Abstracts
An immunoistochemical (IHC) test was developed to detect bovine respiratory syncytial virus (BRSV) in cell cultures and tissues of experimentally infected mice and calves, using a commercial monoclonal antibody (Mab) against human respiratory syncytial virus (HRSV), as a less expensive alternative, instead of producing specific monoclonal antibodies to BRSV. Clinical samples from calves suffering respiratory disease were also submitted to this test. IHC detected BRSV antigens in mouse tracheas (3, 5 and 7 days post-infection) and lungs (5 and 7 days post-infection), and in one of three lungs from experimentally infected calves. Lungs samples from two naturally infected calves were tested and resulted positive for BRSV by the IHC test. These results suggest that this test may be used in the future for diagnosis as well as a useful tool to assess the distribution of BRSV infections in Brazilian herds.
bovine respiratory syncytial virus; immunohistochemistry; calf; mice; experimental infection; monoclonal antibody
Desenvolveu-se um teste de imunohistoquímica (IHQ) para detecção do vírus respiratório sincicial bovino (BRSV) multiplicado em cultivo celular e em tecidos de camundongos e bezerros infectados experimentalmente, utilizando um anticorpo monoclonal comercial contra o vírus respiratório sincicial humano (HRSV), como uma alternativa para eliminar os custos de produção de anticorpos monoclonais específicos para o BRSV. Amostras clínicas de bezerros com sintomatologia respiratória foram analisadas. A técnica mostrou-se eficiente na detecção de antígenos do BRSV em traquéias (3, 5 e 7 dias pós-infecção) e pulmões (5 e 7 dias pós-infecção) dos camundongos infectados e em uma das três amostras de pulmões dos bezerros infectados experimentalmente. Amostras de pulmões de dois animais com infecção natural foram positivas para BRSV. Conclui-se que o teste de IHQ pode ser usado no diagnóstico das infecções por BRSV e na avaliação da distribuição dessas infecções nos rebanhos bovinos brasileiros.
vírus respiratório sincicial bovino; imunoistoquímica; bezerros; camundongos; infecção experimental; anticorpo monoclonal
VETERINARY MEDICINE
Bovine respiratory syncytial virus: immunohistochemichal detection in mouse and bovine tissues using a Mab against human respiratory syncytial virus
Vírus respiratório sincicial bovino: detecção por imunoistoquímica em tecidos de camundongos e bovinos usando AcM contra o vírus respiratório sincicial humano
R.S. AlmeidaI; F.R. SpilkiI; P.M. RoeheII, III; L.M.C. VerinaudI; C.W. ArnsI, * * Corresponding author ( autor para correspondência) E-mail: arns@unicamp.br
IDepartamento de Microbiologia e Imunologia - IB-UNICAMP Caixa Postal 6109 13083-970 - Campinas, SP
IIDepartamento de Microbiologia - UFRGS, Porto Alegre, RS
IIICentro de Pesquisas Veterinárias Desidério Finamor Porto Alegre, RS
ABSTRACT
An immunoistochemical (IHC) test was developed to detect bovine respiratory syncytial virus (BRSV) in cell cultures and tissues of experimentally infected mice and calves, using a commercial monoclonal antibody (Mab) against human respiratory syncytial virus (HRSV), as a less expensive alternative, instead of producing specific monoclonal antibodies to BRSV. Clinical samples from calves suffering respiratory disease were also submitted to this test. IHC detected BRSV antigens in mouse tracheas (3, 5 and 7 days post-infection) and lungs (5 and 7 days post-infection), and in one of three lungs from experimentally infected calves. Lungs samples from two naturally infected calves were tested and resulted positive for BRSV by the IHC test. These results suggest that this test may be used in the future for diagnosis as well as a useful tool to assess the distribution of BRSV infections in Brazilian herds.
