Abstracts
This work evaluates the preservation of bovine placentomes using paraformaldehyde and glutaraldehyde at 4% and solutions of Karnovsky, Bouin and Carnoy for 4, 12 and 24 hours fixation, at room temperature or at 4ºC, before inclusion in plastic resin and paraffin. The best preservation of the specimens was obtained after 12 hours of fixation with those solutions containing aldehydes. With Bouin solution the best time of fixation was 4 hours, while for Carnoy solution the best time of fixation was 24 hours. Inclusion in plastic resin gave better results than in paraffin, and the temperature did not influence the quality of preservation of the specimens of bovine placentomes for evaluation with light microscopy.
Bovine; placentome; light microscopy
Avaliou-se a preservação de placentomas bovinos com as soluções fixadoras paraformaldeído e glutaraldeído a 4% e soluções de Karnovsky, Bouin e Carnoy nos tempos de 4, 12 e 24 horas de fixação, à temperatura ambiente ou a 4ºC, incluídos em resina plástica e parafina. A melhor preservação dos placentomas foi obtida com os fixadores à base de aldeídos, a partir de 12 horas de fixação. Em Bouin, os espécimes foram melhor preservados quando fixados por 4 horas, enquanto que em Carnoy a preservação foi melhor com 24 horas. A inclusão em resina plástica apresentou resultados superiores do que em parafina. A temperatura de fixação, 4ºC ou ambiente, não influenciou na preservação dos espécimes de placentoma para estudos em microscopia óptica.
Bovino; placentoma; microscopia óptica
Bovine placentome preservation for light microscopy evaluation
(Preservação de placentoma bovino para microscopia óptica)
E. Martins1, V.M.V. Martins2, A.P. Marques Júnior3*, A.C. Vasconcelos4,
H. Chiarini-Garcia4, P.F. Malard3
1EPAGRI SA Empresa de Pesquisa Agropecuária de Santa Catarina. Lages, SC
2UDESC Universidade Estadual de Santa Catarina. Lages, SC
3Escola de Veterinária da UFMG
Caixa Postal 567
30123-970 - Belo Horizonte, MG
4Instituto de Ciências Biológicas da UFMG
Recebido para publicação, após modificações, em 17 de novembro de 1999.
Trabalho financiado pela FAPEMIG Proc. 518/96
(*) Autor para correspondência
E-mail: ampinho@vet.ufmg.br
ABSTRACT
This work evaluates the preservation of bovine placentomes using paraformaldehyde and glutaraldehyde at 4% and solutions of Karnovsky, Bouin and Carnoy for 4, 12 and 24 hours fixation, at room temperature or at 4oC, before inclusion in plastic resin and paraffin. The best preservation of the specimens was obtained after 12 hours of fixation with those solutions containing aldehydes. With Bouin solution the best time of fixation was 4 hours, while for Carnoy solution the best time of fixation was 24 hours. Inclusion in plastic resin gave better results than in paraffin, and the temperature did not influence the quality of preservation of the specimens of bovine placentomes for evaluation with light microscopy.
Keywords: Bovine, placentome, light microscopy
RESUMO
Avaliou-se a preservação de placentomas bovinos com as soluções fixadoras paraformaldeído e glutaraldeído a 4% e soluções de Karnovsky, Bouin e Carnoy nos tempos de 4, 12 e 24 horas de fixação, à temperatura ambiente ou a 4oC, incluídos em resina plástica e parafina. A melhor preservação dos placentomas foi obtida com os fixadores à base de aldeídos, a partir de 12 horas de fixação. Em Bouin, os espécimes foram melhor preservados quando fixados por 4 horas, enquanto que em Carnoy a preservação foi melhor com 24 horas. A inclusão em resina plástica apresentou resultados superiores do que em parafina. A temperatura de fixação, 4oC ou ambiente, não influenciou na preservação dos espécimes de placentoma para estudos em microscopia óptica.
