Abstracts
Twelve steers were intraruminally administered a high dose (0.5g/kg BW) of urea to study the damage effect of ammonia poisoning on liver and/or muscles. Blood samples were collected to determine ammonia and activities of gammaGT, AST and CK. Eleven steers were successfully poisoned and treated properly, but one succumbed. Poisoned cattle showed high concentration of ammonia, and higher activities of AST and CK. The higher the ammonia, the greater were the activities of AST (r=0.59) and CK (r=0.61). The correlation between AST and CK was high and significant (r=0.80), but not between AST and gammaGT (r=0.19). The activities of AST and CK were higher after the beginning of the convulsive episodes due to ammonia poisoning. Those results showed that occurred muscle damage instead of liver damage since CK is a typical enzyme from skeletal muscle; AST is found either in skeletal muscle and hepatocytes, while gammaGT is present in hepatic cells.
cattle; ammonia poisoning; urea; muscle damage; liver
Doze novilhos foram experimentalmente intoxicados por administração intrarruminal com alta dose de uréia (0,5g/kg PV) para estudar o efeito da intoxicação por uréia sobre o fígado e/ou musculatura. Foram coletadas amostras de sangue no decorrer do experimento para determinar o teor de amônia e as atividades de gama glutamiltransferase (gamaGT), aspartato aminotransferase (AST) e creatina quinase (CK). Onze novilhos apresentaram quadro clínico típico de intoxicação por amônia, porém um animal sucumbiu. Animais intoxicados apresentaram alto teor de amônia e elevada atividade de CK e AST. Quanto mais elevados foram os teores de amônia maiores foram as atividades de AST (r=0,59) e CK (0,61). A correlação entre AST e CK foi alta e significativa (r=0,80), ocorrendo o inverso com AST e gamaGT (r=0,19). Tanto a atividade de AST como de CK aumentaram significativamente a partir dos episódios convulsivos decorrentes da intoxicação por amônia. Estes resultados indicam que ocorreu uma expressiva lesão muscular, mas não hepática, visto que a CK é uma enzima típica da musculatura estriada; e o AST encontra-se tanto na musculatura estriada como nos hepatócitos, enquanto que a gamaGT está presente somente em células hepáticas.
bovino; intoxicação por amônia; uréia; lesão muscular; fígado
VETERINARY MEDICINE
Ammonia poisoning causes muscular but not liver damage in cattle
Intoxicação por ammonia causa lesões musculares e não hepáticas em bovinos
A.C. AntonelliI; G.A.S. TorresI; P.C. SoaresII; C.S. MoriI; M.C.A. SucupiraI; E.L. OrtolaniI, * * Autor para correspondência (corresponding author) E-mail: ortolani@usp.br
IFaculdade de Medicina Veterinária e Zootecnia - USP Av. Prof. Dr. Orlando Marques de Paiva, 87 05508-900 - São Paulo, SP
IIDepartamento de Medicina Veterinária - UFRPE
ABSTRACT
Twelve steers were intraruminally administered a high dose (0.5g/kg BW) of urea to study the damage effect of ammonia poisoning on liver and/or muscles. Blood samples were collected to determine ammonia and activities of gGT, AST and CK. Eleven steers were successfully poisoned and treated properly, but one succumbed. Poisoned cattle showed high concentration of ammonia, and higher activities of AST and CK. The higher the ammonia, the greater were the activities of AST (r=0.59) and CK (r=0.61). The correlation between AST and CK was high and significant (r=0.80), but not between AST and gGT (r=0.19). The activities of AST and CK were higher after the beginning of the convulsive episodes due to ammonia poisoning. Those results showed that occurred muscle damage instead of liver damage since CK is a typical enzyme from skeletal muscle; AST is found either in skeletal muscle and hepatocytes, while gGT is present in hepatic cells.
