Abstracts
In order to describe seasonal changes in Lyme diseases risk rate at three localities in Serbia, during the period of 2003-2005, a total of 1542 Ixodes ricinus ticks (493 nymphs, 525 females and 524 males) were examined. The prevalence of Borrelia burgdorferi in Ixodes ricinus ticks at the Bovan Lake County were higher than the average for European localities (45.9% for adults and 18.8% for nymphs). In Mt. Avala and Kljajicevo counties adults and nymphs were, respectively, infected at the following percentages: 26.3, 10.7; 16.2 and 7.6%. The outcome indicates a relatively high risk of the contracting Lyme disease in all investigates areas.
tick; Ixodes ricinus; Lyme disease; risk rate
Para estimar a variação sazonal das taxas de risco para doença de Lyme em três localidades da Sérvia foram examinados, no período de 2003-2005, 1542 espécimes do carrapato Ixodes ricinus (493 ninfas, 525 fêmeas e 524 machos). A prevalência de Borrelia burgdorferi em Ixodes ricinus no município de Bovan Lake foi mais alta que a registrada em outras localidades da Europa. Nos municípios de Mt. Avala e Kljajicevo as porcentagens de adultos e ninfas infectadas foram: 26,3 e 10,7; 16,2 e 7,6, respectivamente. Esses resultados indicam um relativo alto risco de se contrair doença de Lyme nas três localidades estudadas.
carrapato; Ixodes ricinus; doença de Lyme; taxa de risco
VETERINARY MEDICINE
Assessment of the risk of contracting Lyme disease in areas with significant human presence
Risco de contrair doença de Lyma em áreas com significativa presença humana
M. Milutinovic; Z. Radulovic; S. Tomanovic
University of Belgrade - Institute for Medical Research Dr. Subotica 4, P.O. Box 102 11129 Belgrade, Serbia
ABSTRACT
In order to describe seasonal changes in Lyme diseases risk rate at three localities in Serbia, during the period of 2003-2005, a total of 1542 Ixodes ricinus ticks (493 nymphs, 525 females and 524 males) were examined. The prevalence of Borrelia burgdorferi in Ixodes ricinus ticks at the Bovan Lake County were higher than the average for European localities (45.9% for adults and 18.8% for nymphs). In Mt. Avala and Kljajicevo counties adults and nymphs were, respectively, infected at the following percentages: 26.3, 10.7; 16.2 and 7.6%. The outcome indicates a relatively high risk of the contracting Lyme disease in all investigates areas.
Keywords: tick, Ixodes ricinus, Lyme disease, risk rate
RESUMO
Para estimar a variação sazonal das taxas de risco para doença de Lyme em três localidades da Sérvia foram examinados, no período de 2003-2005, 1542 espécimes do carrapato Ixodes ricinus (493 ninfas, 525 fêmeas e 524 machos). A prevalência de Borrelia burgdorferi em Ixodes ricinus no município de Bovan Lake foi mais alta que a registrada em outras localidades da Europa. Nos municípios de Mt. Avala e Kljajicevo as porcentagens de adultos e ninfas infectadas foram: 26,3 e 10,7; 16,2 e 7,6, respectivamente. Esses resultados indicam um relativo alto risco de se contrair doença de Lyme nas três localidades estudadas.
Palavras-chave: carrapato, Ixodes ricinus, doença de Lyme, taxa de risco
INTRODUCTION
Lyme disease is a multi-system tick-borne disorder caused by the spirochete bacteria Borrelia burgdorferi sensu lato (sl), one whose most prominent manifestations affect thin skin, nervous system, musculoskeletal system and heart. Early Lyme disease can cause nonspecific symptoms and diagnosis is often established in late, chronic stages. Assessment of the risk of contracting Lyme disease is an essential component of the design and implementation of treatment and prevention measures.
As the only natural way humans can been infected with B. burgdorferi sl is through a tick bite, predicting the risk is based on the presence of infected ixodid ticks. Risk maps for Lyme disease have been produced by various methods: by detecting the prevalence of borreliae in ixodid ticks (Daniels et al., 1998), by determination of habitat suitability for ixodid tick development (Eisen et al., 2004), or using a geographic information system (Glass et al., 1995; Guerra et al., 2002). Development of new less expensive methods or models for risk assessment is of great interest. Detection of genetic differences between borreliae-infected and uninfected Ixodes ricinus ticks (Radulovic, 2005) point to the real possibility of using some genetic markers as indicators of Lyme disease risk.
