Abstracts
After a serious injury or sudden death, epididymis cauda sperm recovery and cryopreservation may present as the last opportunity to obtain genetic material from a valuable stallion. This study evaluated the viability of cooled equine sperm collected by three different methods: sperm of ejaculated (G1), sperm recovered from the epididymal cauda immediately after orchiectomy (G2) and sperm recovered from the epididymal cauda after storage for 24 hours at 5°C (G3). To obtain G1 sperm, two ejaculates were collected. After 1 week, all stallions underwent a bilateral orchiectomy, and one of the removed epididymides was flushed to obtain G2 sperm. The contralateral epididymis was stored at 5°C for 24 hours before being flushed to obtain G3 sperm. The sperm samples were evaluated immediately after the addition of the refrigeration extender, and after 24 and 48 hours of storage at 5°C. After 24 and 48 hours of storage, the epididymal sperm demonstrated higher motility traits when compared to the ejaculated sperm (P<0.05). These results indicate that sperm recovered from the epididymal cauda of stallions are more resistant to the cooling process, with higher kinetic parameters and plasma membrane integrity when compared to the ejaculated sperm.
horse epididymal spermatozoa; cryopreservation; stallion; sperm viability; seminal plasma
A recuperação de espermatozoides da cauda do epidídimo pode ser a última chance para preservação do germoplasma quando ocorre morte súbita ou lesão grave em garanhões de alto valor genético. O presente trabalho comparou a viabilidade após refrigeração dos espermatozoides do ejaculado (G1), recuperados da cauda do epidídimo imediatamente após a orquiectomia (G2) e recuperados após armazenamento do epidídimo por 24 horas a 5ºC (G3). No G1 foram colhidos dois ejaculados. Uma semana após a colheita dos ejaculados os garanhões foram submetidos à orquiectomia bilateral e realizada a colheita dos espermatozoides da cauda do epidídimo de um testículo de cada garanhão (G2). O testículo contralateral permaneceu a 5°C por 24 horas, antes da recuperação espermática (G3). A análise das amostras foi realizada imediatamente após a adição do meio de refrigeração, e após 24 e 48 horas de armazenamento a 5°C. Após 24 e 48 horas de armazenamento, os espermatozoides do epidídimo demonstraram características de cinética maiores que os do ejaculado (P<0.05). Estes resultados indicam que espermatozoides recuperados da cauda do epidídimo foram mais resistentes ao processo de refrigeração, com maiores parâmetros de cinética espermática e integridade da membrana plasmática quando comparados aos espermatozoides do ejaculado.
espermatozoide do epidídimo de garanhão; criopreservação; garanhão; viabilidade espermática; plasma seminal
VETERINARY MEDICINE MEDICINA VETERINÁRIA
Cooling of ejaculated and epididymal stallion sperm
Refrigeração de espermatozoides do ejaculado e epidídimo em garanhões
G.A. Monteiro; P.N. Guasti; F.P. Hartwig; J.A. Dellaqua Jr.; M.A. Alvarenga; F.O. Papa
Faculdade de Medicina Veterinária e Zootecnia - UNESP-Botucatu, SP
ABSTRACT
After a serious injury or sudden death, epididymis cauda sperm recovery and cryopreservation may present as the last opportunity to obtain genetic material from a valuable stallion. This study evaluated the viability of cooled equine sperm collected by three different methods: sperm of ejaculated (G1), sperm recovered from the epididymal cauda immediately after orchiectomy (G2) and sperm recovered from the epididymal cauda after storage for 24 hours at 5°C (G3). To obtain G1 sperm, two ejaculates were collected. After 1 week, all stallions underwent a bilateral orchiectomy, and one of the removed epididymides was flushed to obtain G2 sperm. The contralateral epididymis was stored at 5°C for 24 hours before being flushed to obtain G3 sperm. The sperm samples were evaluated immediately after the addition of the refrigeration extender, and after 24 and 48 hours of storage at 5°C. After 24 and 48 hours of storage, the epididymal sperm demonstrated higher motility traits when compared to the ejaculated sperm (P<0.05). These results indicate that sperm recovered from the epididymal cauda of stallions are more resistant to the cooling process, with higher kinetic parameters and plasma membrane integrity when compared to the ejaculated sperm.