Palavras-chave: Bovino, placentoma, microscopia óptica
INTRODUCTION
In the studies of animal specimens the steps of fixation and inclusion are critical for a proper preservation of the histologic architecture of the tissue, since these procedures stop autolysis and imobilize the tissue components, keeping the structural relationship between the several elements that give the unique morphology of each tissue. The cellular and tissue constituents, when not fixed and included adequately might show distortion and retraction that difficult the evaluation (Fox et al., 1985). For these reasons the search for an adequate fixation solution is important for histologic studies of different biological tissues.
The selection of the solution for fixation and inclusion is made considering the tissue structure and characteristics to be preserved and evaluated, besides the effects of the time of keeping the specimens and the advantages and disadvantages of each procedure, including the presence of artifacts that might occur. The fixation time, size of the specimens and storage potential are important considerations for obtaining good material for histomorphological, histochemical and immunological evaluations (Swartz & Nusbickel, 1978).
The bovine placenta is a structure formed by the apposition of fetal membranes and maternal tissues, characterized as cotiledonary. The chorionic villi are present in restricted areas of the chorion, the cotiledons, separated by areas of smooth corion, the intercaruncular portion (Ramsey, 1982). The cotiledons develop in specialized areas of the uterine mucosa named caruncles, and together they form the unit called placentome (Amoroso, 1952; Perry, 1981). Histologically the bovine placenta is classified as epitheliochorial, showing six cellular extracts that form the materno-fetal interface: the fetal endothelium, conjunctive tissue and chorionic epithelium and the maternal epithelium, conjunctive tissue and endothelium (Ramsey, 1982). The fetal components intertwine with the intact maternal tissue, from which they can be easily separated (Steven, 1975; Bjorkman, 1982). The study of the mechanisms involved in the placental retention process in the bovine have been based on the histomorphological aspects of the placentome, considering among others the chemotaxy of leucocytes into the cotiledon (Marques Júnior, 1988), the interrelation between maternal and fetal tissues (Bjorkman & Dantzer, 1987; Barreto Filho, 1992; Barreto Filho & Marques Júnior, 1993), the extracellular matrix and the maternal and fetal conjunctive tissues (Elier & Hopkins, 1993).
In most of the works evaluating the histology of the placenta the authors have used Bouin for fixation of the specimens and paraffin for inclusion (Bjorkman & Dantzer, 1987; Marques Júnior, 1988; Barreto Filho, 1992; Santos, 1995), what results in separation of maternal and fetal tissues due to tissue retraction, with prejudice to the histological evaluation. Besides, the evaluation of the interrelation of the maternal and fetal tissue is compromised in studies with light microscopy and stereology.
The tissue inclusion with glycolmethacrylate resins (GMA) is frequently used for studies with light microscopy. Because the GMA is a monomer of small size and soluble in water it penetrates easily between the molecular components of the tissues and polymerize fast in the presence of catalizers, resulting in a bloc firm enough to allow thin sections (Frater, 1979) what increases the resolution. Several authors describe the superiority of the inclusion with GMA in comparison to other inclusion substances (Cole & Sykes, 1974; Woodruff & Greenfield, 1979; Ferreira & Chiarini-Garcia, 1992).
In this work it is evaluated the preservation of bovine placentome in several fixing solutions at room temperature and at 4oC in different times, with inclusion in plastic resin and paraffin.
MATERIAL AND METHODS
Nineteen placentomes were harvested from cows in slaugtherhouses, soon after the killing of the animals, and from each were made several cuts with 0,3 x 0,5cm width, from the apex to the base, starting from the central region. The cuts were immediately placed in several different fixation dilutions.
