Abstracts
The consequences of complete removal of the sclerocorneal limbus epithelium were evaluated, as well as the model for such, in which n-heptanol and superficial keratectomy were used. The association of n-heptanol and keratectomy allowed a complete excision of stem cells. Moreover, the destruction of these cells led to significant tectonic alterations that could be employed as a feasible model for clinical experimentation of corneal transplantation.
dog; stem cell; cornea
As conseqüências da completa remoção do epitélio do limbo esclerocorneano foram avaliadas, bem como o modelo utilizado para tal, em que se empregou o n-heptanol e a ceratectomia superficial. O n-heptanol e a ceratectomia associados possibilitaram a completa excisão das células-tronco. Da destruição dessas células decorrem alterações tectônicas significativas factíveis para estudos dos transplantes de córnea.
cão; células-tronco; córnea
VETERINARY MEDICINE
Excision of sclerocorneal limbus in dogs and resulting clinical events. Study of an experimental model
Excisão do limbo esclerocorneano de cães e eventos clínicos decorrentes. Estudo de um modelo experimental
A.T.J. BrunelliI; F.A.M. VicentiI; A. OriáI; C.F. CamposI; F.A. Doria NetoI; J.L. LausII, * * autor para correspondência ( corresponding author) E-mail: jllaus@fcav.unesp.br
IAluno de pós-graduação FCAV UNESP
IIFaculdade de Ciências Agrárias e Veterinárias - UNESP Via de Acesso Prof. Paulo Donato Castelanne, s/n 14884-900 Jaboticabal, SP
ABSTRACT
The consequences of complete removal of the sclerocorneal limbus epithelium were evaluated, as well as the model for such, in which n-heptanol and superficial keratectomy were used. The association of n-heptanol and keratectomy allowed a complete excision of stem cells. Moreover, the destruction of these cells led to significant tectonic alterations that could be employed as a feasible model for clinical experimentation of corneal transplantation.
Keywords: dog, stem cell, cornea
RESUMO
As conseqüências da completa remoção do epitélio do limbo esclerocorneano foram avaliadas, bem como o modelo utilizado para tal, em que se empregou o n-heptanol e a ceratectomia superficial. O n-heptanol e a ceratectomia associados possibilitaram a completa excisão das células-tronco. Da destruição dessas células decorrem alterações tectônicas significativas factíveis para estudos dos transplantes de córnea.
Palavras-chave: cão, células-tronco, córnea
INTRODUCTION
Ocular surface is composed of corneal and conjunctival epithelia (Tsai et al. 1990). Limbus is a transitional zone between cornea and sclera (Swift et al., 1996). The limbal epithelium prevents both the growth and the migration of conjunctival epithelial cells onto the cornea (Dua, 1998). It differs morphologically from the cornea in that it possesses Langerhans cells and melonocytes, and from the conjunctiva in that it lacks a layer of goblet cells (Chen and Tseng, 1990). The limbus is considered a specialized conjunctiva, which is composed of over 10 layers of cells (Wagoner, 1997; Akped and Foster, 1999).
The corneal epithelium constantly renews and regenerates. In the dog, the renewal occurs in about one week (Schoenau and Pippi, 1993). The cells of its outer layer are constantly desquamating and being replaced by basal cells that proliferate when in contact with the basal membrane. In 1983, Thoft et al., cited by Lauweryns et al. (1993), proposed the X, Y, Z hypothesis, which would explain how the maintenance and integrity of the corneal epithelium surface is processed. The desquamated epithelial cells (Z component) would be continuously renewed by the proliferation of basal cells (X component) and by the proliferation and centripetal migration of the limbal cells (stem cells - Y component). This hypothesis states that for a steady state in the regeneration to be maintained, X + Y must equal to Z.
The stem cells are mainly located in the basal portion of the limbus (Haamann et al., 1998), as it was demonstrated by a model of exclusive expression of 64 KD (cytokeratin 3) and 55KD (cytokeratin 12) keratins, using monoclonal antibodies (Schermer et al., 1986). Cytokeratin 3 (K3) is present only in the peripheral part of the corneal epithelium. The absence of K3 in the limbal cells indicates that they are less differentiated than the epithelium of the cornea. Cotsarelis et al. (1989) confirmed the presence of stem cells in the limbus by administering tritiated thymidine, via the stimulation of corneal and limbal cells with a tumor promoter. The authors indicated that limbal epithelium has therefore a higher potential of proliferation when compared with central cornea.