I. ricinus is the principal vector of Lyme disease in Europe. As B. burgdorferi sl spirochetes are present in all examined I. ricinus populations (Hubálek and Halouzka, 1998), the risk of contracting Lyme disease exists throughout the geographic distribution of this species, including Europe, North Africa, and parts of Asia. There are wide temporal and spatial fluctuations in the risk rate, depending on the abundance of questing I. ricinus ticks, the prevalence of borreliae in I. ricinus ticks, the intensity of infection of ticks with borreliae, and the sex and stage ratio in tick populations. The importance of these values for Lyme disease risk assessment has alredy been reported (Hubálek et al., 1991, 1994, 2004; Matuschka et al., 1992; Jensen and Frandsen, 2000; Jensen et al., 2000; Nahimana et al., 2004; Cisak et al., 2005). Research carried out on Lyme disease in Serbia so far pointed out to the significant prevalence of borreliae in I. ricinus ticks (Milutinovic, 2000; Milutinovic et al., 2004).
In this study, it was recorded important parameters for assessment of Lyme disease risk at three spatially distant and ecologically different localities in Serbia during a three-year period in order to describe seasonal changes in risk rates and compare them between localities.
MATERIAL AND METHODS
The study was performed at three localities in Serbia, namely: Kljajicevo, Mt. Avala, and Bovan Lake. Ticks were collected within sampling areas (one for each locality) having diameters of 200m. While the sampling area at the Kljajicevo locality, which is situated below the Tele ka loess plateau on the Pannonian plain, consists of agricultural land and sparse deciduous forest (including part of an area used for pheasant breeding), the Mt. Avala and Bovan Lake localities represent recreational areas in the vicinity of Belgrade and Ni
ka loess plateau on the Pannonian plain, consists of agricultural land and sparse deciduous forest (including part of an area used for pheasant breeding), the Mt. Avala and Bovan Lake localities represent recreational areas in the vicinity of Belgrade and Ni , respectively. There is a significant occupational human exposure to tick bites within the study areas. During the period of questing activity of ticks (from March to November), farmers, hunters, and gamekeepers are present at the Kljajicevo locality, forestry workers at the Mt Avala locality, and anglers and shore guards at the Bovan Lake locality. As for the Mt. Avala and Bovan Lake localities, attendance of a huge number of picnickers during holidays and summer vacations is usual. It should be noted that the waters of Sakinac (included in the study area), a spring situated below Mt. Avala, attracts many visitors.
, respectively. There is a significant occupational human exposure to tick bites within the study areas. During the period of questing activity of ticks (from March to November), farmers, hunters, and gamekeepers are present at the Kljajicevo locality, forestry workers at the Mt Avala locality, and anglers and shore guards at the Bovan Lake locality. As for the Mt. Avala and Bovan Lake localities, attendance of a huge number of picnickers during holidays and summer vacations is usual. It should be noted that the waters of Sakinac (included in the study area), a spring situated below Mt. Avala, attracts many visitors.
RESULTS
The breaking of diapause in ticks and start of active questing for a host are linked with changes of ecological factors in the habitat. Successions of periods of activity and periods of dormancy in the species I. ricinus are caused by physiological changes that are directly dependent upon temperature and relative atmospheric humidity in the external environment (Table 1).
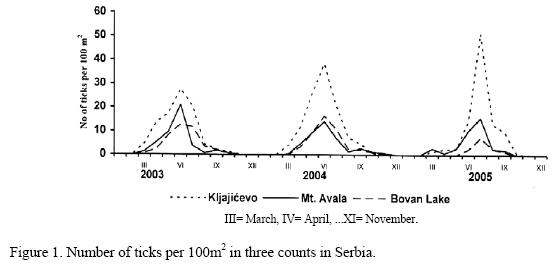
In tracing of seasonal abundance of host-seeking I. ricinus ticks during the three-year period of study at the Kljajicevo, Mt. Avala and Bovan Lake localities, it was noted that the questing activity of ticks started in early spring, during March and April, and continued until late autumn (Fig. 1 and 2). Regarding abundance within localities, seasonal and annual variations were detected for both adults and nymphs. Generally, ticks were more abundant in spring and less abundant in autumn.