The cuts were fixed by immersion in the following fixing solutions: a) paraformaldehyde at 4% in phosphate buffer 0.1M, pH 7.2; b) glutaraldehyde at 4% in sodium cacodilate buffer 0.1M, pH 7.2; c) Karnovsky solution (paraformaldehyde at 4%, glutaraldehyde at 2.5% in sodium cacodilate buffer 0.1M, pH 7.2 and 7.4); d) Carnoy solution (60ml etanol PA, 30ml chloroform, 10ml glacial acetic acid); and e) Bouin solution (25ml formalin, 75ml saturated picric acid solution and 5ml of glacial acetic acid). The process of fixation was done at room temperature and at 4oC, and for each treatment three parts of the placentomes specimens were fixed, composed of two fragments fixed during 4, 12 and 24 hours.
Fragments of each placentome were infiltrated and included in paraffin, while other fragments were similarly treated in plastic resin with glycolmethacrylate. After inclusion the fragments were cut into 3mm width sections using a microtome with glass blade. For both inclusion methods the staining was hematoxilin-eosin (HE), as described by Luna (1968).
The microscopic characteristics considered were integrity of the tissues, integrity of the structural relationship between fetal and maternal tissues, cellular border integrity and staining affinity.
RESULTS AND DISCUSSION
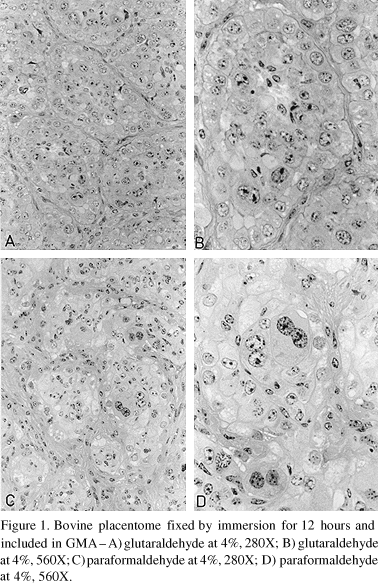
The best preservation of the epithelial and mesenchimal structures of the placentome were obtained with the fixing solutions containing aldehydes (paraformaldehyde at 4% or glutaraldehyde at 4%) (Fig. 1), or the association of both (Karnovsky solution) for 12 hours of fixation (Fig. 2A, 2B). The maternal epithelial cells and the trophoblastic binucleate cells were well preserved, showing clear nuclear and cellular borders well stained. These same fixing solutions when used for a period of 4 hours did not preserve the tissues adequately, leaving them flacid and allowing the loosening and separation of some tissues during microtomy. Besides, in these specimens, when possible to make cuts, it was found cytoplasm with turbidity, condensed chromatin and apparent decrease of the nuclear volume, cariolysis and lack of staining affinity of the nuclei, mainly in the fetal tissue, besides that in several specimens the fetal portion was missing. These observations indicate that the most adequate time for fixation of bovine placentome samples is 12 hours, in fixing solutions containing aldehydes. Swartz & Nusbickel (1978) evaluating the preservation of chicken embryos found differences between the fixing solution with acid and neutral formalin, with the first one being superior and with time of fixation varying with the nature of the tissue. Of all tissues fixed in neutral formalin the best time of fixation was 12 hours. Similar findings were also registered in this study when preserving the placentome in paraformaldehyde at 4%.
The Carnoy solution provided the best time of fixation for 24 hours (Fig. 2C, 2D), with minimal retraction of the fetal tissue. However, the cytoplasmic border was not so distinct, making difficult to analyse the cells individually. In the specimens fixed for 4 and 12 hours it was found chromatin condensation and intense caryolysis, with the cytoplasm becoming granular and showing amorph masses of ill defined borders, characterisitic of autolyis.
The specimens fixed with Bouin solution showed best preservation with 4 hours fixation (Fig. 3A, 3B), allowing good identification of the cell structures, with clear cellular delimitation. However, there was intense retraction of the fetal tissue and clothing of structural proteins. With 12 and 24 hours fixation the tissue was friable and with intense loss during the microtomy procedures. Although the results were satisfactory with 4 hours fixation, not considering the tissue retraction and protein clothing, it is necessary to emphasize the need for effective control of the time of fixation, in order not to affect the quality of the cuts during microtomy.
