteration of a fruit or vegetable due to the operations of selection, washing, classification, peeling, cutting or slicing, sanitization, rinsing, draining, wrapping and cooling to obtain a fresh product that does not need further preparation (Puschmann et al., 2006). These operations are responsible for the immediate physical, chemical, physiological and microbiological responses such as tissue cell destructuring (Hodges et al., 2008), loss of natural shine and dehydration (Piagentini et al., 2002), increases in respiration rates and ethylene evolution, pigment losses (Bhagwat et al., 2004) and vitamin C losses (Gil et al., 2006), change in taste and firmness, formation of strange odors (Beaulieu, 2006) and enzyme activity involved in darkening.
The strawberry is appreciated for its characteristic color, flavor and aroma. The non-climacteric fruit has a high respiratory rate with high moisture content (90%) and sugar content (glucose 4%, fructose 5% and sucrose 0.9%), an ideal substrate for proliferation of microorganisms, fungi and yeasts such as Botrytis cinerea (Prasanna et al., 2007).
Microorganism growth in minimally processed products can be controlled using quality raw material, sanitization, proper drainage and low temperature (Del Aguila et al., 2006). Sanitization reduces the number of microorganisms in the food and will depend on the disinfectant concentration and exposure time (Sant'ana et al., 2002). Drainage removes excess water or water and cell juice from the fruit surface preventing liquid accumulation inside the packages and the later microorganism development.
Generally, immersion in aqueous solution is not recommended in minimal processing of perishable fruit. However, Costa et al. (2006) recorded up to eight days of shelf life in minimally processed strawberries, sanitized with chlorine solution.
The adaptation of the plant tissue after harvest, preparation and obtaining of the end product can be defined by quality indicators such as appearance and nutritional value. Thus the objective of the present study was to study the physical, chemical, physiological and microbiological changes during the minimal processing steps for strawberries.
MATERIAL AND METHODS
Obtaining the raw material
Strawberries of the Camarosa, Dover and Tudla cultivars produced in a greenhouse in an experimental area at the Crop Science Department of the Federal University of Viçosa were collected in plastic trays (before 8 a.m.) and taken to a fruit and vegetable minimal processing unit of the Plant Biology Department, for sampling for color (¾ red) and absence of damage.
Assessing the minimal processing flow gram
1. Calyx and penduncle removal - the calyx was removed by hand and the penduncle using a sharp blade, 3 mm from the base. The fruits were divided into two lots, with and without calyx, placed on PET trays (177x122x40 mm) with 150 g fruits, without a lid, wrapped in poly vinyl chloride film (PVC, 15 μm) and kept in a vertical display with forced air circulation (Metalfrio), at 5±0.5ºC, under 90-95 % RH. The green matter, soluble solids, titratable acidity, strawberry pH and the SST/ATT ratio were analyzed every three days, starting on day one, for 13 days (Costa, 2009).
2. Quick cooling - Recently collected fruits were cooled quickly (water and ice, 5±1°C), for 15 minutes and compared with uncooled strawberries (environment, 22±2°C, for the controlled experiment.
3. Sanitization - strawberries were immersed in chlorine aqueous solution (Sumaveg®) at 5±1°C, at the concentrations of 0.5 (tap water); 200 and 400 mg L-1, then rinsed with 5 mg L-1 chlorine at a 5±1°C. The following sanitization times were tested: 30 seconds, 5 and 10 minutes for a fixed sanitization concentration.
4. Drainage - after the sanitization and rinsing steps, the strawberries were submitted to drainage times of 20, 40 and 120 minutes, on perforated trays at the processing environment temperature, 18±2°C under 80±5 % RH.
5. Cutting - after the drainage step, one part of the strawberries was cut (in half, longitudinally) using sharp blades and the other part of the fruits was kept whole to form the control experiment.
Physical, chemical, physiological and microbiological analysis
1. Electrolyte leakage - described by Simon (1977) and adopted by Costa (2009) was estimated using the leakage curve of the cell juice from epidermis tissue and pulp (5 mm2 per 2 mm of thickness) removed from the equatorial region of the strawberries using blades up to 2 g sample. The sample was placed on a glass recipient (117 mL) with a lid containing 40 mL deionized water and the leakage readings were taken in CD-850 conductivity every hour for four hours. The samples were then submitted to eight sessions of 30 seconds in a microwave oven (Electrolux ME 28s) with a 30 second interval with the door open between each session to prevent overflowing. The leakage was obtained according to Stuart (1939):  , where: E% = electrolyte leakage, %; E4h = leakage in 4 h; and, Et = total leakage.