Keywords: bovine respiratory syncytial virus, immunohistochemistry, calf, mice, experimental infection, monoclonal antibody
RESUMO
Desenvolveu-se um teste de imunohistoquímica (IHQ) para detecção do vírus respiratório sincicial bovino (BRSV) multiplicado em cultivo celular e em tecidos de camundongos e bezerros infectados experimentalmente, utilizando um anticorpo monoclonal comercial contra o vírus respiratório sincicial humano (HRSV), como uma alternativa para eliminar os custos de produção de anticorpos monoclonais específicos para o BRSV. Amostras clínicas de bezerros com sintomatologia respiratória foram analisadas. A técnica mostrou-se eficiente na detecção de antígenos do BRSV em traquéias (3, 5 e 7 dias pós-infecção) e pulmões (5 e 7 dias pós-infecção) dos camundongos infectados e em uma das três amostras de pulmões dos bezerros infectados experimentalmente. Amostras de pulmões de dois animais com infecção natural foram positivas para BRSV. Conclui-se que o teste de IHQ pode ser usado no diagnóstico das infecções por BRSV e na avaliação da distribuição dessas infecções nos rebanhos bovinos brasileiros.
Palavras-chave: vírus respiratório sincicial bovino, imunoistoquímica, bezerros, camundongos, infecção experimental, anticorpo monoclonal
INTRODUCTION
Bovine respiratory syncytial virus (BRSV), which belongs to the Pneumovirus genus, Paramyxoviridae family, is one of the most important pathogens of respiratory tract in nursing beef and dairy calves (Larsen, 2000). This virus is widely distributed in most countries, mainly in intensive cattle production, including Brazil. The first viral isolation in this country was performed in 1995, from the nasotracheal secretions of calves raised in Southern and Southeastern regions (Arns et al., 2003). Moreover, although serological tests for BRSV showed 95% positivity (Gonçalves et al., 1993), the exact prevalence and importance of such viral infection in production losses in Brazilian herds is not known so far.
There are frequent difficulties in the laboratory for diagnosis of infections caused by respiratory syncytial viruses (RSVs) due to limited growth of virus in cell cultures or in most experimental animals, as well as of the lability viral particle under environmental conditions. Currently, the diagnosis of BRSV infections is most commonly done by clinical and histopathological examinations, by the detection of virus-specific antibodies in paired sera using methods such as seroneutralization and by virus isolation (Westenbrink et al., 1985; Westenbrink et al., 1987; Dubovi, 1993; Driemeier et al., 1997). However, difficulties with these tests include the long time needed to obtain results (Dubovi, 1993), reports of asymptomatic infections that are serologically undetectable (Collins et al., 1996) and evidence indicating the existence of persistent infections (Ames, 1993).
The aim of this work was to develop an immunohistochemical (IHC) test for BRSV detection, using reagents commercially available as a monoclonal antibody (Mab) against human respiratory syncytial virus (HRSV) which could eliminate the costs linked to development of reagents, mainly those related to monoclonal antibodies production and, consequently, improve the diagnosis of this virus in Brazil.
MATERIAL AND METHODS
The Brazilian strain BRSV-25-BR, isolated at the Laboratório de Virologia Animal at the Instituto de Biologia, Universidade Estadual de Campinas - Campinas, SP (LVA-IB-UNICAMP; Arns et al., 2003), was used in this study.
Four-week-old specific-pathogen-free (SPF) female Balb/c mice, purchased from the Centro Multidisciplinar de Investigação Biológica (CEMIB-UNICAMP), were used for experimental inoculations. Mice were distributed in two groups, one containing 16 animals and the other eight animals (negative control). Good quality water and food were provided ad libitum to the animals. All mice in the first group were intranasally inoculated with 100ml of the virus with an infectious dose of 104.3 tissue culture infective doses per millilitre (TCID50/ml). The negative controls received the same volume of supernatant from uninfected chicken embryo related cells (CER). The animals were daily observed for clinical signs and euthanised using humanitarian procedures avoiding suffering. Lungs and tracheal tissues were collected from the infected and control groups on the days 3, 5, 7 and 10 post-infection (dpi) (four mice/day) and processed for immunohistochemistry (IHC). These inoculations were done in triplicate, at different periods of time, resulting in a total of 48 virus-infected and 24 mock-infected mice.