Keywords: horse epididymal spermatozoa, cryopreservation, stallion, sperm viability, seminal plasma
RESUMO
A recuperação de espermatozoides da cauda do epidídimo pode ser a última chance para preservação do germoplasma quando ocorre morte súbita ou lesão grave em garanhões de alto valor genético. O presente trabalho comparou a viabilidade após refrigeração dos espermatozoides do ejaculado (G1), recuperados da cauda do epidídimo imediatamente após a orquiectomia (G2) e recuperados após armazenamento do epidídimo por 24 horas a 5ºC (G3). No G1 foram colhidos dois ejaculados. Uma semana após a colheita dos ejaculados os garanhões foram submetidos à orquiectomia bilateral e realizada a colheita dos espermatozoides da cauda do epidídimo de um testículo de cada garanhão (G2). O testículo contralateral permaneceu a 5°C por 24 horas, antes da recuperação espermática (G3). A análise das amostras foi realizada imediatamente após a adição do meio de refrigeração, e após 24 e 48 horas de armazenamento a 5°C. Após 24 e 48 horas de armazenamento, os espermatozoides do epidídimo demonstraram características de cinética maiores que os do ejaculado (P<0.05). Estes resultados indicam que espermatozoides recuperados da cauda do epidídimo foram mais resistentes ao processo de refrigeração, com maiores parâmetros de cinética espermática e integridade da membrana plasmática quando comparados aos espermatozoides do ejaculado.
Palavras-chave: espermatozoide do epidídimo de garanhão, criopreservação, garanhão, viabilidade espermática, plasma seminal
INTRODUCTION
The use of cooled semen during artificial insemination (AI) is the most commonly used technique in equine reproduction. The cryopreservation of equine semen has gained popularity because it prolongs storage time. It also offers additional benefits, including an easier way to deliver and preserve genetic material. The marketing and application possibilities of semen have also increased significantly since the advent of cryopreservation (Nunes et al., 2006). The preservation of spermatozoa at low temperatures reduces its catabolism and prevents further damage to cells (Squires, 1999; Ball, 2008). The use of cooled semen in AI has a similar pregnancy rate when compared to fresh semen (Jasko, 1992; D arenius, 1998). Some stallion's sperm respond more favorably to cooling than others (Brinsko et al., 2000). This finding depends not only on the quality of the semen but also on the composition of the seminal plasma and the cell membrane (Aurich, 2005).
Despite the benefits during mating, seminal plasma has been reported to have deleterious effects on in vitro preservation of cooled semen (Pickett et al., 1975; Rigby et al., 2001; Todd et al., 2001). Many studies have questioned the necessity of seminal plasma in the fertilization process because the sperm recovered from epididymal cauda show a high conception rate (Papa et al., 2008; Monteiro et al., 2011a). In addition, studies have reported that seminal plasma can damage semen during the cooling and freezing process, and it may also decrease the fertility of both fertile (Moore and Hibbit, 1976; Aurich et al., 1996) and subfertile stallions (Monteiro et al., 2011b). It is suggested that seminal plasma may cause biochemical changes that lead to increased plasmatic membrane permeability, which promotes cellular injury (Moore and Hibbit, 1976; Moustafa and Mezaros, 1981).
The recent successful fertility rates achieved in horses using sperm recovered from the epididymal cauda highlight the importance of studying the effects of cryopreservation on these cells (Papa et al., 2008; Monteiro et al., 2011a). Such studies become even more relevant when it is considered that the harvesting of sperm from epididymal cauda may allow for a final opportunity to obtain semen from deceased stallions.
The aim of this study was to compare the viability of cooled equine sperm collected using an artificial vagina (Control, G1), with the sperm recovered from the epididymal cauda immediately after orchiectomy and the sperm recovered from the epididymal cauda after storage for 24 hours at 5°C.