, where: E% = electrolyte leakage, %; E4h = leakage in 4 h; and, Et = total leakage.
2. Standard filamentous fungi and yeast count - the surface spreading technique was used, inoculating 0.1 mL of the 10-1, 10-2 and 10-3 dilutions in triplicate containing Agar, potato and dextrose - BDA (Oxoid®), acidified with 10% tartaric acid, pH 3.5 and incubated at 25°C, for 5 to 7 days (Beuchat & Cousin, 2001).
3. Quantity of water absorbed - estimated by gravimetry, on 0.01 g precision scales from the differences in fresh matter before strawberry sanitization and after sanitization, rinsing and drainage.
4. Carbon dioxide quantification - about 50 g strawberries were kept in 170 mL hermetically sealed flasks for 0, 0.5, 1, 2, 4, 8 and 12 hours to make the accumulation curve and identify the ideal sampling time. The quantification was estimated according to Costa (2005), with a 1.0 cm3 aliquot of the atmosphere of the flasks in ultrafine syringes (29 G1/2" needles) and injected in a GC-14B gas chromatographer (Shimadzu, Kyoto), with a heat conductivity detector and column filled with Porapak-Q. The drag gas was nitrogen with 30 cm3/min flow and 85 mA electric current. The temperature of the column, injector and detector were adjusted to 40, 100 and 180°C, respectively.
5. Sample extraction for vitamin C, anthocyanin and phenol compounds - the cell juice was extracted in a Comfort CE55 - Walita® domestic centrifuge and filtered through four layers of gauze to quantify the vitamin C, anthocyanins and phenol compounds.
5.1. Vitamin C - according to Strohecker & Henning (1967), using the colorimetric method of 2.4-dinitrophenylhydrazine (DNPH) with adaptations. About 2 g sample were diluted in 100 mL 0.5% oxalic acid, 20 mL was filtered and 300 μg active carbon were added and left to rest for 15 minutes. From the clarified sample 400 μL were reacted with 3.6 mL 0.5% oxalic acid, 60 μL 2.6-0.2% dichlorophenolindophenol (DCPIP), 1 mL 2.4-DNPH 2% and 20 μL 10% thiourea. The reaction took place in a water bath, 100°C for 10 minutes and was interrupted in a water bath with ice for 10 minutes. Then, 5 mL 85% sulfuric acid were added slowly and left to rest for 10 minutes. The standard curve was prepared with ascorbic acid and the readings made on a Hitachi U-2000 spectrophotometer at 520 nm.
5.2. Anthocyanins - described by Giusti & Wrosltad (2001) by pH difference, with adaptations. The method is based on two buffer systems: potassium chloride 0.025 M, pH 1.0 sodium acetate, 0.4 M pH 4.5. For each system, 5 mL buffer plus 200 μL sample were used to obtain absorbency readings between 0.100 and 1.200, at the maximum wave lengths of the visible (Aλmáx.vis. = 497 nm) and at 700 nm. The difference in absorbency between the buffer systems was calculated according to the following equation:
The pigment content was estimated from the most abundant anthocyanin in the strawberry (pelargonidin-3-glucoside), by the equation:  where: At = anthocyanin, mg/100 g fresh matter; ΔA = difference in absorbency, (ApH1,0 - ApH4,5); PM = molecular weight of the pelargonidina-3-glucoside, 451,2; f = dilution factor for the strawberry, 25x; ε = coefficient of molar absorptivity, 15600.
where: At = anthocyanin, mg/100 g fresh matter; ΔA = difference in absorbency, (ApH1,0 - ApH4,5); PM = molecular weight of the pelargonidina-3-glucoside, 451,2; f = dilution factor for the strawberry, 25x; ε = coefficient of molar absorptivity, 15600.
5.3. Total phenol compounds - according to Waterhouse (2006) by Folin-Ciocalteu, reacting 25 µL of the sample in 1.575 µL distilled water and 100 µL Folin-Ciocalteu, then shaken and left to rest for five minutes. After adding 30 µL 20% sodium carbonate, the solution was kept in a water bath at 40°C for 30 minutes. In basic culture the phosphowolframic and phosphomolybdic acids reduce when oxidizing phenol compounds giving rise to blue wolframic oxides (W8O23) and molybdenum (Mo8O23). The standard curve was prepared with galic acid and absorbency reading at 765 nm.