Keywords: cattle, ammonia poisoning, urea, muscle damage, liver
RESUMO
Doze novilhos foram experimentalmente intoxicados por administração intrarruminal com alta dose de uréia (0,5g/kg PV) para estudar o efeito da intoxicação por uréia sobre o fígado e/ou musculatura. Foram coletadas amostras de sangue no decorrer do experimento para determinar o teor de amônia e as atividades de gama glutamiltransferase (gGT), aspartato aminotransferase (AST) e creatina quinase (CK). Onze novilhos apresentaram quadro clínico típico de intoxicação por amônia, porém um animal sucumbiu. Animais intoxicados apresentaram alto teor de amônia e elevada atividade de CK e AST. Quanto mais elevados foram os teores de amônia maiores foram as atividades de AST (r=0,59) e CK (0,61). A correlação entre AST e CK foi alta e significativa (r=0,80), ocorrendo o inverso com AST e gGT (r=0,19). Tanto a atividade de AST como de CK aumentaram significativamente a partir dos episódios convulsivos decorrentes da intoxicação por amônia. Estes resultados indicam que ocorreu uma expressiva lesão muscular, mas não hepática, visto que a CK é uma enzima típica da musculatura estriada; e o AST encontra-se tanto na musculatura estriada como nos hepatócitos, enquanto que a gGT está presente somente em células hepáticas.
Palavras-chave: bovino, intoxicação por amônia, uréia, lesão muscular, fígado
INTRODUCTION
Cattle raised under extensive management on subtropical or tropical environment need to be supplemented with any source of protein or nitrogen, principally during the dry season, because protein is the main limiting nutrient for ruminants grazing during the dry or winter season. Preformed proteins are expensive and mainly used to human nutrition so the cheapest way to supplement protein to cattle is through the use of nonproteic nitrogen (NPN) (Froslie, 1977). The less expensive and most important source of NNP nowadays is urea, which can replace up to 35% of ration protein nitrogen for ruminants (Helmer and Bartley, 1971). More than 20 million Brazilian beef and dairy cattle are supplemented with urea yearly (Kitamura et al., 2003).
The rumen microorganisms hydrolyze urea into ammonium (NH4+) and ammonia (NH3) to synthesize their own protein. At a pH 7.0, approximately 99% of the compound is ammonium and 1% is ammonia, but as the pH becomes more alkaline the ammonia level gets higher (Visek, 1984). Usually, most of the urea is converted in the rumen into ammonium that is available to the rumen microbiota, while the small amount of ammonia produced is absorbed by rumen wall into the blood for the reason that ammonia is lipidsoluble (Bartley et al., 1976; Froslie, 1977). Normally, the liver can detoxify ammonia into urea efficiently synthesizing in the hepatocytes by Krebs & Henseleit cycle urea, which is 40 times less toxic than ammonia (Visek, 1968). Chalmers et al. (1971) made evident that 100g of liver could detoxify 150mg of ammonia, which is equivalent to 6-7mg of ammonia per 100ml of portal blood. But at higher concentrations in the blood, ammonia will overwhelm hepatocytes capacity of detoxification, and will increase its levels in blood, cerebrospinal fluid and other tissues because cells membranes of mammals are highly permeable to ammonia although their cells tolerates moderate levels of ammonia, resulting in ammonia poisoning (Davidovich et al., 1977; Visek, 1984). The primary mechanism of ammonia poisoning is inhibiting the Krebs cycle, which will lower energy production leading to anaerobic glycolysis and systemic metabolic acidosis (Haliburton and Morgan, 1989).
Ammonia poisoning may occur periodically in ruminants that consume large quantities of urea when they are not adapted to it, when feeds are inappropriately mixed with urea, or in a low energy, low protein, and high roughage diets with high urea levels. The onset of clinical picture may start in a matter of minutes to hours after consumption of urea and in most cases it is acute and can cause heavy mortality (Ortolani et al., 2000). Clinical signs most often observed in ammonia poisoning are restlessness, dullness, weakness, muscle tremors profuse salivation, rumen atony, bloat, dyspnea, incoordination, vocalization, lung edema, tonic-clonic convulsion, and finally death by heart failure (Payne, 1989; Radostits et al., 2002; Kitamura et al., 2003).
Although it is recognized that hyperammoniemia causes biochemical disarrange in cellular metabolism and intense cellular damage to the brain and lungs, few studies were carried out regarded to hepatic and muscle tissues, that during the poisoning accumulate large amounts of ammonia (Payne, 1989).
