Abstracts
A trial was carried out to investigate the susceptibility of seven strains of mice to Strongyloides venezuelensis primary and secondary experimental infections, in order to provide the basis for genetic studies about resistance. Twelve six-week-old male inbred mice of the A/J, BALB/c, CBA/J, C3H/Hepos, C57BL/6, DBA/2 and NIH strains were infected s.c. with 2000 infective larvae. The mean worm counts (± SD) in the small intestine six days after infection were, in increasing order: 28 (± 19) in NIH; 647 (± 228) in BALB/c; 709 (± 425) in DBA/2; 731 (± 151) in C3H/Hepos, 801 (± 174) in CBA/J; 1024 (± 267) in C57BL/6 and 1313 (± 483) in A/J. C57BL/6 mice showed the highest fecal egg counts and NIH, the lowest. No eggs in fecal exams or nematodes in small intestines were recovered from animals reinfected 14 days after primary infection. NIH strain was highly resistant to primary infection by S. venezuelensis. The most susceptible of the other six strains appeared to be the C57BL/6 strain which presented a high nematode counting in intestine and the highest egg output.
Strongyloides venezuelensis; mice; inbred; resistance
Foi investigada a susceptibilidade de sete linhagens isogênicas de camundongos à infecção experimental, primária e secundária, por Strongyloides venezuelensis a fim de servir de base para estudos genéticos sobre a resistência. Foram utilizados 12 camundongos machos, com seis semanas de idade, das seguintes linhagens isogênicas: A/J, BALB/c, CBA/J, C3H/Hepos, C57BL/6, DBA/2 e NIH. Os animais foram inoculados, via sub-cutânea, com 2000 larvas infectantes. As contagens médias (± desvio padrão) de parasitas no intestino delgado dos camundongos seis dias após a infecção, em ordem crescente, foram: 28 (± 19) na linhagem NIH; 647 (± 228) na BALB/c; 709 (± 425) na DBA/2; 731 (± 151) na C3H/Hepos, 801 (± 174) na CBA/J; 1024 (± 267) na C57BL/6 e 1313 (± 483) na A/J. Os camundongos C57BL/6 apresentaram as mais elevadas contagens de ovos de S. venezuelensis por grama de fezes (OPG) e os NIH, as mais baixas. Não foram detectados ovos nos exames de fezes e não foram encontrados parasitas no intestino delgado dos animais re-infectados 14 dias após a infecção primária. A linhagem NIH apresentou elevada resistência contra as infecções primárias por S. venezuelensis. Entre as outras seis linhagens, uma das mais susceptíveis foi a linhagem C57BL/6.
Strongyloides venezuelensis; camundongo; isogênico; resistência
Strongyloides venezuelensis infection susceptibility of seven inbred strains of mice
[Susceptibilidade de sete linhagens isogênicas de camundongos à infecções por Strongyloides venezuelensis]
A.F.T. Amarante, T.C.G. Oliveira-Sequeira*
Departamento de Parasitologia, IB, Universidade Estadual Paulista-Botucatu
Caixa Postal 510
18618-000 - Botucatu, SP
Recebido para publicação em 8 de junho de 2001.
*Autor para correspondência
E-mail: sequeira@ibb.unesp.br
ABSTRACT
A trial was carried out to investigate the susceptibility of seven strains of mice to Strongyloides venezuelensis primary and secondary experimental infections, in order to provide the basis for genetic studies about resistance. Twelve six-week-old male inbred mice of the A/J, BALB/c, CBA/J, C3H/Hepos, C57BL/6, DBA/2 and NIH strains were infected s.c. with 2000 infective larvae. The mean worm counts (± SD) in the small intestine six days after infection were, in increasing order: 28 (± 19) in NIH; 647 (± 228) in BALB/c; 709 (± 425) in DBA/2; 731 (± 151) in C3H/Hepos, 801 (± 174) in CBA/J; 1024 (± 267) in C57BL/6 and 1313 (± 483) in A/J. C57BL/6 mice showed the highest fecal egg counts and NIH, the lowest. No eggs in fecal exams or nematodes in small intestines were recovered from animals reinfected 14 days after primary infection. NIH strain was highly resistant to primary infection by S. venezuelensis. The most susceptible of the other six strains appeared to be the C57BL/6 strain which presented a high nematode counting in intestine and the highest egg output.