6. Statistical analysis - Analysis of variance was carried out using the SAEWG 9 program. The F test was applied to assess the significance and the Tukey test was used at the level of 5% probability whenever necessary. The cultivar and the replication for each experimental arrangement varied in function of the quantity of raw material available.
RESULTS AND DISCUSSION
Strawberry storage after removing the calyx
Removing the calyx had no significant effect for green matter, SST, ATT, pH and the SST/ATT ratio compared to the fruits with the calyx (Table 1A). There was, however, significant effect of storage time for green matter, SST, pH and the SST/ATT ratio.
At the end of the 13 days, the loss of accumulated green matter was 0.8% in the fruit with and without calyx (Table 1A). This value may have been due to the type of packaging used, a PET tray with PVC film (15) that functioned as a physical barrier to water vapor loss. Furthermore, using low temperature, 5±0.5ºC and high relative humidity, 90-95%, contributed to reducing green matter loss from the strawberries.
It is believed that the presence of the calyx accelerates water loss from the calyx + pseudofruit set, because it raises the fruit transpiration rate (personal communication). However, the green matter loss observed in the Dover strawberry was minimal (Table 1A). This performance cannot be adopted as a general rule because in addition to temperature, relative humidity and packaging type, the cultivar may present calyx with variable morphological pattern, as observed visually in the Camarosa and Oso Grande fruits, with larger bracts in expansion compared to Dover (data not shown).
Generally, strawberries are used for fresh consumption with the calyx free from dirt and contaminations (Cantillano, 2004). In industry, the strawberries can be used with or without the calyx.
In the fresh strawberry, a turgid and clean calyx indicates freshness and attractiveness and can be used by consumers as a desirable characteristic. In eggplant, for example, the calyx is used as a quality indicator by producers and consumers (Moretti & Pineli, 2005).
The SST content remained practically constant throughout the storage period, ranging from 7.3 to 6.4%, with greatest decrease on the second day of analysis (Table 1A). This characteristic is of commercial interest, especially for fresh strawberries, because the consumer prefers sweeter fruit (Fumis et al., 2003).
The pH increased in the fruit with and without calyx from 3.17 to 3.94 during storage (Table 1A). The Dover strawberry was considered acid, not very sweet or even sour. The main acids present in the fruit, citric and malic, can directly affect the flavor, cell pH and coloring by anthocyanins (Fumis et al., 2003; Cantillano, 2004).
The SST/ATT ratio decreased after the second day, ranging from 10.6 to 9.3 and remained practically constant until the 13th day (Table 1A) with values above the commercially required (8.75) characterized by sweet fruits with low acidity.
Strawberry under cooling, sanitization, drainage and cutting
a) Eletrolyte leakage
Electrolyte leakage up to 4 h four was the most indicated, with discrete sensitivity among the cultivars Camarosa (9%), Tudla (11%) and Dover (13%) (Figure 1A).
The relative growth rate in volume of the strawberry is a result of cell division and filling or of their joint action, and the contribution of each factor is relative to the species, variety or cultivar. Furthermore, the genetic difference among cultivars influences the structural and mechanical development of the tissue (Havis, 1943) and strawberries consisting structurally of small cells are more resistant (Havis, 1943) to post-harvest handling and minimal processing.
Electrolyte leakage in non-cooled strawberries (22±2°C) and without sanitization was greater compared to the sanitized fruit (Figure 1b) and this difference was smaller in the strawberries submitted to cooling (5±1°C). The difference was around 5% among the non- sanitized fruits at 22±2°C and the cooled fruits, showing that cooling (water plus ice) reduced the field heat in the fruits.
Strawberries cooled quickly after harvest, in a forced air chamber at 1°C for one hour, and stored for one week at 1°C plus one day at 20°C, had attractive coloring, less green matter loss, greater firmness, minimal vitamin C and sugar content loss compared to strawberries when there was a six hour delay in cooling and exposure to 30°C (Nunes et al., 1995).
Soft and succulent fruit consist of cells with high solute content, a condition that can generate turgor pressure above the limit of cell wall resistance (Simon, 1977) making them sensitive and vulnerable to leakage. In the case of the sanitized strawberries, the epidermis and adjacent cells may have increased the turgor pressure, and thus acquired less mechanical resistance. In this sense, sanitization may be harmful but not sufficiently to prevent its use in minimal processing of strawberries or fruits with similar sensitivity.