A light injury in the striated muscle causes both cellular enzymes creatine kinase (CK) and aspartate aminotransferase (AST) leak to the serum. Although the serum CK activity can be very high after a muscular injury its half life is very short approximately 2 to 4h, conversely the serum AST activity is lower but its half life longer, about 3d. Thus to monitor any striated muscle injuries it is recommended to analyze serum CK activity complemented with AST (Kaneko et al., 1997). To evaluate liver damage in adult cattle both serum gamma glutamyltransferase (gGT) and AST levels are proposed, while the serum increase of AST can be unspecific the gGT is primarily seen elevated only in liver diseases (Kaneko et al., 1997).
The objective of this study was to determine if experimental ammonia poisoning causes liver and/or muscle damage in cattle, as seen by the leakage of their cellular enzymes to serum.
MATERIALS AND METHODS
Twelve yearling crossbred (Holstein-Gir) healthy steers (body weight range 200-250 kg), from a single herd located in the city of Aluminio, Sao Paulo State were used in this experiment. They were housed indoors in individual tie stalls and provided, for two months before the trial, the following basal diet with 9.6% of dry matter of crude protein: 30% commercial concentrate and 70% coast-cross hay (Cynodon dactylon (L) Pers), and water (ad libitum). This diet did not contain urea or any source of NPN. In order to administer urea, a fistula to the rumen was surgically made, and fitted and capped with a rubber cannula, during the period of adaptation. The steers were restrained of food for 16 hours before the experiment.
Animals received 0.5g/kg BW of urea and 2.0g/kg BW of cornstarch, separately. Each animal was used only once in the trial. The urea and the cornstarch were administered within the ventral sac of the rumen by a plastic tube through the rubber cannulla, and then the rumen content was stirred by hand to assure the complete and uniform spreading of the urea.
Venous blood samples were drawn from jugular vein to obtain plasma and serum in the following moments: before giving urea, at the occurrence of the 1st clinical sign, muscle tremors, sternal recumbency, and at the 1st convulsive episode for determination of plasma ammonia and the serum activities of AST, gGT and CK. All samples were analyzed in the multiple clinical chemistry autoanalyzer1 1 Liasys â, AMS, Rome, Italy using commercial diagnostic kits.
All animals were treated properly when a convulsive episode was first observed or at the end of 240min. The rumen contents were removed using a siphon, and 10l of isotonic saline solution and 4 litres of rumen content obtained from a healthy steer were administered through the fistula. Animals that exhibited a very severe signs of toxicity such as depressive mental state or the presence of multiple convulsive episodes were treated intravenously with 1ml/kg BW of commercial solution of urea cycle amino acids2 2 Ornitargin â Lab. Baldacci , 1mg/kg BW furosemide3 3 Zalix â Lab. Intervet , and 20ml/kg BW isotonic saline solution as recommended by Kitamura et al. (2002).
Descriptive statistics are expressed as means and standard deviation. The data were assessed at first by one-way analysis of variance and the means compared by Duncan test. The coefficient of correlation was assessed between all variables, and according to Little and Hills (1978). A high correlation was considered when r>0.60; a medium correlation when 0.30<r<0.60; and a low correlation when r<0.30, with a P<0.0001 value. Results were considered significant at the P<0.05 value (Sampaio, 1998). The statistical analyses were performed using the MINITAB statistical software (Minitab, 2000).
RESULTS
At the beginning of the experiments, all steers were healthy, alert and active. The 12 tests resulted in 11 definite toxic cases, with only one steer just exhibiting mild fasciculation and had an uneventful recover. The other 11 steers had typical clinical signs of ammonia poisoning that resulted in convulsive episodes.
The concentration of blood ammonia increased progressively (P<0.04) (Table 1). The first clinical signs occurred when blood ammonia reached mean values of 782mmol/l. As the clinical picture evolved, when the cattle showed muscle tremors the blood ammonia gGT concentration rose, reaching an average value of 1136mmol/l, with significant changes when the animals were lying in sternal recumbency, reaching 1272mmol/l. Blood ammonia reached its peak at convulsion, with mean values of 1632mmol/l.