Stem cells are responsible for tissue replacement and regeneration (Kruse et al., 1990; Akped and Foster, 1999). They are essential in maintaining the integrity of corneal surface, by promoting its renewal in healthy states and reepithelialization in wound healing processes (Huang and Tseng, 1991; Swift et al., 1996; Haamann et al., 1998; Dua and Azuara-Blanco, 1999).
Limbal deficiency or loss of stem cells are characterized by a reduction of proliferative capacity, resulting in abnormal corneal surface (Akped and Foster, 1999). In such occasions, there is growth of the conjunctival epithelium onto the corneal surface (conjunctivalization), as well as chronic inflammation, superficial vascularization, calcification, ulceration, melting and perforation of the cornea (Swift et al., 1996; Dua and Azuara-Blanco, 1999; Dua and Azuara-Blanco, 2000). When corneal surface is covered by conjunctival epithelium, goblet cells can be seen in this specialized part of the fibrous tunic (Dua, 1998).
Taking into account that partial or total destruction of the stem cells of the sclerocorneal limbus results in very significant clinical alterations of the cornea, as well as in healing of damaged tissues, this article was conceived to investigate the proposal of an experimentation model of limbal destruction, as well as the unexpected consequences, aiming at researches of limbal or corneal transplantation procedures.
MATERIAL AND METHODS
A total of 18 adult mongrel male and female healthy dogs were used. In order to have homogeneous groups, all dogs included in this research underwent clinical assessment. For such, they were submitted to routine clinical evaluation, which included physical examination, complete blood count, slit-lamp biomicroscopy1 1 Portable stit-lamp SL- 14 Kowa. , and binocular indirect ophthalmoscopy2 2 Indirect ophthalmoscope FCV- 2000 Eyetec. , as well as applanation tonometry3 3 Tono-pen XL, Mentor®. , Schirmer's tear test4 4 Schirmer's tear test, Ophthalmos® - Ophthalmos Indústrias e Comércio de Produtos Farmacêuticos Ltda. , and fluorescein test5 5 Fluorescein Strips Ophthalmos® - Ophthalmos Indústrias e Comércio de Produtos Farmacêuticos Ltda. .
The bioethical handling of animals followed the rules given by the Association for Research in Vison and Ophthalmology (ARVO) National Institutes of Health Publications No 85-23: Revised 1985, according to the NÜREMBERG code (Goldim, 1995).
Food and water were withheld for an 8-hour period prior to the operative procedures. Animals were pre-medicated with intravenously levomepromazine6 6 Neozine®- Laboratório Rhodia. at 1mg/kg of body weight, followed by anesthetic induction with sodium thiopental7 7 Thiopentax®- Laboratório Cristália Produto químico e Farmacêutico Ltda. at 12.5mg/kg of body weight given intravenously. Anesthesia was thus maintained with halogenated anesthetic agent8 8 Fluotane® - Laboratório Astrazeneca do Brasil Ltda. in oxygen, delivered via a closed circle system, in the third plane of the third stage of anesthesia, after Guedel classification.
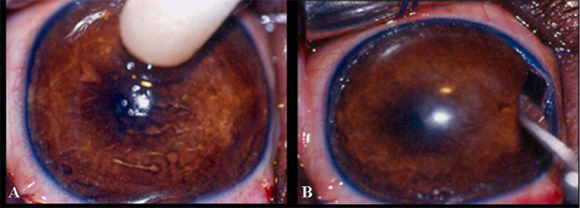
The destruction of stem cells was performed in only one eye (right eye). For such, a sterile cotton tip wet with n-heptanol solution9 9 N-heptanol solution Laboratório MerckSchuchardt, Hohenbrunn, Munique. was applied to the eye in a circular fashion, beginning centrally and extending to the bulbar conjunctiva (Fig. 1A), for 120 seconds. The area was rinsed with sterile saline solution10 10 Sterile 0.9% saline solution - JP Indústria Farmacêutica S.A. for 120 seconds to remove excess n-heptanol. Since the procedure alone is not able to completely remove stem cells, 360º lamellar keratectomy was employed as an ancillary method (Tsai et al., 1990).