Electrolyte leakage ranged from 38 to 43% in the cut strawberries with and without cooling, respectively, while in the whole fruit, this variation was 34 and 36% in the same cooling order (Figure 1C). Thus using cooling reduced the stress induced by cutting especially the electrolyte leakage resulting from cell breakdown.
The cells of the fruits did not break easily in situ, because they were under pressure from the neighboring cell layers or protected by resistant epidermis, cuticle or skin cells. Once the skin was removed, the pulp cells become vulnerable to any hypertonic condition (Simon, 1977). The strawberry has a thin and fragile epidermis that may make it more sensitive to cell leakage when submitted to minimal processing conditions such as washing, sanitization, rinsing and cutting.
b) Microorganism growth
The sanitization concentrations and sanitization times did not differ on the strawberry microbiota (Figure 2). Regarding the sanitization concentrations tested, the decrease in the number of log UFC was 0.3 for 200 and 400 mg L-1 chlorine compared to the non-sanitized and sanitized fruits with 0.5 mg L-1 (Figure 2). At the 5 and 10 minute sanitization times, the reduction was 0.5 log UFC g-1 compared to the non-sanitized and strawberries sanitized for 30 seconds, with 200 mg L-1 chlorine (Figure 2). This showed that the microbiota present on the strawberry during the processing was insufficient to damage the fruit safety.
Microorganism incidence on strawberries and other low pH fruit (3.5 and 4) is due to filamentous fungi and yeast. Furthermore, this pH is low enough to inhibit the growth of other microorganisms such as bacteria (Park et al., 2005).
The microbiological changes that occur at the start and during fruit and other vegetable storage vary with the microbiota of each product and are directly linked to factors such as hygiene during handling and processing location, temperature, relative humidity and water quality (Porte & Maia, 2001).
C) Drainage
Drainage was essential in the strawberry minimal processing and removed the excess water from the fruit surface by 2.5%, regardless of the sanitization time (Figure 2). The water available in the Dover strawberry, sanitized and rinsed for 30 seconds, fell to 0.7 and 0.3% in drainage for 20 and 40 minutes, respectively (Figure 2A). In the fruits sanitized and rinsed for five minutes, drainage for 20 and 40 minutes resulted in water absorbed with 1.3 and 1%, respectively (Figure 2B). Sanitization and rinsing for 10 minutes permitted that the water absorbed in the strawberry reached 1.7 and 0.6% in drainage for 20 and 40 minutes, respectively (Figure 2C).
Drainage for 120 minutes, in all the sanitization and rinsing times, in addition to removing water adhering to the strawberry, also removed water from the pulp or the first epidermis cell layers (Figure 2). Thus this condition becomes damaging to the strawberry appearance, anticipating the senescence process.
Thus the strawberries sanitized and rinsed for 30 seconds and drained for 20 minutes permitted the minimum of water adhering to the fruit surface, besides making the process quick compared to the other drainage times studied.
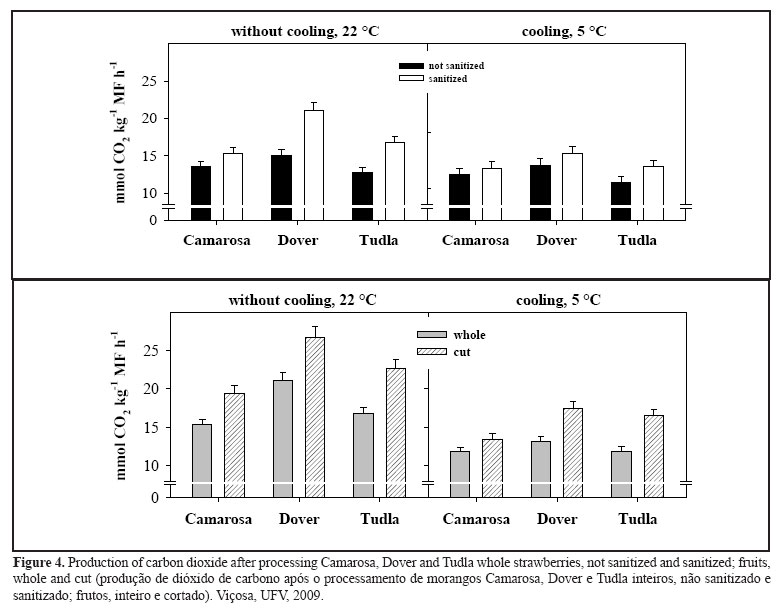
d) Carbon dioxide production
Non-cooled and non- sanitized strawberries had a lower respiratory rate compared to the sanitized fruit, with differences of 12, 13 and 23% for the Camarosa, Dover and Tudla cultivars, respectively (Figure 3). This difference fell to 5, 8 and 11% for Camarosa, Dover and Tudla, respectively, when submitted to cooling (Figure 3), highlighting the importance of cooling in reducing the field heat in the fruit.