MATERIAL AND METHODS
Eight stallions of different breeds, four Brazilian Jumping Horses, two Lusitano and two Mangalarga Marchador, ranging in age from 4 to 6 years, were used in this study. Initially, three ejaculates from each stallion were collected, with an interval of 2 days, to eliminate possible damaged cells from the epididymal cauda and to stabilize the sperm parameters. These same animals provided the sperm for the G1, G2 and G3 samples.
For G1, two ejaculates were collected from each stallion over a 1-week interval using an artificial vagina. One week after the last semen collection using the artificial vagina, the same stallions underwent a bilateral orchiectomy. In G2, the harvesting of the sperm from the epididymal cauda was performed immediately after the surgery, alternating between right and left epididymides. In G3, the contralateral testis and epididymis was stored in a passive refrigeration container (Botutainer®) at 5°C for 24 hours, before the harvest of the epididymal sperm. Recovery of the cauda epididymal sperm was performed using the flushing technique, as reported by Garde et al. (1994) and Bruemmer et al. (2002). In all groups, the refrigeration extender (Botu-Sêmen®) was added to the standard concentration of 50 million (50 x 106) viable sperm per milliliter. After the dilution, the samples were stored in a passive cooling system (Botutainer®), which has a refrigeration rate of 0.03ºC per minute from room temperature to its stabilization at 5ºC. The samples were stored for up to 48 hours.
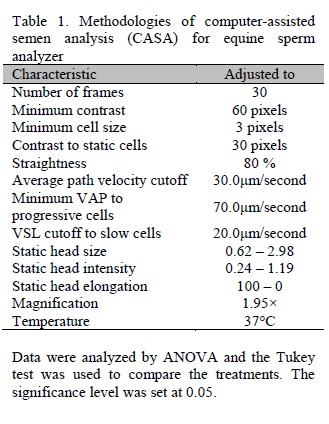
Ejaculated sperm collected by an artificial vagina and sperm recovered from the epididymal cauda were evaluated by the computadorized method CASA (HTM-IVOS 12), immediately after the addition of the refrigeration extender, and after 24 and 48 hours of storage at 5°C. The CASA setup is described in Table 1. The following parameters were evaluated: total motility (TM, %), progressive motility (PM, %), angular progressive velocity (VAP, μm/s), progressive velocity (VSL, μm/s) and percentage of rapid sperm (RAP, %). An aliquot of each sample was evaluated for plasma membrane integrity (PMI; %) using epifluorescence microscopy (Harrison and Vickers, 1990).
Data were analyzed by ANOVA and the Tukey test was used to compare the treatments. The significance level was set at 0.05.
RESULTS
As shown in Table 2, no differences in the sperm kinetic parameters in the fresh samples were observed. However, the membrane integrity (PMI) in G1 samples was significantly lower (P<0.05) when compared to sperm recovered from the cauda epididymis immediately after orchiectomy (G2).
In evaluating the sperm stored for 24 hours at 5°C (Table 3), there were no differences in the VAP and VSL traits between the tested groups. However, the TM, RAP and PMI traits were significantly lower (P<0.05) for G1 samples when compared to G2 and G3 samples. In addition, the PM was higher in the G2 when compared to the G1 samples.
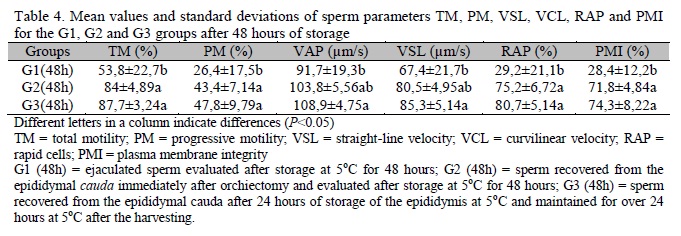
For the groups evaluated after 48 hours of cooling at 5°C (Table 4), TM, PM, RAP and PMI were significantly lower (P<0.05) for G1 when compared to G2 and G3 samples. Moreover, the VAP and VSL traits were higher in the G3 samples when compared to G1 samples.