During the period of 2003-2005, a total of 1542 I. ricinus ticks (493 nymphs, 525 females, and 524 males) were examined by dark-field microscopy for borreliae presence. Out of this number, 62 (12.6%) nymphs and 308 (29.4%) adults were infected with B. burgdorferi sl. The obtained results show seasonal prevalence of borreliae in questing I. ricinus ticks (Table 2). There were no statistically significant differences of infection rates between males and females at any of the investigated localities. On the other hand, statistically significant differences between adults and nymphs were detected in most annual samples, and differences of total samples for each locality were highly significant (Kljajicevo, P=0.008; Mt. Avala, P=0.0001; Bovan Lake, P<0.00001). Seasonal and interannual variation in infection rates were statistically insignificant, in contrast to statistically significant differences between localities (for nymphs P=0.006 and for adults P<0.00001).
As for the approximate intensity of infection, the showed that the number of I. ricinus ticks harbored a small number of borreliae, while the proportion of highly infected adults by localities was as follows: Kljajicevo - 1.2 %, Mt. Avala - 4.3 % and Bovan Lake - 12.7 % (Table 3). There was only one highly infected nymph, which was collected at the Bovan Lake locality. The seasonal distribution of borreliae infection intensity in nymphs and adults (Table 4) represents information very important for assessment of the risk of Lyme disease in human and animal populations.
The main reasons for omitting I. ricinus larvae in this study were as follows: low borreliae infection rate (the overall mean prevalence for Europe was about 1.9 % in the review of Hubálek and Halouzka (1998); rare fidings of larvae on humans (Hubálek et al., 2004); and the possibilities of stochastic errors in abundance measuring due to aggregation of larvae after egg eclosion.
DISCUSSION
By capturing ticks with the aid of a flag, it is possible to collect only individuals that are actively questing for a host. In addition to active individuals, the total abundance of ticks also includes ones that are in diapause and ones in the litter that are making restitution for fluids lost during the active periods. The abundance of active ticks constitutes information more significant than total tick abundance in assessing the degree of risk to the human population, since only active ticks represent a danger to man.
Two peaks are evident in the seasonal abundance of active adults of I. ricinus at the Kljajicevo, Mt. Avala, and Bovan Lake localities, the higher spring peak occurring during the period of May-June and the lower autumn peak occurring during the period of September-October, a pattern that is characteristic of populations of this species in the Temperate Zone (Milutinovic, 1992; Milutinovic and Bobic, 1997; Korenberg, 2000; Perret et al., 2000; Milutinovic and Radulovic, 2002). Seasonal dynamics in the abundance of active nymphs of I. ricinus at the investigated localities achieves a peak during the period of June-July.
Ecological conditions of the habitat dictate seasonal changes in the number of active ticks, which deviate significantly from the seasonal dynamics of total abundance of the population. Active ticks of the species I. ricinus at the investigated localities were present from April to October, although a significant number of active individuals were also recorded in March at the Kljajicevo and Mt. Avala localities. Only a few active individuals were captured during November of 2004 at the Mt. Avala locality.
The greatest tick abundance was recorded at the Kljajicevo locality, where more than 25 adults per 100m2 on average were collected during the seasonal peaks. The abundance of adults during the seasonal peaks at the other two localities varied significantly between years and ranged from about 10 individuals per 100m2 in June of 2005 to more than 30 individuals per 100m2 in June of 2004 at the Mt. Avala locality, and from 12 individuals per 100m2 in June of 2003 to close to 30 individuals per 100m2 in May of 2005 at the Bovan Lake locality. Interannual variations in the abundance of ticks result from significant interannual differences in the main ecological factors that determine their activity: temperature, atmospheric humidity, and precipitation. The abundance of adults was close to the number recorded by Walker (2001) at a deciduous forest locality in the south of Scotland during the period of 1996-1999, but significantly higher than that recorded in Sweden, namely one adult per 100m2 on average (Mejlon, 2000). The abundance of nymphs established during the seasonal peaks was lower than expected and 10 times less than the abundance recorded in Wales during the period of 1995-2000 (Randolph et al., 2002). Whereas other authors recorded 10-20 times greater abundance of nymphs in relation to adults at the localities they investigated (Mejlon, 2000; Walker, 2001; Randolph et al., 2002), approximately equal numbers of adults and nymphs on average were captured at the Kljajicevo, Mt. Avala, and Bovan Lake localities. As the main hosts of juvenile stages of I. ricinus, Matuschka et al. (1991) cited small rodents and lizards, which were present in large numbers at the localities they investigated. Nymphs easily found a host and quickly concluded the questing period. Adults prefered to parasitize larger mammals and birds, whose numbers at the investigated localities were small, a circumstance that extended the duration of their questing period. Differences in the duration of active periods were the main factor accounting for capture of a smaller number of nymphs in relation to adults.