Although the cuts of placentomes included in GMA can be fixed in any fixing solution (Cole & Sikes, 1974; Bennett et al., 1976; Woodryff & Greenfield, 1979), it was observed that the specimens preserved in nonaldehyde fixing solutions showed less quality of the material, making difficult the evaluation of the cellular structures and the distinction between the fetal and maternal tissues. The aldehyde fixing solutions preserved the tissue and their structures with better details and better resolution, similar to the findings by Ferreira & Chiarini-Garcia (1992) that studied tissue and cells of fish intestine. The best tissue preservation with aldehyde fixing solutions allows a more acurate evaluation of the fetal and maternal structures, essential for the precision of the stereologic studies.
With the inclusion of the placentome cuts in glycolmethacrylate the distortion and retraction of the fetal and maternal tissues were minimal when compared with the results obtained with inclusion in paraffin (Fig. 3C, 3D), allowing better morphological evaluation of the placentome. These observations are similar to those made by Swartz & Nusbickel (1978) when using glycolmethacrylate to include chicken embryos. They found that the glycolmethacrylate, although giving more stability to the tissue also decreased artifacts frequently found with other methods. Another reason for the better results with glycolmethacrylate could be that it does not require the high temperature necessary for inclusion with paraffin resulting in tissue damage and distortion. The tissue inclusion with glycolmetacrylate also allows the maintenance of the enzymatic action in the interior of the cell, a feature that permits hystochemical investigation of the tissue (Troyer & Nusbickel, 1975). Evaluating the fixation of tissues in isotonic paraformaldehyde at 4% and inclusion in glycolmethacrylate Junqueira et al. (1995) found that this method of inclusion allows the obtention of tissue images without distortion and with better resolution under light microscopy, with the quality of the images similar to the obtained with low increase in electronic microscopy.
Although the techniques used for the staining of tissues included in paraffin need some adjustments when using them in tissues included in plastic resins (Bennett et al., 1976; Swartz & Nusbickel, 1978; Franklin & Martin, 1980) the high quality of the material obtained in the present study suggests the use of plastic resin based in glycolmethacrylate for hystological evaluation of bovine placentomes.
Despite the temperature of fixation recommended is 4oC (Prophet, 1994), apparently there was no such influence in the preservation of specimens of bovine placentomes for studies in light microscopy, since no microscopic difference was found between the treatment at 4oC and at room temperature.
Among all the fixing solutions and inclusion media used the best results were obtained with the use of aldehyde fixing solutions for 12 hours in association with the inclusion in glycolmethacrylate. They allowed a good justaposition between the fetal and maternal tissues in sections up to 1mm wide without distortion of the tissue structure, allowing the observation of the chorionic villi adhered to the maternal epithelium. The procedure used allowed better stereologic evaluation than that obtained routinely with material fixed in Bouin and included in paraffin.
CONCLUSIONS
The results obtained in the present study allow to recommend the fixation of bovine placentomes in paraformaldehyde or glutaraldehyde at 4% and inclusion in plastic resins for histomorphological studies with light microscopy.
BIBLIOGRAPHY
- AMOROSO, E.C. Placentation. In PARKES, A.S. (Ed.) Marshalls physiology of reproduction 3.ed. London: Longmans Green, 1952. v.2, p.127-311.
- BARRETO FILHO, J. B. Aspectos morfofisiológicos da placentaçăo do zebu (Bos taurus indicus). Belo Horizonte: Escola de Veterinária da UFMG, 1992. 106p. (Tese, Mestrado).
- BARRETO FILHO, J. B., MARQUES JÚNIOR, A. P. Aspectos histológicos do placentomo da vaca zebu (Bos taurus indicus ) no pós-parto. Rev. Bras. Reprod. Anim., v.19, p.161-164, 1995.