Keywords: Strongyloides venezuelensis, mice, inbred, resistance
RESUMO
Foi investigada a susceptibilidade de sete linhagens isogênicas de camundongos à infecção experimental, primária e secundária, por Strongyloides venezuelensis a fim de servir de base para estudos genéticos sobre a resistência. Foram utilizados 12 camundongos machos, com seis semanas de idade, das seguintes linhagens isogênicas: A/J, BALB/c, CBA/J, C3H/Hepos, C57BL/6, DBA/2 e NIH. Os animais foram inoculados, via sub-cutânea, com 2000 larvas infectantes. As contagens médias (± desvio padrão) de parasitas no intestino delgado dos camundongos seis dias após a infecção, em ordem crescente, foram: 28 (± 19) na linhagem NIH; 647 (± 228) na BALB/c; 709 (± 425) na DBA/2; 731 (± 151) na C3H/Hepos, 801 (± 174) na CBA/J; 1024 (± 267) na C57BL/6 e 1313 (± 483) na A/J. Os camundongos C57BL/6 apresentaram as mais elevadas contagens de ovos de S. venezuelensis por grama de fezes (OPG) e os NIH, as mais baixas. Não foram detectados ovos nos exames de fezes e não foram encontrados parasitas no intestino delgado dos animais re-infectados 14 dias após a infecção primária. A linhagem NIH apresentou elevada resistência contra as infecções primárias por S. venezuelensis. Entre as outras seis linhagens, uma das mais susceptíveis foi a linhagem C57BL/6.
Palavras-chave: Strongyloides venezuelensis, camundongo, isogênico, resistência
INTRODUCTION
Livestock gene maps are likely to remain sparsely populated when compared to those of human and mouse where enormous mapping efforts are under way. Thus, in research identifying genes contributing to differences in resistance against parasites, the approach would be to use species with dense physical and genetic maps, such as mice. The availability of a range of genetic resources in this species such as inbred lines, congenic lines, recombinant inbred lines, and well-defined mutants allows the establishment of suitable pedigrees for a wide range of traits. These resources, plus the short generation interval, mean that the genetic basis of many traits can be established quickly (Beh & Maddox, 1996). The conservation of karyotypes between animals also means that it is possible to find a locus influencing a trait in one species and use this information to locate the homologous gene in another species (O'Brien et al., 1993).
Crossing of resistant and susceptible strains can provide valuable information on the dominant or recessive nature of resistance to diseases, as well as indicate the numbers of genes involved and their approximate locations in the genome (Balmain & Nagase, 1998). This approach has been used in mice to map genes that regulate resistance against infections by Plasmodium chabaudi (Fortin et al., 1997; Foote et al., 1997), Leishmania spp. (Roberts et al., 1997; Beebe et al., 1997), Trypanosoma brucei rhodesiense (Seed & Sechelski, 1995), and Trypanosoma congolense (Kemp et al., 1996; Kemp et al., 1997). However, there is a lack of information on the genetic basis of resistance against nematode infections. Therefore, this trial was carried out with seven strains of mice to find one resistant and one susceptible to Strongyloides venezuelensis infections for future genetic studies.
MATERIALS AND METHODS
Six-week-old male inbred mice, 12 from each of the following strains: A/J, BALB/c, CBA/J, C3H/Hepos, C57BL/6, DBA/2, and NIH, were used in this experiment. All mice were purchased from the Centro de Bioterismo Unicamp, except the NIH strain which was obtained from the Biotério do Laboratório de Gnotobiologia e Nutrição - Instituto de Ciências Biológicas - ICB/UFMG.
The strain of S. venezuelensis was isolated from a wild rat in the early 80s in Botucatu, São Paulo State, and has been maintained in the laboratory by repeated passages in Wistar rats. Filariform larvae (L3) were obtained from fecal cultures and adjusted to the appropriate number in distilled water to infect the animals.
Mice were infected subcutaneously with 2000 larvae. The intensity of infection was determined by counting the number of eggs per gram of feces (EPG) by a modified McMaster technique (Gordon & Whitlock, 1939), where each egg counted represented 100 eggs/g. Fecal samples were taken daily until the 14th day after infection. To collect the sample, the animals were randomly allocated in two boxes (six animals per box), where they stayed for three hours. The feces obtained constituted a sample for fecal egg counts (FEC). To eliminate the possibility of mice re-infecting themselves from fecal sources during the experimental period, their cages were changed daily.