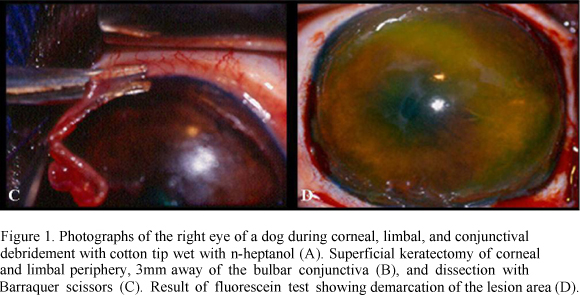
Keratectomy was started after routine preparation, protection, and antiseptic treatment of the operative field with 10% buffered polyvinylpyrrolidone iodine11 11 Marcodine Tópico® -Innovatec -Divisão de Cristália Produtos Farmacêuticos Ltda. solution (PVPI) diluted in saline solution (1/50). Blepharostat and mechanical fixation of the ocular bulb were then done. Under six-time magnification provided by the operating microscope12 12 Microscope MC-M 900-D.F. Vasconcellos-S.A. , the limbal zone was excised in a 360º lamellar pattern, removing approximately 2mm of the cornea and 3mm of the conjunctiva. To allow this procedure, it was used a no.66 Beaver blade13 13 #66 Beaver blade Lâmina Alcon Surgical blade®. (Fig. 1B) and Barraquer scissors (Fig. 1C). During the operation, ocular surface was systematically irrigated with sterile 0.9% saline solution10. After surgery, fluorescein test was performed to delimit the excised area (Fig. 1D).
After the surgical maneuvers, the animals wore Elizabethan type collars. All subjects were prophylactically given topical tobramicine14 14 Tobrex®- Alcon laboratórios do Brasil. and nonsteroidal anti-inflammatory based on flurbiprofen15 15 Ocufen® Laboratório Allergan-Frumtost. , both at regular 6-hour intervals for 14 consecutive days.
To reduce ciliary spasm, atropine16 16 Atropine 1%®- Laboratório Allergan- Lok. was instilled at 12-hour intervals for 3 days, followed by use, every 24 hours, for three more days. It was also instituted analgesic and anti-inflammatory therapy with oral meloxicam17 17 Maxicamâ Laboratório Ouro Fino at 0.1mg/kg of body weight, daily, for 5 days.
Animals were evaluated once a day, for 30 days. They were examined for signs of neovascularization, opacity, pigmentation and granulation of the cornea, and conjunctivalization. To do so, the criteria of subjective quali-quantitative analysis was adopted, in which NIHIL represented an absence of any of the events, (+) mild manifestations of signs, (++) moderate signs, and (+++) severe signs. Slit-lamp1 biomicroscopy and fluorescein test were used for evaluation of the eye after 3, 7, 14, and 30 days of limbal destruction.
RESULTS
In the first three days, corneal edema was mild in 83.4% of the animals, and moderate in 16.7%. In the subsequent moments (days 7 and 14) this phenomenon evolved progressively, being mild in 33.3% of the animals, moderate in 22.2% of the dogs, and severe in 44.4% of the animals. At day 30, 55.6% of the animals manifested it mildly, 33.3% in a moderate fashion, and 5.6% in a severe pattern. It was not present in 5.6% of the animals.
In all animals, corneal neovascularization developed 7 days after destruction of the limbus. In 83.3% of the cases, it was localized in the peripheral cornea, while in 16.7% the neovessels were about 4mm away from the limbus. At day 14, the vessels were limited to 2mm away from the limbus in 33.3% of the dogs, to 2mm in 33.3%, and to 6mm in the remaining (33.3%). At day 30, this clinical sign was still present in all animals. Most of the dogs (72.2%) presented vessels directed toward the corneal center. In 27.8% of the animals, the distance of the corneal neovessels to the limbus was not greater than 4mm.