The activity of CK also increased progressively throughout the clinical picture of ammonia poisoning (P<0.01), but only after the muscle tremors started with 90U/l. When the steers were lying down in sternal recumbency, the activity of CK were higher than when the muscle tremors started (137U/l), but it reached higher activity levels at convulsion, with 1481U/l.
Values for AST activity remained at physiological ranges (mean 37U/l) until sternal recumbency, but at the beginning of convulsion there was a sudden increased, reaching mean values of 60U/l being significant different from the initial values (P<0.002). No significant differences between the results of gGT activities throughout the experiment were observed (Table 1).
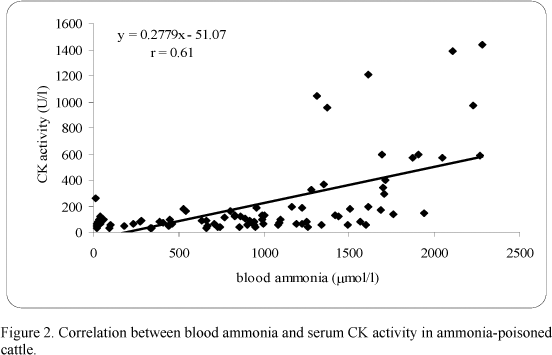
There was a significant correlation between blood ammonia and AST (r=0.59) (Fig. 1), and a high correlation between blood ammonia and CK (r=0.61) (Fig. 2), but an extremely low correlation between blood ammonia and gGT (r=0.01). A high correlation between AST and CK (r=0.80) (Fig. 3) was estimated. Conversely, a low non-significant correlation between AST and gGT (r=0.19), and CK and gGT (r=0.19) was found.
DISCUSSION
The high correlation between AST and CK but not between AST and gGT suggested that ammonia poisoning definitely caused muscle but not liver damage. Those lesions might be due to the severity of convulsive episodes and the pathologic recumbency throughout the clinical picture, and so far the higher values for AST and CK activities were detected after the convulsions.
Froslie (1977) reported in his classical review about ammonia poisoning in ruminants that muscle damage may occur sporadically, and no mention was made about liver damage. In a study with sheep, Roller et al. (1982) evaluated the activities of lactate dehydrogenase (LDH), alkaline phosphatase (ALP), AST and CK throughout ammonia poisoning, describing a less intense increase in the first three enzymes and a higher elevation in the activity of CK. The authors mentioned that transaminases would be elevated in hepatic diseases, cardiac necrosis and skeletal muscle damage, leading to an ambiguous conclusion that might exist liver damage. The authors did not emphasized LDH is present in skeletal muscle and ALP in the intestines, and catarrhal enteritis or even a haemorrhagic inflammation in the abomasum and jejunum may occur due to ammonia poisoning (Froslie, 1977).
Singer and McCarty (1971) stated that sheep undergoing ammonia poisoning that died showed massive myocardic and skeletal muscle haemorrhage, and no macroscopic lesion was detected in the liver, with only a mild swelling of the hepatocytes. According to Meyer et al. (1995), mild injuries in the hepatocytes almost never cause enzymes releasing in this cells.
The presence of AST in many tissues makes this serum enzyme a nonspecific but sensitive marker of soft tissue damage, but precludes its use as an organ-specific enzyme, and although CK is a best marker for detect muscle damage, AST is frequently used to complement CK changes (Kaneko et al., 1997).
The continuous muscle tremors, the pathological recumbency as the several convulsive episodes, typical clinical signs of ammonia poisoning, may represent a strenuous exercise to the animal which could lead to an activity-induced injury of skeletal muscle. This condition often occurs when muscles are active after a period of inactivity or when muscles are exposed to extraordinary conditions. In some situations it results in increased levels of intracellular enzymes such as creatine kinase, with or without structural evidence of muscle damage ( Swenson and Reece, 1993).
Recebido para publicação em 21 de outubro de 2004
Aceito em 20 de setembro de 2006
- BARTLEY, E.E.; DAVIDOVICH, A.; BARR, G.W. et al. Ammonia toxicity in cattle. I. Rumen and blood changes associated with toxicity and treatments methods. J. Anim. Sci., v. 43, p.835-841, 1976.