Previous results showed that the maximum numbers of worms are recovered 6-7 days after infection (Oliveira-Sequeira & Amarante, 2001). So, six animals from each strain were randomly chosen and sacrificed for nematode counts six days after infection. FEC were carried out on samples taken from the remaining animals. They were re-infected by the same route with 2000 L3 14 days after the first infection. Similar procedures such as daily FEC and worm counts six days after the second infection were performed.
The animals were killed by cervical dislocation and the small intestines were removed and divided into two pieces. Each segment was inverted over a thin wire support. These were then placed in 20ml tubes and incubated in saline for four hours at 37ºC. The wire supports with intestines were then shaken and removed from tubes. Supernatants were discarded and the sediment was preserved with formaldehyde 5%. All nematodes were counted using stereoscopic microscopy.
The data were analysed using a one-way analysis of variance. Significant differences between the group means were determined by Tukey test (Ott, 1992). Statistical significance was taken as P<0.05. Mean FEC and small intestine nematode count values of each strain six days after infection were used to fit a linear regression model and to plot a regression line. Pearson correlation coefficient was also calculated among these data. All analyses were performed using the Minitab Version 11 software.
RESULTS
Eggs of S. venezuelensis started to appear in fecal samples five days after subcutaneous infection in all strains of mice, except NIH that started eliminating eggs within six days (Fig. 1). The patent infection period ranged from five (CBA/J) to 10 days (DBA/2 and C57BL/6) (Table 1). The NIH strain showed the lowest FEC and the C57BL/6, the highest (Fig. 1). Trends in fecal egg output differed slightly among strains. For example, A/J strain showed a peak in FEC five days post-infection and C57BL/6 by eight days post-infection (Fig. 1). In most strains, peak in FEC occurred seven days post-infection.
NIH mice presented the lowest mean intestinal S. venezuelensis count, significantly lower than those recorded in all the other strains (P<0.05). A/J and C57BL/6 showed the highest worm count: 1313 (±483) and 1024 (±267), respectively (Table 1). The count for A/J was significantly (P<0,05) higher than BALB/c, DBA/2 and C3H/Hepos.
No eggs in fecal exams or nematodes in small intestine were recovered from animals reinfected 14 days after primary infection.
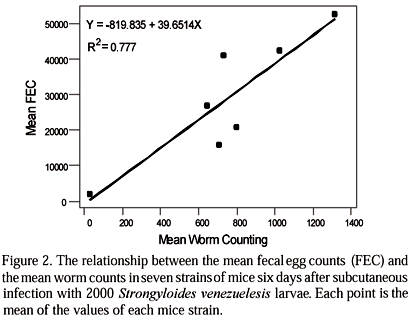
Fig. 2 shows a linear regression model and a plot of regression line using mean FEC and worm count values of each strain six days after infection. The correlation coefficient between these two parameters was high (r=0.88). This result showed that FEC is a good predictor of the nematode burden in mice infected with S. venezuelensis.
DISCUSSION
The NIH strain proved to be highly resistant against primary infection by S. venezuelensis in comparison with the other six mice strains. It showed a low worm count and low egg output in feces. Martins et al. (2000) also found low FEC in germ free and conventional NIH mice infected with 500 S. venezuelensis larvae. The conventional NIH strain, the same used in this trial, showed a low FEC (the highest FEC was less than 800) and a mean of only 16.2 worms in the intestine seven days after infection (Martins et al., 2000).
The NIH strain is described as also resistant to T. muris, T. spiralis and N. dubius infections (Wakelin, 1975; Else & Wakelin, 1988; Goyal & Wakelin, 1993; Wakelin & Donachie, 1983; Behnke & Wakelin, 1977). NIH mice are considered more immunoresponsive to infection by Toxocara canis than CD1 mice (Abo-Shehada et al., 1991). The NFR/N and NFS/N, inbred strains derived from NIH Swiss mice, have shown rapid expulsion of T. spiralis when previously immunised, while mice of the Balb/c, CBA, A/He, C3H, SJL and C57BL were "nonresponders" (Bell et al., 1982).