Three five-month-old calves (139, 151 and 165), seronegative for BRSV, were maintained in isolation units at FEPAGRO - Saúde Animal (Eldorado do Sul, RS). Good quality water and food supply were provided to the animals on adequate levels. Animal experiments were performed in accordance with the Brazilian federal regulations and the statements of animal welfare proposed by the Brazilian College of Animal Experimentation (COBEA). For infection, calves were sedated with 1% acepromazine (0.1mg/kg) and intratracheally inoculated with 20ml of the virus BRSV-25-BR strain (104.3 DICC50/ml). Six days later, the animals were euthanised and the lungs and tracheal tissues were collected and processed for IHC.
Lungs from two calves with respiratory symptoms were received for diagnosis at FEPAGRO-Saúde Animal. Calves were serologically negative for bovine herpesvirus type 1, bovine viral diarrhoea virus and bovine leukaemia virus, but their serological status for BRSV was unknown. The first calf (B1) was one-year-old and had anorexia, apathy, fever, copious sero-mucous nasal discharge, dyspnoea, tracheal stridors, pulmonary sibyls and pain at palpation of ventral thoracic region. At necropsy, it presented severe interstitial pneumonia, parenchymal and sub-pleural emphysema, typical of BRSV acute pneumonia. The second calf (B2) was seven-month-old, showed mild and recurrent respiratory symptoms. The lungs from this animal showed no macroscopic injury.
An immunoperoxidase assay was firstly standardized, using continuous cell line CER, cultured on 13mm diameter coverslips1 1 Thermanox ®; Nunc, Inc., IL, USA . The monolayers were subsequently inoculated with the strain BRSV-25-BR (104.3 DICC50/ml; multiplicity of infection 0.01). Then, the plates were incubated at 37ºC until the observation of initial cytopathic effect (ECP; after 2 days), at which point the cells were fixed with 80% acetone for 30 min at 4ºC.
Tissue animal samples were fixed in 4% paraformaldehyde2 2 Sigma-Aldrish Co., MO, USA , embedded in paraffin wax, and 4µm thick sections were cut and mounted on microscope slides.
The coverslips were re-hydrated in PBS-T (8.0g NaCl, 0.2g KCl, 1.44g Na2HPO4, 0.24g KH2PO4, 50µl Tween 20, H2O) or tissue sections were de-paraffinated in a graded series of xylene and ethanol. After treatment with PBS-T containing 3% H2O2 for 15 min, at room temperature, the sections were three times boiled in citrate buffer, pH 6.0, in a microwave oven, 3 min each and, then, blocked with 3% normal goat serum and incubated overnight at 4ºC with a solution of monoclonal antibodies against human RSV F and N proteins (primary antibody, NCL-RSV33 3 Novocastra Laboratories Ltd, Newcastle, UK ) at a dilution of 1:600 in PBS-T containing 1% normal goat serum. Biotinylated anti-mouse IgG antibody4 4 Vector Laboratories, Inc., CA, USA was subsequently added to the sections, which were incubated for 1h, at 37ºC. The signal was amplified by incubation with an avidin-biotinylated peroxidase system (Vectastain® Elite ABC Kit4 4 Vector Laboratories, Inc., CA, USA ) for 1h, at 37ºC. The reaction was stained by adding diaminobenzidine (DAB substrate kit for peroxidase4 4 Vector Laboratories, Inc., CA, USA ) to detect the immunoreactive sites. The sections were, then, counter-stained with Harris' haematoxylin and mounted in Entellan®5 5 Merck, Darmstadt, Germany for examination by light microscopy.
Vector® M.O.M.4 4 Vector Laboratories, Inc., CA, USA solution was used for all blocking steps and the reagent dilutions for reactions in mouse tissue were done according to the recommendations of the manufacturer.
RESULTS
Immunoperoxidase assay detected BRSV antigens in all BRSV-infected CER cell cultures and showed no positive reaction in uninfected ones. Some of infected CER cells showed an atypical sign on nuclei (Fig. 1). The immunoperoxidase assay described here generated a very strong signal in BRSV-infected cells, with no background reaction. In addition, non-specific reactions were absent in the negative control coverslips.