- CHALMERS, M.I.; JAFFRAY, A.E.; WHITE, F. Movements of ammonia following intraruminal administration of urea or casein. Proceed. Nutr. Soc., v.30, p.7-17, 1971.
- DAVIDOVICH, A.; BARTLEY, E.E.; BECHTLE, R. M. et al. Ammonia toxicity in cattle. III. Absorption of ammonia gas from the rumen and passage of urea and ammonia from rumen to the duodenum. J. Anim. Sci., v.45, p.551-558, 1977.
- FROSLIE, A. Feed-related urea poisoning in ruminants. Folia Vet. Lat., v.7, p.17-37, 1977.
- HALIBURTON, J. C.; MORGAN, S. E. Nonprotein nitrogen-induced ammonia toxicosis and ammoniated feed toxicity syndrome. Vet. Clin. N. Am.: Food Anim. Pract., v.5, p.237-249, 1989.
- HELMER, L.G.; BARTLEY, E.E. Progress in the utilization of urea as a protein replacer for ruminants: a review. J. Dairy Sci., v.54, p.25-51, 1971.
- KANEKO, J.J.; HARVEY, J.W.; BRUSS, M.L. Clinical biochemistry of domestic animals. 5.ed. London: Academic, 1997. 932p.
- KITAMURA, S.S.; ANTONELLI, A.C.; MARUTA, C.A. et al. A model for ammonia poisoning in cattle. Vet. Hum. Toxicol., v.45, p.274-277, 2003
- KITAMURA, S. S.; ORTOLANI, E. L.; ANTONELLI, A. C. Ammonia poisoning in cattle fed dietary urea: basic concepts and new findings. Rev. Educ. Cont. CRMV-SP, v.5, p.292-298, 2002.
- LITTLE, T.M.; HILLS, F.J.. Agricultural experimentation: design and analysis New York: John Wiley, 1978. 350p.
- MEYER, D. J.; COLES, E.H.; RICH, L.J. Medicina de laboratório veterinária São Paulo: Roca, 1995. 320p.
- MINITAB. The student edition of MINITAB statistical software adapted for education: 13.0 release user's manual New York: Wesley, 2000. 624p.
- ORTOLANI, E.L.; MORI, C.S.; FILHO, J.A.R.. Ammonia toxicity from urea in a Brazilian dairy goat flock. Vet. Hum. Toxicol., v. 42, p.87-89, 2000.
- PAYNE, J.M. Metabolic and nutritional diseases of cattle Oxford: Blackwell Scientific Publications, 1989. 149p.
- RADOSTITS, O.M.; BLOOD, D.C.; GAY, C.C. Veterinary medicine: a textbook of the diseases of cattle, sheep, pig, goats and horses London: Baillière Tindall, 1994. 1765p.
- ROLLER, M.H.; RIEDEMANN, G.S.; ROMKEMA, G.E. et al. Ovine blood chemistry values measured during ammonia toxicosis. Am. J. Vet. Res., v.43, p.1068-1071, 1982.
- SAMPAIO, I.B.M. Estatística aplicada à experimentação animal Belo Horizonte: Fundação de Ensino e Pesquisa em Medicina Veterinária e Zootecnia, 1998. 221p.
- SINGER, R.H.; McCARTY, R.T. Pathologic changes resulting from acute ammonium salt poisoning in sheep. Am. J. Vet. Res., v.32, p.1239-1246, 1971.
- SWENSON, M.J.; REECE, W.O. Dukes' physiology of domestic animals 11.ed. Ithaca: Cornell University, Press, 1993. 962p.
- VISEK, W.J. Some aspects of ammonia toxicity in animals cells. J. Dairy Sci., v.51, p.286-295, 1968.
- VISEK, W.J. Ammonia: Its effects on biological systems, metabolic hormones, and reproduction. J. Dairy Sci., v.67, p.481-498, 1984.
Publication Dates
-
Publication in this collection
02 May 2007 -
Date of issue
Feb 2007
History
-
Accepted
20 Sept 2006 -
Received
21 Oct 2004