The respiration rate in the strawberries, cooled or not, was greater in the cut fruit than in the whole fruit, regardless of the cultivar (Figure 3). In the cooled fruits, the respiratory rate was lower in the whole fruits than the cut fruits, in all the cultivars used. In this case, the used of cooling functioned as an excellent tool to control respiration in the strawberry.
Cutting in minimal processing is a very intense stress to the tissue because some physiological manifestations are due to the rupture of the cell structures such as increase in carbon dioxide production (Porte & Maia, 2001). In some minimally processed products, the respiratory rates can increase three to seven-fold compared to the intact tissue, with accelerated oxygen intake. Thus respiration in fruits and vegetables can serve as an indicator of catabolic alteration, especially when the tissue is peeled or cut (Maistro, 2001).
e) Vitamin C, anthocyanins and phenol compounds
The vitamin C contents in the whole Dover and Tudla strawberries were greater, about 8 to 12%, respectively, compared to the cut strawberries (Table 1B).
Vitamin C predominates in the strawberry in the form of ascorbic acid with an average content of 60 mg/100 g MF and can vary with the cultivar and ripening stage (Pazinato, 1999; Cordenunsi et al., 2005).
In minimal processing, vitamin C can serve as an excellent quality indicator because it is labile and susceptible to degradation in the presence of light and oxygen so that its content can be affected at the cutting stage. Ascorbate oxidation by ascorbic oxidase increases under adverse conditions such as high temperatures, metallic ions, physical and chemical damage and with exposure to products that contain halogens in the molecule such as hydrochloride salts used in sanitization (Lee & Kader, 2000).
The cut Camarosa strawberry was outstanding compared to the whole fruits with anthocyanin and phenol compound contents of around 15 and 5% more, respectively (Table 1B). The Dover strawberry had low anthocyanin contents compared to Camarosa, with practically equal contents in the whole and cut fruit (Table 1B). The anthocyanin contents found in the whole and cut Seascape strawberry was around 45 mg/100 g MF (Gil et al., 2006), close to that estimated for Camarosa (40-50 mg 100/g MF) and greater than the contents detected in Dover (25 mg 100/g MF), showing that the anthocyanin content is a characteristic intrinsic to the cultivar. The Dover strawberries had a higher anthocyanin content compared to the Campineiro and Oso Grande cultivars in the study by Cordenunsi et al. (2005).
The anthocyanins and the factors that affect their synthesis, content and stability account for the color in the strawberry, and pelargonidin-3-glucoside is the most common anthocyanin in strawberries (Lopes da Silva et al., 2003).
Regarding the phenol compound contents, the Dover strawberry performed similarly to the Camarosa (Table 1B). The phenol compound contents in the strawberry can vary with the cultivar and the extraction and quantification methods, and can function as anti-oxidants through the phenolic acids and the anthocyanins themselves (Kosar et al., 2004). The phenol compound content estimated in the whole and cut Seascape strawberry was 55 mg/100 g (Gil et al., 2006), below that estimated for Camarosa and Dover, close to 95 and 80 mg/100 g MF, respectively.
Strawberry minimal processing did not result in drastic loss in the visual and nutritional quality of the fruit, especially of the vitamin C, anthocyanin and phenol compound contents and was shown to be an economically viable market alternative and promising for strawberry producers.
The strawberry minimal processing flow chart was defined starting with operations of cooling with water and ice, removing the calyx and penduncle, sanitization, rinsing, draining, cutting and wrapping. Future studies are very important on the performance of minimally processed strawberries under cooled storage.
ACKNOWLEDGMENTS
The authors thank the CNPq, CAPES and FAPEMIG for the financial resources.
REFERENCES
BEAULIEU JC. 2006. Effect of cutting and storage on acetate and nonacetate esters in convenient, ready-to-eat fresh-cut melons and apples. HortScience 41: 65-73.
BEUCHAT LR; COUSIN MA. 2001. Yeasts and molds. In: DOWNES FP; ITO K (eds). Compendium of methods for the microbiological examination of foods. 4 ed. Washington DC: APHA. p. 209-215.
BHAGWAT AA; SAFTNER RA; ABBOTT JA. 2004. Evaluations of wash treatments for survival of foodborne pathogens and maintenance of quality characteristics of fresh-cut apple slices. Food Microbiology 21: 319-326.