Corneal pigmentation was observed after 30 days in 61.1% of the animals in a mild pattern, and in 11.1% of the dogs in a moderate fashion. In the remaining (27.8%), pigmentation of the cornea was not seen.
Seven days after destruction of the limbus, corneal conjunctivalization started in 27.8% of the animals. At day 14, such sign was observed in 66.7% of the evaluated animals. During the period, conjunctivalization was not seen in 33.3% of the dogs. At day 30, all animals presented 360º corneal conjunctivalization.
Granulation tissues were observed after 7 days, being mild (22.2%), moderate (11.1%), or severe (22.22%). In 44.4% of the animals, it was not present in the period. After 14 days, it was absent in 33.3% of the dogs, mildly present in 16.7% of the subjects, moderately present in 5.6%, and severely present in 50% of the dogs. At day 30, 61.1% of the dogs did not show granulation tissue. The manifestation was mild in 16.7% of the animals, moderate in 5.6%, and severe in 16.7% of the dogs.
Superficial ulcerative keratitis was observed until day 14. One dog presented severe keratitis. The most representative clinical signs were mucopurulent ocular discharge, deep corneal neovessels, severe corneal edema, granulation, and thickening of the cornea. At day 30, it was noted a diminishment of clinical manifestation. The clinical evolution is presented in Fig. 2 (A, B, C, D, E, and F).
DISCUSSION
There are no reports proposing a model of limbal destruction in dogs. It was opted for studying a model for this species, whose corneal and limbal anatomic features not always admit safe extrapolation of results of previous researches on human being.
Several models have been proposed aiming at simulating diseases of the ocular surface. The effectiveness of clinical and surgical therapies is best evaluated when a species-to-species comparison is done. The experimental destruction of the limbus is performed to produce corneal alterations that can simulate what occurs accidentally, so that future studies regarding therapeutic variants are possible.
Several protocols have been described, highlighting chemical (n-heptanol, iodine, sodium hydroxide solution) and mechanical debridement (corneal scarification, superficial keratectomy) (Kruse et al., 1990). Chemical debridement using n-heptanol removes corneal epithelium with no damage to the basal membrane (Cintron et al., 1979, cited by Kruse et al., 1990).
It was performed lamellar dissection followed by the use of n-heptanol to evaluate the reserve of stem cells (Huang and Tseng, 1991) and to develop vascularization of the corneas (Tsai et al., 1990). Superficial lamellar keratectomy of the conjunctiva, limbus, and peripheral cornea, according to description of Huang and Tseng (1991), was performed in the current study after chemical debridement using n-heptanol for 120 seconds. Studies from Kruse et al. (1990) using only n-heptanol for 120 seconds were not able to completely destruct stem cells, despite all of them were removed. In accordance with results obtained by Kruse et al. (1990) after chemical and mechanical debridement, the animals of this research presented progressive corneal vascularization and conjunctivalization.
The growth of conjunctival epithelium toward the cornea (conjunctivalization) results from loss of the limbal epithelium and its stem cells (Chen and Tseng, 1990). The start of the conjunctivalization was observed after 7 days of destruction of the limbus, so that at day 30, it was seen in all animals.
Tsai et al. (1990) reported that vascularization results from removal of stem cells and presents varying degrees according to the extension of removal. These authors observed corneal neovascularization owing to complete limbal destruction in all evaluated subjects. The findings of this study included centripetal vascular neoformation in 72.2% of the animals, while in the remaining dogs it reached 4mm beyond the limbus.
Edema led to partial loss of corneal transparency in all animals during the initial periods. At day 30, edema was still observed in most of the animals, corroborating description of Swift et al. (1996) in rabbits.
In the absence of stem cells, severe corneal granulation occurs, which confirms the importance of those cells and their function (Tseng et al., 1990). In this research, exuberant granulation was observed in 38.9% of the dogs after 30 days of limbal destruction.
CONCLUSIONS
The experimental model using n-heptanol associated with superficial keratectomy, involving 3mm of conjunctival-limbal surface and 2mm of corneal periphery, showed satisfactory destruction of stem cells of the sclerocorneal limbus of dogs, leading to the development of corneal neovascularization and conjunctivalization. The results substantiate that the model herein evaluated is feasible for future studies concerning techniques of corneal and limbal transplantation, aiming at treating lesions in which significant loss of stem cells is determined.