Based on FEC, the C57BL/6 strain showed the highest susceptibility toS. venezuelensis infection. These results are in agreement with the literature which describes findings of its highest susceptibility to S. venezuelensis (Sato & Toma, 1990), Strongyloides ratti (Dawkins et al., 1980) and Trichuris muris (Else & Wakelin, 1988). This strain was also susceptible to Trichinella spiralis infections while NIH was resistant (Goyal & Wakelin, 1993; Wakelin & Donachie, 1983).
However, opposite results are described for infections by the larval stages of cestodes. C57BL/6 or C57BL/10 strains, susceptible to most nematodes, are shown highly resistant to infections by Mesocestoides corti (White et al., 1982; Lammas et al., 1990) and Taenia taeniaeformis (reviewed by Mitchell, 1979; Mitchell et al., 1980) while the NIH strain, resistant to nematodes, is considered susceptible to Mesocestoides corti infection (Lammas et al., 1990).
After a primary infection by subcutaneous inoculation with various doses of S. venezuelensis into C57BL/6 mice, about 50% of the initial dose of infective larvae became intestinal adult worms (Khan et al., 1993; Kobayashi et al., 1998). Similar results were observed in C57BL/6 mice in this trial.
Secondary infection did not produce a patentinfection in any of the strains tested in this trial. Sato & Toma (1990) also did not detect eggs of S. venezuelensis in feces of BALB/c mice receiving a secondary challenge infection 14 days after the first. These results show that a solid immune response develops after a primary infection in mice independent of susceptibility to primary infections.
From the six inbred mice strains studied, C57BL6 proved to be highly susceptible to S. venezuelensis infection while NIH was highly resistant. The extreme genetic variability between these strains to resistance makes crosses between them an excellent rodent model to map quantitative trait loci affecting resistance against S. venezuelensis. Therefore, these two strains will be very useful in studies on the genetic basis of variation in resistance against primary infections by S. venezuelensis.
ACKNOWLEDGEMENTS
Thetechnical assistance of Mrs. M.A.B. Gomes is gratefully acknowledged. The authors are also grateful to Dr. J. R. Nicoli and Mrs. Jacqueline I. A. Leite for providing the NIH strain of mice.
- ABO-SHEHADA, M.N.; AL-ZUBAIDY, B.A.; HERBERT, I.V.Acquired immunity to Toxocara canis infection in mice. Vet. Parasitol., v.38, p.289-298, 1991.
- BALMAIN, A.; NAGASE, H.Cancer resistance genes in mice: models for the study of tumour modifiers. Trends Genet., v.14, p.139-144, 1998.
- BEEBE, A.M.; MAUZE, S.; SCHORK, N.J.et al. Serial backcross mapping of multiple loci associated with resistance to Leishmania major in mice. Immunity, v.6, p.551-557, 1997.
- BEH, K.J.; MADDOX, J.F. Prospects for development of genetic markers for resistance to gastrointestinal parasite infection in sheep. Int. J. Parasitol., v.26, p.879-897, 1996.
- BEHNKE, J.M.; WAKELIN, D. Nematospiroides dubius: stimulation of acquired immunity in inbred strains of mice. J. Helminthol., v.51, p.167-176, 1977.
- BELL, R.G.; MCGREGOR, D.D.; ADAMS, L.S.Trichinella spiralis: characterization and strain distribution of rapid expulsion in inbred mice. Exp. Parasitol., v.53, p.301-314, 1982.
- DAWINKS, H.J.S.; GROVE, D.I.; DUNSMORE, J.D.et al. Strongyloides ratti: susceptibility to infection and resistance to reinfection in inbred strains of mice as assessed by excretion of larvae. Int. J. Parasitol., v.10, p.125-129, 1980.
- ELSE, K.; WAKELIN, D.The effects of H-2 and non-H-2 genes on the expulsion of the nematode Trichuris muris from inbred and congenic mice. Parasitology, v.96, p.543-550, 1988.
- FOOTE, S.J.; BURT, R.A.; BALDWIN, T.M. et al. Mouse loci for malaria-induced mortality and the control of parasitaemia. Nat. Genet., v.17, p.380-381, 1997.
- FORTIN, A.; BELOUCHI, A.; TAM, M.F.et al. Genetic control of blood parasitaemia in mouse malaria maps to chromosome 8. Nat. Genet., v.17, p.382-383, 1997.