The prevalence of B. burgdorferi in I. ricinus ticks at the Bovan Lake locality (45.9% for adults and 18.8% for nymphs) was exceptionally high and higher than the average for European localities given by Hubalek and Halouzka (1998) (21.1% for adults and 13.8% for nymphs). At the Mt. Avala locality, the percentage of infected adults (26.3%) was higher, while the percentage of infected nymphs (10.7%) was lower than the cited averages. The prevalence of B. burgdorferi in I. ricinus ticks at the Kljajicevo locality (16.2% for adults and 7.6% for nymphs) was lower than at the two preceding localities.
Differences between the investigated localities with respect to the number of infected ticks are statistically significant (on the boundary of statistical significance in the case of nymphs) and consistent with the distance between localities. The Mt. Avala locality is approximately midway between the Kljajicevo and Bovan Lake localities. Distance between localities is not a precondition for the existence of differences in the prevalence of borreliae in I. ricinus ticks. Investigating samples of I. ricinus ticks from four neighboring localities on the territory of Belgrade, Milutinovic et al. (2004) detected significant differences between them in the percentage of infected ticks.
In samples of I. ricinus ticks from all three of the localities investigated in the present study, the percentage of borrelia-infected nymphs was significantly lower than the percentage of infected adults. Similar results were obtained by Hubalek et al. (1991) at two neighboring localities in the Czech Republic (3.8% infected nymphs and 10.6% infected adults at locality A vs. 29.1% infected nymphs and 35.9% infected adults at locality B). Bukowska et al. (2003) recorded 21.1% infected adults and 9.9% infected nymphs during 2000 and 2001 at 10 localities in the immediate vicinity of Szczecin (Poland). At two localities in the south of Sweden, Mejlon (2000) established the presence of 10.1 and 6.9% infected nymphs and 18 and 19.1% infected adults of I. ricinus, respectively.
Low relative abundance of infected nymphs at the investigated localities does not mean a lower risk of infection after their bites. Due to their small size, nymphs after biting often remain unnoticed by the host for a longer period of time. Sood et al. (1997) indicate a positive correlation between the duration of attachment of an infected tick and infection of the host.
The significance of intensity of tick infection with borreliae for risk assessment is attributable to the shorter period of attachment to the host needed by a highly infected tick in order to successfully transmit the disease agent. In highly infected ticks, transmission of borreliae to the host with the saliva during sucking is faster than in the case of weakly infected ticks. The highest prevalence of borreliae in ticks and significantly more highly infected adults were recorded at the Bovan Lake locality in comparison with the other two localities.
In addition to nymphs of I. ricinus infected with B. burgdorferi, Hubalek et al. (1994) also cite infected females of this species as being potentially dangerous to the human population. Males of I. ricinus most often do not feed at all. For assessment of the risk to the human population of infection with borreliae, only the abundance of infected nymphs and females is of any significance.
The abundance of males and females did not differ significantly at the investigated localities, nor did the percentage of their infection in the tested samples. Insignificant variation in the percentage of infected ticks at the investigated localities during 2003, 2004, and 2005, together with the recorded seasonal dynamics in the activity of ticks at these localities, make it possible to define the periods of greatest risk to the human population (Hubalek et al., 1994). In view of the average prevalence and infection intensity recorded at each locality, as well as values of absolute abundance, it is evident that the greatest risk existed at the Bovan Lake locality during May of 2005, when 14 infected adults per 100m2 were captured, an average of four of which were highly infected. Despite the significantly greater abundance of ticks (especially nymphs) recorded at the Kljajicevo locality, the risk of contracting Lyme disease is not as high at this locality due to the lower prevalence and small number of highly infected ticks: close to four infected adults per 100m2, an average of 0.3 of which were highly infected, and about four slightly or moderately infected nymphs during the seasonal peaks of abundance.