CANTILLANO RFF. 2004. Fisiologia e manejo na colheita e pós-colheita de morangos. In: SIMPÓSIO NACIONAL DO MORANGO, 2; ENCONTRO DE PEQUENAS FRUTAS E FRUTAS NATIVAS DO MERCOSUL, 1. Palestras... Pelotas: Embrapa Clima Temperado. p. 146-161.
CORDENUNSI BR; GENOVESE MI; NASCIMENTO JRO; HASSIMOTTO NMA; SANTOS RJ; LAJOLO FM. 2005. Effetcs of temperature on the chemical composition and antioxidante activity of three strawberry cultivars. Food Chemistry 91: 113-121.
COSTA FB. 2005. Injúria mecânica e qualidade pós-colheita da banana Prata Anã produzida no Norte de Minas Gerais. Viçosa: UFV. 49p. (Tese mestrado).
COSTA FB. 2009. Fisiologia e conservação de cultivares de morangos inteiros e minimamente processados. Viçosa: UFV. 115p. (Tese doutorado).
COSTA FB; SIMÕES AN; MOREIRA SI; SOUZA DD; FREITAS MA; SANTOS RHS; PUSCHMAN R. 2006. Processamento mínimo de morango cultivado organicamente. In: ENCONTRO NACIONAL SOBRE PROCESSAMENTO MÍNIMO DE FRUTOS E HORTALIÇAS, 4. Resumos... São Pedro: USP/ESALQ.
DEL AGUILA JS; SASAKI FF; HEIFFIG LS; ONGARELLI MG; GALLO CR; JACOMINO AP; KLUGE RA. 2006. Determinação da microflora em rabanetes minimamente processados. Horticultura Brasileira 24: 75-78.
FUMIS TF; SAMPAIO AC; PALLAMIN ML; OLIVEIRA OM. 2003. Avaliação tecnológica de nove cultivares de morango na região de Bauru-SP. In: CONGRESSO BRASILEIRO DE OLERICULTURA, 43. Resumos... Recife: SOB (CD-ROM).
GIL MI; AGUAYO E; KADER AA. 2006. Quality changes and nutrient retention in fresh-cut versus whole fruits during storage. Journal of Agricultural and Food Chemistry 54: 4284-4296.
GIUSTI MM; WROSLTAD RE. 2001. Anthocyanins: characterization and measurement with UV-visible spectroscopy. In: WROSLTAD RE (ed). Current protocols in food analytical chemistry. New York: John Wiley & Sons. p. 1-11.
HAVIS AL. 1943. A developmental analysis of the strawberry fruit. American Journal of Botany 30: 311-314.
HODGES DM; TOIVONEN PMA. 2008. Quality of fresh-cut fruits and vegetables as affected by exposure to abiotic stress. Postharvest Biology and Technology 48: 155-162.
KOSAR M; KAFKAS E; PAYDAS S; HÜSNÜ CAN; BASER K. 2004. Phenolic composition of strawberry genotypes at different maturation stages. Journal of Agricultural and Food Chemistry 52: 1586-1589.
LEE SK; KADER AA. 2000. Preharvest and postharvest factors influencing vitamin C content of horticultural crops. Postharvest Biology and Technology 20: 207-220.
LOPES DA SILVA F; DE PASCUAL TERESA S; RIVAS GONZALO J; SANTOS BUELGA C. 2002. Identification of anthocyanin pigments in strawberry (cv Camarosa) by LC using DAD and ESI-MS detection. European Food Research and Technology 214: 248-253.
MAISTRO LC. 2001. Alface minimamente processada: uma revisão. Revista de Nutrição 14: 219-224.
MORETTI CL; PINELI LO. 2005. Qualidade química e física de berinjelas submetidas a diferentes tratamentos pós-colheita. Ciência e Tecnologia de Alimentos 25: 339-344.
NUNES MCN; BRECHT JK; MORAIS AMMB; SARGENT SA. 1995. Physical and chemical quality characteristics of strawberry after storage are reduced by a short delay to cooling. Postharvest Biology and Technology 6: 17-28.
PARK S; STAN SD; DAESCHEL MA; ZHAO Y. 2005. Antifungical coatings on fresh strawberries (Fragaria x ananassa) to control mold growth during cold storage. Journal of Food Science 70: 202-207.