ACKNOWLEDGMENTS
Research supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPQ).
Recebido em 10 de setembro de 2004.
Aceito em 25 de outubro de 2005
- AKPED, E.K.; FOSTER, C.S. Limbal stem cells transplantation. Int. Ophthalmol. Clin., v.39, p.71-81, 1999.
- CHEN J.J.Y.; TSENG, S.C.G. Corneal epithelial wound healing in partial limbal deficiency. Invest. Ophthalmol. Visual Sci., v.31, p.1301-1314, 1990.
- CITRON, C.; HASSINGER, I.; KUBLIN, C.I. et al. A simple method for the removal of rabbit corneal epithelium utilizing n-heptanol. Ophtalmic Res., v.11, p.90, 1979.
- COTSARELIS, G.; CHEN, S.Z.; DONG, G. et al. Existence of slow-cycling limbal epithelial basal cells that can be preferentially stimulated to proliferate: implications on epithelial stem cells. Cell, v.57, p.201-209, 1989.
- DUA, H.S. The conjuntival in corneal epithelial wound healing. Br. J. Ophthalmol., v.82, p.1407-1411, 1998.
- DUA, H.S.; AZUARA-BLANCO, A. Allo-limbal transplantation in patients with limbal stem cell deficiency. Br. J. Ophthalmol., v.83, p.414-419, 1999.
- DUA, H.S.; AZUARA-BLANCO, A. Limbal stem cells of corneal epithelium Surv. Ophthalmol., v.44, p.415-425, 2000.
- GOLDIM, J.R. Pesquisa em saúde e direitos dos animais Porto Alegre: HCPA, 1995. 28p.
- HAAMANN, P.; JENSEM, O.M.; SCHIMIDT, P. Limbal autograft transplantation. Acta Ophthalmol. Scand., v.76, p.117-118, 1998.
- HUANG, A.J.W.; TSENG, S.C.G. Corneal epithelial wound healing in the absence of limbal epithelium. Invest. Ophthalmol. Visual Sci., v.32, p.96-105, 1991.
- KRUSE, F.E.; CHEN, J.J.Y.; TSAI, R.J. et al. Conjunctival transdifferentiation is due to the incomplete rmoval of limbal basal epithelium. Invest. Ophthalmol. Visual Sci., v.31, p.1903-1913, 1990.
- LAUWERYNS, B.; van den OORD, J.J.; de VOS, R. et al. A new epitelial cell type in the human cornea. Invest. Ophthalmol. Visual Sci., v.34, p.1983-1990, 1993.
- SCHERMER, A.; GALVIN, S.; SUN, T.T. Differentiation-related expression of a major 64K corneal keratin in vivo and in culture suggests limbal location of corneal epithelial stem cells. J. Cell Biol., v.103, p.49-62, 1986.
- SCHOEUNAU, L.S.F.; PIPPI, N.L. Aspectos morfológicos e funcionais da córnea: uma breve revisão. Hora Vet., v.12, p.49-53, 1993.
- SWIFT, G.J.; AGGARWAL, R.K.; DAVIS, G.J. et al. Survival of rabbit limbal stem cell allografts. Transplantation, v.62, p.568-574, 1996.
- TSAI, R.J.; SUN, T.T.; TSENG, S.C.G. Comparison of limbal and conjunctival autograft transplantion in corneal surface reconstruction in rabbits. Ophthalmology, v.97, p.446-455, 1990.
- TSENG, S.C.G.; CHEN, J.J.; HUANG, A.J. et al. Classification of conjunctival surgereis for corneal disease based on stem cell concept. Ophthalmol. Clin. North Am., v.3, p.595-611, 1990.
- WAGONER, M.D. Chemical injuries of the eye: current concepts in pathophysiology and therapy. Surv. Ophthalmol., v.41, p.275-314, 1997.
Publication Dates
-
Publication in this collection
19 Apr 2006 -
Date of issue
Feb 2006
History
-
Accepted
25 Oct 2005 -
Received
10 Sept 2004