Here, it seems appropriate to mention the findings of Radulovic (2005) concerning differences in Mdh and a-Gpdh allele frequencies in I. ricinus ticks infected and uninfected with borreliae: he indicated higher frequencies of rare alleles in infected specimens. Statistically significant differences were confirmed only in females and pertained to the a-Gpdh gene locus. These results point to the real possibility of using some genetic markers as indicators of the risk of contracting for Lyme disease. Since approximately the same frequencies of a-Gpdh alleles were established at the Kljajicevo, Mt. Avala, and Bovan Lake localities, it would seem logical to expect equal prevalence and intensity of infection with borelliae in these populations of I. ricinus. However, these results are contrary to expectations. Many factors affect allele frequencies, and the use of a-Gpdh frequency as an indicator of Lyme disease risk must not be completely rejected. Rather, additional investigations are needed to confirm the indicated possibility.
CONCLUSIONS
The outcome of this study indicates a relatively high risk of the contracting Lyme disease in all investigated areas. The highest risk was recorded at Bovan Lake locality, followed by Mt. Avala and Kljajicevo localities, respectively.
ACKNOWLEDGEMENT
This work was supported by a grant from the Ministry of Science and Environmental Protection of the Republic of Serbia (Project No. 1726 and 145002).
Recebido em 24 de outubro de 2006
Aceito em 26 de novembro de 2007
E-mail: majam@imi.bg.ac.yu
- BUKOWSKA, K.; KOSIK-BOGACKA, D.; KUZNA-GRYGIEL, W. The occurrence of Borrelia burgdorferi sensu lato in the populations of Ixodes ricinus in forest areas of Szczecin during 2000-2001. Ann. Agric. Environ. Med, v.10, p.5-8, 2003.
- CISAK, E.; CHMIELEWSKA-BADORA, J.; ZWOLINSKI, J. et al. Risk of tick-borne bacterial diseases among workers of Roztocze national park (south-eastern Poland). Ann. Agric. Environ. Med., v.12, p.127-132, 2005.
- DANIELS, T.J.; BOCCIA, T. M.; VARDE, S. et al. Geographic risk for Lyme disease and human granulocytic ehrlichiosis in southern New York State. Appl. Environ. Microbiol, v.64, p.4663-4669,1998.
- EISEN, L.; EISEN, R.J.; CHANG, C.-C. et al. Acarologic risk of exposure to Borrelia burgdorferi spirochaetes: long-term evaluations in north-western California, with implications for Lyme borreliosis risk-assessment models. Med. Vet. Entomol, v.18, p.38-49, 2004.
- GLASS, G.E.; SCHWARTZ, B.S.; MORGAN, J.M. III. et al. Environmental risk factors for Lyme disease identified with geographic information systems. Am. J. Public Health, v.85, p.944-948, 1995.
- GUERRA, M.; WALKER, E.; JONES, C. et al. Predicting the risk of Lyme disease: Habitat suitability for Ixodes scapularis in the north central United States. Emerging Infect. Dis, v.8, p.289-297, 2002.
- HUBÁLEK, Z.; HALOUZKA, J. Prevalence rates of Borrelia burgdorferi sensu lato in host-seeking Ixodes ricinus ticks in Europe. Parasitol. Res, v.84, p.167-172, 1998.
- HUBÁLEK, Z.; HALOUZKA, J.; JURICOVÁ, Z. A comparison of the occurrence of borreliae in nymphal and adult Ixodes ricinus ticks. Zbl. Bak, v.275, p.133-137, 1991.
- HUBÁLEK, Z.; HALOUZKA, J.; JURICOVÁ, Z. Borreliae in Ixodes ricinus ticks feeding on humans. Med. Vet. Entomol, v.18, p.228-231, 2004.
- HUBÁLEK, Z.; HALOUZKA, J.; JURICOVÁ, Z. et al. Seasonal distribution of borreliae in Ixodes ricinus ticks. Zbl. Bak, v.280, p.423-431, 1994.
- JENSEN, P.M.; FRANDSEN, F. Temporal risk assessment for Lyme borreliosis in Denmark. Scand. J. Infect. Dis, v.32, p.539-544, 2000.