- GORDON, H.M.; WHITLOCK, H.V. A new technique for counting nematode eggs in sheep feces. J. Coun. Scient. Ind. Res., v.12, p.50-52., 1939.
- GOYAL, P.K.; WAKELIN, D. Influence of variation in host strain and parasite isolate on inflammatory and antibody responses to Trichinella spiralis in mice. Parasitology, v.106, p.371-378, 1993.
- KEMP, S.J.; DARVASI, A.; SOLLER, M.et al. Genetic control of resistance to trypanosomiasis. Vet. Immunol. Immunopathol., v.54, p.239-243, 1996.
- KEMP, S.J.; IRAQI, F.; DARVASI, A.et al. Localization of genes controlling resistance to trypanosomiasis in mice. Nat. Genet., v.16, p.194-196, 1997.
- KHAN, A.I.; HORII, Y.; TIURIA, R.et al. Mucosal mast cells and the expulsive mechanisms of mice against Strongyloides venezuelensis Int. J. Parasitol., v.23, p.551-555, 1993.
- KOBAYASHI, T.; TSUCHIYA, K.; HARA, T.et al. Intestinal mast cell response and mucosal defence against Strongyloides venezuelensis in interleukin-3-hyporesponsive mice. Parasite Immunol., v.20, p.279-284, 1998.
- LAMMAS, D.A.; MITCHELL, L.A.; WAKELIN, D.Genetic influences upon eosinophilia and resistance in mice infected with Mesocestoides corti Parasitology, v.101, p.291-299, 1990.
- MARTINS, W.A.; MELO, A.L.; NICOLI, J.R.et al. A method of decontaminating Strongyloides venezuelensis larvae for the study of strongyloidiasis in germ-free and conventional mice. J. Med. Microbiol., v.49, p.387-390, 2000.
- MITCHELL, G.F. Responses to infection with metazoan and protozoan parasites in mice. Adv. Immunol., v.28, p.451- 511, 1979.
- MITCHELL, G.F.; RAJASEKARIAH, G.R.; RICKARD, M.D.A mechanism to account for mouse strain variation in resistance to the larval cestode, Taenia taeniaeformis. Immunology, v. 39,p.481-489, 1980.
- O'BRIEN, S.J.; WOMACK, J.E.; LYONS, L.A.et al. Anchored reference loci for comparative genome mapping in mammals. Nat. Genet., v.3, p.103-112, 1993.
- OLIVEIRA-SEQUEIRA, T.C.G.; AMARANTE, A.F.T. Dynamics of Strongyloides venezuelensis infection and relationship between fecal egg counts and parasite burden in Swiss mice. Rev. Bras. Med. Vet., v.23, p.99-102, 2001.
- OTT, R.L. An introduction to statistical methods and data analysis 4 ed. Wadsworth, Belmont, 1992. 1051p.
- ROBERTS, L.J.; BALDWIN, T.M.; CURTIS, J.M.et al.Resistance to Leishmania major is linked to the H2 region on chromosome 17 and to chromosome 9. J. Exp. Med., v.185,p.1705-1710, 1997.
- SATO, Y.; TOMA, H. Strongyloides venezuelensis infections in mice. Int. J. Parasitol., v.20, p.57-62, 1990.
- SEED, J.R.; SECHELSKI, J. The inheritance of factors controlling resistance in mice infected with Trypanosoma brucei rhodesiense. J. Parasitol., v.81, p.653-657, 1995.
- WAKELIN, D. Genetic control of immune responses to parasites: immunity to Trichuris muris in inbred and random-bred strains of mice. Parasitology, v.71, p.51-60, 1975.
- WAKELIN, D.; DONACHIE, A.M.Genetic control of eosinophilia. Mouse strain variation in response to antigens of parasite origin. Clin. Exp. Immunol., v.106, p.239-246, 1983.
- WHITE, T.R.; THOMPSON, R.C.A.; PENHALE, W.J.A comparative study of the susceptibility of inbred strains of mice to infection with Mesocestoides corti. Int. J. Parasitol., v.12, p.29-33, 1982.
Publication Dates
-
Publication in this collection
16 Dec 2002 -
Date of issue
June 2002
History
-
Received
08 June 2001