Infected Balb/c mice showed no clinical signs after virus inoculation. However, macroscopic lesions involving severe hemorrhage were observed in the lungs sections of one infected mouse (euthanised at 10 dpi) (data not shown). Histopathological examination revealed infiltration of mononuclear cells with marked thickening of the alveolar walls and mucosal edema, periarterial edema, mononuclear infiltrate in the lamina propria, multifocal proliferative tracheitis, and hyperplasia of the tracheal mucous glands (data not shown). IHC gave a positive reaction in tracheal tissue from mice 3, 5 and 7 dpi and in lung samples from mice euthanised 5 and 7 dpi. The reactions were characterized by staining of glandular epithelial cells (Fig. 2).
Macroscopic lesions compatible with BRSV-infections were seen in two calves (139 e 165). The lesions present in the calf 139 were mild, characterised by interstitial pneumonia and emphysema. The calf 165 showed more severe interstitial multifocal pneumonia, severe interstitial and subpleural emphysema, including consolidated lung areas (previously described by Almeida et al., 2005). BRSV antigens were detected by the IHC assay in pulmonary cells from only one of the experimentally infected calves (165). None of the tracheal samples was positive for BRSV antigens (Fig. 3).
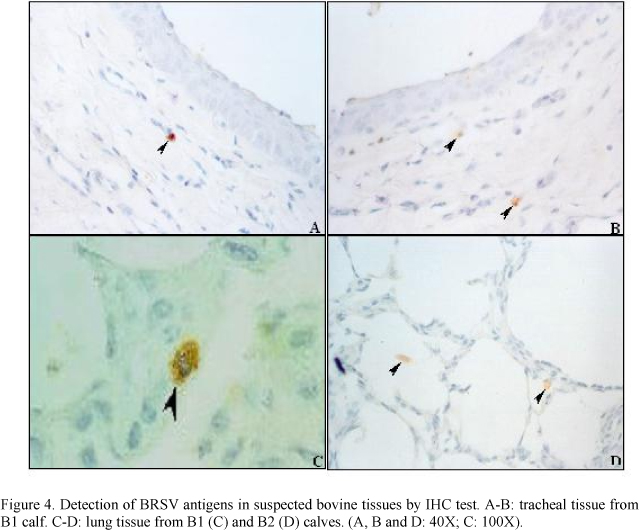
The lungs from two calves and tracheal sample from one calf suspected to have BRSV natural infection were positive for BRSV antigens by IHC (Fig. 4). One of them (B1) allowed confirmation by viral isolation (data not shown).
It was noted in all reactive coverslips, during the standardization of immunoperoxidase assay in CER cell cultures, that some infected cells with nuclei or perinuclear region also intensely stained, in addition to the cytoplasm. This could represent an abnormal reaction since BRSV is a RNA virus and, therefore, does not require reaching the nucleus for replication. Studies with monoclonal and polyclonal antibodies have detected N viral protein in the cytoplasmic and perinuclear regions of cells infected with porcine reproductive and respiratory viruses and coronaviruses (Rowland et al., 1999; Chen et al., 2002). Since a monoclonal antibody specific for the RSV N protein was used, it is possible that the same phenomenon described in other studies also occurred here. The interaction of viruses with the nucleus, nuclear subdomains and proteins does not appear to be restricted to viruses that use the nucleus as a site of replication. Many positive and negative strand RNA viruses, whose primary site of replication is the cytoplasm, sequester nuclear factors in order to facilitate their replication and, by altering the nuclear-cytoplasmic trafficking, can disrupt host cell functions and cellular responses to viral infections (reviewed by Hiscox, 2003). However, the perinuclear reaction could be triggered by viral glycoproteins present in the rough endoplasmic reticulum where they are produced. More studies should be done to analyze the relevance of this result.
DISCUSSION
Masot et al. (2000) reported that immunodetection requires a large number of molecules in order to obtain positive reactions, which is in agreement with the small number of cells that were positive in infected Balb/c mice tissue sections. In Balb/c mice, the most commonly used model for human RSV the inoculation of 104 plaque-forming units into the respiratory tract resulted in an infection that yielded only 104 plaque forming units per gram of lung tissue at the peak of virus replication (Collins et al., 1996). In RSV-infected cotton rats, which are more permissive than mice, virus detection using in situ hybridisation analysis in lung tissues showed that only a small fraction of the cells were infected (Murphy et al., 1990). In another study in which Balb/c mice were infected with BRSV and sacrificed 3, 5, 7 and 10 days post-infection, a positive immunohistochemical reaction was detected in only three lung samples 5 days post infection using a different protocol from that described here (Almeida et al., 2004).