- BENNET, H. S., WYRICK, A. D., LEE, S. W. et al. Science and in preparing tissues embedded in plastic for light microscopy, with special reference to glycol methacrylate, glass knives and simple stains. Stain Technol, v.51, p.7197, 1976.
- BJORKMAN, N. Placentaçăo. In: DELLMAN, H.D., BROWN, E.M. Histologia veterinária Rio de Janeiro: Guanabara Koogan, 1982, p.279-294.
- BJORKMAN, N., DANTZER, V. Placentation. In: DELLMAM H.D., BROWN, E.M. Textbook of veterinary histology. 3. ed. Philadelphia, Lea & Febiger,1987. p.340-360
- COLE, M. B., SYKES, S. M. Glycol methacrylate in light microscopy: a routine method for embedding and sectioning animal tissues. Stain Technol., v.49, p.387-400, 1974.
- ELIER, H., HOPKINS, F. M. Successful treatment of retained placenta with umbilical cord injections of collagenase in cows. J. Am. Vet. Med. Assoc., v.203, p.436-443, 1993.
- FERREIRA, R. M. A., CHIARINI-GARCIA, H. Efeito da fixaçăo e do meio de inclusăo na preservaçăo histológica do intestino da traíra, Hoplias malabaricus (Bloch, 1794). Rev. Bras. Cięn. Morfol., v.9, p.3237, 1992.
- FOX, C.H., JOHNSON, F.B., WHITINNG, J. et al. Formaldehyde fixation. J. Histochem. Cytochem., v.33, p.845853, 1985.
- FRANKLIN, R.M., MARTIN, M.T. Staining and histochemistry of undecalcified bone embedded in a watermiscible plast. Stain Technol, v.55, p.313321, 1980.
- FRATER, R. Rapid removal of acid from glycol methacrylate for improved histological embedding. Stain Technol, v.54, p.241243, 1979.
- JUNQUEIRA, L.C.U., SILVA, M.D.A., TORLONI, H. A simple procedure to obtain one-micrometer sections of routinely embedded paraffin material. Stain Technol., v.64, p.39-42, 1995.
- LUNA, L.G. Manual of histologic staining methods of the Armed Forces Institute of Pathology 3.ed. New York: McGraw Hill, 1968.
- MARQUES JÚNIOR, A. P. Leucocyte chemotaxis activity by cotiledons of dairy cows with normal delivery and retained placenta Urbana: University of Illinois, 1988. 182p. (Tese, Doutorado).
- PERRY, J.A. The mammalian fetal membranes. J. Reprod. Fertil., v.62, p.321-335, 1981.
- PROPHET, E.B. Fixation. In: PROPHET, E.B., MILLS, B., ARRINGTON, J.B. et al (eds.) Laboratory methods in histotechology Washington: Armed Forces Institute of Pathology, 1994. p.2528.
- RAMSEY, E.M. The placenta - human and animal. New York: Praeger, 1982. 187p.
- SANTOS, R.L. Estudo morfológico da placenta de vacas leiteiras com liberaçăo normal e com retençăo. Belo Horizonte: Escola de Veterinária da UFMG, 1995. 102p. (Tese, Mestrado).
- STEVEN, D.H. Comparative placentation. London: Academic, 1975. p.25-57.
- SWARTZ, W.J., NUSBICKEL, F.R. Histologic investigation of glycol methacrylate embedded chiek embryonic tissue. J. Microscopy, v.115, p.181185, 1978.
- TROYER, H., NUSBICKEL, F. R. Enzime histochemistry of undecalcified bone and cartilage embedded in glycol methacrylate. Acta Histochem, v.53, p.198207, 1975.
- WOODRUFF, J.M., GREENFIELD, S. A. Advantages of glycol methacrylate embedding systems for light microscopy. J. Histotechnol., v.22, p.164-167, 1979.
Publication Dates
-
Publication in this collection
14 Aug 2000 -
Date of issue
Apr 2000
History
-
Received
17 Nov 1999