PIAGENTINI AM; GÜMES DR; PIROVANI ME. 2002. Sensory characteristics of fresh-cut spinach preserved by combined factors methodology. Journal of Food Science 67: 1544-1549.
PORTE A; MAIA LH. 2001. Alterações fisiológicas, bioquímicas e microbiológicas de alimentos minimamente processados. Boletim do Centro de Pesquisa e Processamento de Alimentos, B. CEPPA 19: 105-118.
PRASANNA V; PRABHA TN; THARANATHAN RN. 2007. Fruit ripening phenomena - An overview. Critical Reviews in Food Science and Nutrition 47: 1-19.
PUSCHMANN R; COSTA FB; SIMÕES AN; SILVA EO. 2006. História e atualidades sobre pesquisa com processamento mínimo de frutas e hortaliças no Brasil. In: ENCONTRO NACIONAL SOBRE PROCESSAMENTO MÍNIMO DE FRUTOS E HORTALIÇAS, 4. Resumos... São Pedro: USP/ESALQ.
SANT'ANA A; AZEVEDO DP; COSTA M. 2002. Análise de perigos no processamento mínimo de vegetais. Revista Higiene Alimentar 16: 80-84.
SIMON EW. 1977. Leakage from fruit cells in water. Journal of Experimental Botany 28: 1147-1152.
STROHECKER RL; HENNING HM. 1967. Analisis de vitaminas: metodos comprobados. Madrid: Paz Montalvo, 428p.
STUART NW. 1939. Comparative cold hardiness of scion roots from fifty apple varieties. Proceedings of the American Society for Horticultural Science 37: 330-334.
WATERHOUSE A. 2006. Folin-Ciocalteu micro method for total phenol in wine. Disponível em: http://waterhouse.ucdavis.edu/phenol/folinmicro.htm. Acesso em 21 de setembro de 2009.
(Recebido para publicação em 23 de dezembro de 2009; aceito em 10 de maio de 2011)
(Received on December 23, 2009; accepted on May 10, 2011)
- BEAULIEU JC. 2006. Effect of cutting and storage on acetate and nonacetate esters in convenient, ready-to-eat fresh-cut melons and apples. HortScience 41: 65-73.
- BEUCHAT LR; COUSIN MA. 2001. Yeasts and molds. In: DOWNES FP; ITO K (eds). Compendium of methods for the microbiological examination of foods 4 ed. Washington DC: APHA. p. 209-215.
- BHAGWAT AA; SAFTNER RA; ABBOTT JA. 2004. Evaluations of wash treatments for survival of foodborne pathogens and maintenance of quality characteristics of fresh-cut apple slices. Food Microbiology 21: 319-326.
- CANTILLANO RFF. 2004. Fisiologia e manejo na colheita e pós-colheita de morangos. In: SIMPÓSIO NACIONAL DO MORANGO, 2; ENCONTRO DE PEQUENAS FRUTAS E FRUTAS NATIVAS DO MERCOSUL, 1. Palestras.. Pelotas: Embrapa Clima Temperado. p. 146-161.
- CORDENUNSI BR; GENOVESE MI; NASCIMENTO JRO; HASSIMOTTO NMA; SANTOS RJ; LAJOLO FM. 2005. Effetcs of temperature on the chemical composition and antioxidante activity of three strawberry cultivars. Food Chemistry 91: 113-121.
- COSTA FB. 2005. Injúria mecânica e qualidade pós-colheita da banana Prata Anã produzida no Norte de Minas Gerais Viçosa: UFV. 49p. (Tese mestrado).
- COSTA FB. 2009. Fisiologia e conservação de cultivares de morangos inteiros e minimamente processados Viçosa: UFV. 115p. (Tese doutorado).
- COSTA FB; SIMÕES AN; MOREIRA SI; SOUZA DD; FREITAS MA; SANTOS RHS; PUSCHMAN R. 2006. Processamento mínimo de morango cultivado organicamente. In: ENCONTRO NACIONAL SOBRE PROCESSAMENTO MÍNIMO DE FRUTOS E HORTALIÇAS, 4. Resumos.. São Pedro: USP/ESALQ.
- DEL AGUILA JS; SASAKI FF; HEIFFIG LS; ONGARELLI MG; GALLO CR; JACOMINO AP; KLUGE RA. 2006. Determinação da microflora em rabanetes minimamente processados. Horticultura Brasileira 24: 75-78.