Although the IHC assay gave a positive reaction in the two suspected animals, only one lung sample from experimentally infected calves was positive. BRSV has been detected by some authors using immunoenzymatic techniques around 6 days after experimental infection, which is the peak of BRSV viral excretion (West et al., 1998; Schreiber et al., 2000; Tjørnehøj et al., 2003). However, we were unable to detect the BRSV by the IHC described in this study, although two inoculated animals were positive by RT-nested-PCR (Almeida et al., 2005). This is in agreement with other studies, which demonstrated that PCR techniques usually show higher sensitivity when compared to IHC (Valarcher et al., 1999; Tjørnehøj et al., 2003). In this view, it is possible that the IHC is not sensitive enough to detect low amounts of the virus. Other studies found false negative results by direct immunofluorescence during BRSV outbreaks (Baker et al., 1986; Kimman et al., 1986; Schreiber et al., 2000) as well as after experimental infection (West et al., 1998).
The experimental inoculation way seems to be crucial for the induction of disease (Tjørnehøj et al., 2003). Aerosol transmission has been demonstrated by Mars et al. (1999). Tjørnehøj et al. (2003) indicated that the inoculation with aerosol for 10 min is probably quite close to the field exposure. The inoculation protocol adopted here may have originated a low infection degree, which was possible to be detected only by a more sensitive technique such as PCR and not by IHC, considering that the only positive sample was originated from the animal most severely affected after BRSV inoculation. Furthermore, two cases of natural infection were detected by the technique.
The advantage of the technique standardized here was the application of monoclonal antibodies directed to conserved proteins of human respiratory syncytial virus, which are commercially available and have relatively low cost. Therefore, there is no need to produce or to use specific BRSV Mabs, that is more expensive. The IHC test standardized here represents a reliable tool for substitution of the laborious BRSV isolation and a good alternative to the laboratorial diagnosis of this virus in Brazil.
ACKNOWLEDGEMENTS
The authors thank Joyce Camargo and Geneci Davi for technical assistance.
Recebido em 9 de novembro de 2004
Aceito em 9 de agosto de 2006
Apoio: CNPq, FAPESP, FAPERGS
- ALMEIDA, R.S.; DOMINGUES, H.G.; COSWIG, L.T.; et al. Detection of bovine respiratory syncytial virus in experimentally infected balb/c mice. Vet. Res, v.35, p.189-197, 2004.
- ALMEIDA, R.S.; SPILKI, F.R.; ROEHE, P.M. et al. Detection of Brazilian bovine respiratory syncytial virus strain by a reverse transcriptase-nested-polymerase chain reaction in experimentally infected calves. Vet. Microbiol., v.105, p.131-135, 2005.
- AMES, T.R. The epidemiology of BRSV infection. Vet. Med Sci., v.13, p.881-885, 1993.
- ARNS, C.S.; CAMPALANS, J.; COSTA, S.C.B. et al. Characterization of bovine respiratory virus isolated in Brazil. Braz. J. Med. Biol. Res., v.36, p.213-218, 2003.
- BAKER, J.C.; WERDIN, R.E.; AMES, T.R. et al. Study on the etiologic role of bovine respiratory syncytial virus in pneumonia of dairy calves. Am. J. Vet. Res, v.47, p.246-253, 1986.
- CHEN, H.; WURM T.; BRITTON, P. et al. Interation of the coronavirus nucleoprotein with nucleolar antigens and the host cell. J. Virol, v.76, p.5233-5250, 2002.
- COLLINS, P.L.; MCINTOSH, K.; CHANOCK, R.M., Respiratory syncytial virus. In: FIELDS, B.N. (Ed). Virology Philadelphia: Lippincott-Raven Publishers, 1996. p.1313-1351.
- DRIEMEIER, D.; GOMES, M.J.P.; MOOJEN, V. et al. Manifestação clínico-patológica de infecção natural pelo vírus respiratório sincicial bovino (BRSV) em bovinos de criação extensiva no Rio Grande do Sul, Brasil. Pesq. Vet. Bras, v.17, p.77-81, 1997.