- JENSEN, P.M.; HANSEN, H.; FRANDSEN, F. Spatial risk assessment for Lyme borreliosis in Denmark. Scand. J. Infect. Dis, v.32, p.545-550, 2000.
- KORENBERG, E.I. Seasonal population dynamics of Ixodes ticks and tick-borne encephalitis virus. Exp. Appl. Acarol, v.24, p.665-681, 2000.
- MATUSCHKA, F.R.; FISCHER, P.; HEILER, M.S. et al. Stage-associated risk of transmission of the Lyme disease spirochete by European Ixodes ticks. Parasitol. Res, v.78, p.695-698, 1992.
- MATUSCHKA, F.R.; FISCHER, P.; MUSGRAVE, K. et al. Hosts on which nymphal Ixodes ricinus most abundantly feed. Am. J. Trop. Med. Hyg, v.44, p.100-107, 1991.
- MEJLON, H. Host-seeking activity of Ixodes ricinus in relation to the epidemiology of Lyme borreliosis in Sweden . 2000. Dissertatinon Faculty of Science and Technology, Uppsala University.
- MILUTINOVIC, M. Ecological investigations of ticks (Acarina, Ixodoidea, Ixodidae) of Serbia 1992. (Dissertation) - Faculty of Biology, University of Belgrade [in Serbian]
- MILUTINOVIC, M. Diversity and ecology of the Ixodid ticks (Acari: Ixodidae) in central Serbia, Yugoslavia. Arch. Biol. Sci., v.52, p.39-46, 2000.
- MILUTINOVIC, M.; BOBIC, B. Ecological investigations on ticks (Acari, Ixodidae) of east Serbia, with emphasis on Ixodes ricinus and Hyalomma savignyi Arq. Bras. Med. Vet. Zootec, v.49, p.531-541,1997.
- MILUTINOVIC, M.; RADULOVIC, Z. Ecological notes on ticks (Acari: Ixodidae) in Serbia (central regions). Acta Vet, v.52, p.49-58, 2002.
- MILUTINOVIC, M.; RADULOVIC, Z.; JOVICIC, V. et al. Population dynamics and Borrelia burgdorferi infection rate of Ixodes ricinus ticks in the Belgrade area. Acta Vet, v.54, p.219-225, 2004.
- MILUTINOVIC, M.; RADULOVIC, Z.; TOMANOVIC, S. et al. Seasonal distribution of borreliae in Ixodes ricinus ticks in the Belgrade region, Serbia. Arch. Biol. Sci, v.58, p.183-186, 2006.
- NAHIMANA, I.; GERN, L.;. BLANCl, D.S. et al. Risk of Borrelia burgdorferi infection in western Switzerland following a tick bite. Eur. J. Clin. Microbiol. Infect. Dis, v.23, p.603-608, 2004.
- PERRET, J.L.; GUIGOZ, E.; Rais, O. et al. Influence of saturation deficit and temperature on Ixodes ricinus tick questing activity in a Lyme borreliosis endemic areas (Switzerland). Parasitol. Res, v.86. p.554-557, 2000.
- RADULOVIC, Z. Ecological and genetic study of malate dehydrogenase and a-glycerophosphate dehydrogenase variability in Ixodes ricinus (Linnaeus, 1758) populations 2005. Thesis. Faculty of Biology, University of Belgrade [in Serbian]
- RANDOLPH, S.E.; GREEN, R.M.; HOODLESS, A.N. et al. An empirical quantitative framework for the seasonal population dynamics of the tick Ixodes ricinus Int. J. Parasitol., v.32, p.979-989, 2002.
- SOOD, S.K.; SALZMAN, M.B.; JOHNSON, B.J.B. et al. Duration of tick attachment as a predictor of the risk of Lyme disease in an area in which Lyme disease is endemic. J. Infect. Dis., v.175, p.996-999, 1997.
- WALKER, A.R. Age structure of a population of Ixodes ricinus (Acari: Ixodidae) in relation to its seasonal questing. Bull. Entomol. Res, v.91, p.69-78, 2001.
Publication Dates
-
Publication in this collection
05 May 2008 -
Date of issue
Feb 2008
History
-
Received
24 Oct 2006 -
Accepted
26 Nov 2007