- FUMIS TF; SAMPAIO AC; PALLAMIN ML; OLIVEIRA OM. 2003. Avaliação tecnológica de nove cultivares de morango na região de Bauru-SP. In: CONGRESSO BRASILEIRO DE OLERICULTURA, 43. Resumos... Recife: SOB (CD-ROM).
- GIL MI; AGUAYO E; KADER AA. 2006. Quality changes and nutrient retention in fresh-cut versus whole fruits during storage. Journal of Agricultural and Food Chemistry 54: 4284-4296.
- GIUSTI MM; WROSLTAD RE. 2001. Anthocyanins: characterization and measurement with UV-visible spectroscopy. In: WROSLTAD RE (ed). Current protocols in food analytical chemistry New York: John Wiley & Sons. p. 1-11.
- HAVIS AL. 1943. A developmental analysis of the strawberry fruit. American Journal of Botany 30: 311-314.
- HODGES DM; TOIVONEN PMA. 2008. Quality of fresh-cut fruits and vegetables as affected by exposure to abiotic stress. Postharvest Biology and Technology 48: 155-162.
- KOSAR M; KAFKAS E; PAYDAS S; HÜSNÜ CAN; BASER K. 2004. Phenolic composition of strawberry genotypes at different maturation stages. Journal of Agricultural and Food Chemistry 52: 1586-1589.
- LEE SK; KADER AA. 2000. Preharvest and postharvest factors influencing vitamin C content of horticultural crops. Postharvest Biology and Technology 20: 207-220.
- LOPES DA SILVA F; DE PASCUAL TERESA S; RIVAS GONZALO J; SANTOS BUELGA C. 2002. Identification of anthocyanin pigments in strawberry (cv Camarosa) by LC using DAD and ESI-MS detection. European Food Research and Technology 214: 248-253.
- MAISTRO LC. 2001. Alface minimamente processada: uma revisão. Revista de Nutrição 14: 219-224.
- MORETTI CL; PINELI LO. 2005. Qualidade química e física de berinjelas submetidas a diferentes tratamentos pós-colheita. Ciência e Tecnologia de Alimentos 25: 339-344.
- NUNES MCN; BRECHT JK; MORAIS AMMB; SARGENT SA. 1995. Physical and chemical quality characteristics of strawberry after storage are reduced by a short delay to cooling. Postharvest Biology and Technology 6: 17-28.
- PARK S; STAN SD; DAESCHEL MA; ZHAO Y. 2005. Antifungical coatings on fresh strawberries (Fragaria x ananassa) to control mold growth during cold storage. Journal of Food Science 70: 202-207.
- PIAGENTINI AM; GÜMES DR; PIROVANI ME. 2002. Sensory characteristics of fresh-cut spinach preserved by combined factors methodology. Journal of Food Science 67: 1544-1549.
- PORTE A; MAIA LH. 2001. Alterações fisiológicas, bioquímicas e microbiológicas de alimentos minimamente processados. Boletim do Centro de Pesquisa e Processamento de Alimentos, B. CEPPA 19: 105-118.
- PRASANNA V; PRABHA TN; THARANATHAN RN. 2007. Fruit ripening phenomena - An overview. Critical Reviews in Food Science and Nutrition 47: 1-19.
- PUSCHMANN R; COSTA FB; SIMÕES AN; SILVA EO. 2006. História e atualidades sobre pesquisa com processamento mínimo de frutas e hortaliças no Brasil. In: ENCONTRO NACIONAL SOBRE PROCESSAMENTO MÍNIMO DE FRUTOS E HORTALIÇAS, 4. Resumos.. São Pedro: USP/ESALQ.
- SANT'ANA A; AZEVEDO DP; COSTA M. 2002. Análise de perigos no processamento mínimo de vegetais. Revista Higiene Alimentar 16: 80-84.
- SIMON EW. 1977. Leakage from fruit cells in water. Journal of Experimental Botany 28: 1147-1152.
- STROHECKER RL; HENNING HM. 1967. Analisis de vitaminas: metodos comprobados Madrid: Paz Montalvo, 428p.
- STUART NW. 1939. Comparative cold hardiness of scion roots from fifty apple varieties. Proceedings of the American Society for Horticultural Science 37: 330-334.
- WATERHOUSE A. 2006. Folin-Ciocalteu micro method for total phenol in wine. Disponível em: http://waterhouse.ucdavis.edu/phenol/folinmicro.htm Acesso em 21 de setembro de 2009.
Publication Dates
-
Publication in this collection
05 Jan 2012 -
Date of issue
Dec 2011
History
-
Received
23 Dec 2009 -
Accepted
10 May 2011