- DUBOVI, E.J. Diagnosing BRSV infection: A laboratory perspective. Vet. Med. Sci., v.13, p.888-893, 1993.
- GONÇALVES, I.P.D.; JOST, H.C.; SOGLIO, A.D. et al. Detection of bovine respiratory syncytial virus in calves of Rio Grande do Sul, Brazil. Cien. Rural, v.23, p.389-390, 1993.
- HISCOX, J.A. The interaction of animal cytoplasmic RNA viruses with the nucleus to facilitate replication. Virus Res., v.95, p.13-22, 2003.
- KIMMAN, T.G.; ZIMMER, G.M.; STRAVER, P.J. et al. Diagnosis of bovine respiratory syncytial virus infections improved by virus detection in lung lavage samples. Am. J. Vet. Res, v.47, p.143-147, 1986.
- LARSEN, L.E. Bovine respiratory syncytial virus (BRSV): a review. Acta Vet. Scand., v.41, p.1-24, 2000.
- MARS, M.H.; BRUSCHKE, C.J.M.; VAN OIRSCHOT, J.T. Airborne transmission of BHV1, BRSV and BVDV among cattle is possible under experimental conditions. Vet. Microbiol, v.66, p.197-207,1999.
- MASOT, A.T.; KELLING, C.L.; LÓPEZ, O. et al. In situ hybridization detection of bovine respiratory syncytial virus in the lung of experimentally infected lambs. Vet. Pathol, v.37, p.618-625, 2000.
- MURPHY, B.R.; PRINCE, G.A.; LAWRENCE, L.A. et al. Detection of respiratory syncytial virus (RSV) infected cells by in situ hybridization in the lungs of cottons rats immunized with formaline-inativated virus or purified RSV F and G glycoproteins subunit vaccine and challenged with RSV. Virus Res, v.16, p.153-162, 1990.
- ROWLAND, R.R.; KERVIN, R.; KUCKLEBURG, C. et al. The localization of porcine reproductive and respiratory syndrome virus nucleocapsid protein to the nucleolus of infected cells and identification of a potential nucleolar localization signal sequence. Virus Res, v.64, p.1-12, 1999.
- SCHREIBER, P.; MATHEISE J.P.; DESSY F. et al. High mortality rate associated with bovine respiratory syncytial virus (BRSV) infection in Belgian white blue calves previously vaccinated with an inactivated BRSV vaccine. J. Vet. Med. B, v.47, p.535-550, 2000.
- TJØRNEHØJ, K.; UTTENTHAL, Å.; VIUFF, B. et al. An experimental infection model for reproduction of calf pneumonia with bovine respiratory syncytial virus (BRSV) based on one combined exposure of calves. Res. Vet. Sci., v.74, p.55-65, 2003.
- VALARCHER, J.F. ; BOURHY, H.; GELFI, J. et al. Evaluation of a nested reverse transcription-PCR assay based on the nucleoprotein gene for diagnosis of spontaneous and experimental bovine respiratory syncytial virus infections. J. Clin. Microbiol., v.37, p.1858-1862, 1999.
- WEST, K.; BORGDAN, J.; HAMEL, A. et al. A comparison of diagnostic methods for the detection of bovine respiratory syncytial virus in experimental clinical specimens. Can. J. Vet. Res, v.62, p.245-250, 1998.
- WESTENBRINK, F.; BRINKHOF, J.M.A.; STRAVER, P.J. et al. Comparison of a newly developed enzyme-linked immunosorbent assay with complement fixation and neutralization tests for serology of bovine respiratory syncytial virus infection. Res.Vet. Sci, v.38, p.334-340, 1985.
- WESTENBRINK, F.; KIMMAN, T.G. Immunoglobulin M-especific enzyme-linked immunosorbent assay for serodiagnosis of bovine respiratory syncytial virus infections. Am. J. Vet. Res., v.48, p.1132-1137, 1987.
Publication Dates
-
Publication in this collection
14 Mar 2007 -
Date of issue
Dec 2006
History
-
Accepted
09 Aug 2006 -
Received
09 Nov 2004