DISCUSSION
No differences were noted in TM, PM and RAP between G1 and G2 samples. These results agree with those reported by Monteiro et al. (2011a), where a similarity in the kinetic parameters between the ejaculated and epididymal sperm was observed before freezing.
However, after 24 and 48 hours of storage, the epididymal sperm demonstrated higher motility parameters when compared to the ejaculated sperm. Data previously reported by Aurich et al. (1996) demonstrated that, in stallions with a decreased resistance to freezing, deleterious effects on sperm viability were noted when more than 20% of the seminal plasma was present. Sperm viability in the present study may have been better because sperm recovered from the epididymal cauda does not have any contact with seminal plasma. These findings are similar to those reported by Monteiro et al. (2011b), who observed increased viability in the epididymal sperm of subfertile stallions, both before freezing and after thawing, when compared to ejaculated sperm.
The increased success reported when using epididymal sperm suggests that seminal plasma leads to a decrease in sperm motility parameters during storage at low temperatures. This hypothesis is supported by studies that demonstrated that certain proteins of the seminal plasma can promote biochemical changes, causing damage to the plasma membrane, leading to decreases in sperm freezability and fertility (Moore and Hibbit, 1976; Moustafa and Mezaros, 1981).
The seminal plasma of stallions has a high concentration of sodium when compared to the other species (Mann, 1981). This peculiarity can affect the sperm quality because high levels of Na++ and K+ can induce spontaneous lipid peroxidation in the sperm's membrane (Alvarez and Storey, 1982). Therefore, it is possible that the electrolytes in the seminal plasma may have contributed to the decrease in motility parameters and membrane integrity of the ejaculated sperm in this study. This hypothesis is supported by Kareskoski et al. (2005) who demonstrated that the pre-sperm fluid of stallions has a high concentration of Cl- and Na++, and these substances are reported to be detrimental to sperm survival during the cooling and storage process (Nishikawa, 1959; Padilla and Foote, 1991; Kareskoski et al., 2006). Additionally, Monteiro et al. (2011) observed higher amount of bent tails and consequently lower total and progressive motility of ejaculated sperm when compared to epididymal sperm.
In the present study, G1 samples demonstrated lower rates of membrane integrity in relation to epididymal sperm from G2 and G3. These findings agree with those reported by Barrier-Battut et al. (2010) who demonstrated that the removal of seminal plasma, before cooling to 4°C for 48 hours, increased the stability of the plasma membrane. These results were obtained by evaluating the number of reactive sperm after a hyposmotic test.
CONCLUSIONS
Sperm recovered from the epididymal cauda of stallions are more resistant to the process of cooling, and the kinetic parameters and PMI increases as compared to ejaculated sperm. Furthermore, this study demonstrates that even after the death of a stallion, it is possible to preserve genetic material through the process of cooling.
ACKNOWLEDGMENTS
The authors acknowledge FAPESP for their financial support.
Recebido em 25 de novembro de 2011
Aceito em 4 de dezembro de 2012
E-mail: monteiroga@yahoo.com.br
- ALVAREZ, J.G.; STOREY, B.T. Spontaneous lipid peroxidation in rabbit epididymal spermatozoa: its effect on sperm motility. Biol. Reprod, v.27, p.1102-1108, 1982.
- AURICH, J.E.; KUHNE, A.; HOPPE, H. et al Seminal plasma affects membrane integrity and motility of equine spermatozoa after cryopreservation. Theriogenology, v.46, p.791-797, 1996.
- AURICH, C. Factors affecting the plasma membrane function of cooled-stored stallion spermatozoa. Anim. Reprod. Sci, v.89, p.65-65, 2005.
- BALL, B.A. Oxidative stress, osmotic stress and apoptosis: Impacts on sperm function and preservation in the horse. Anim. Reprod. Sci, v.107, p.257-257, 2008.
- BARRIER-BATTUT, I.; BONNET, C.; GIRAUDO, A. et al Removal of seminal plasma by centrifugation, before cooled storage, enhances membrane stability of stallion spermatozoa. Anim. Reprod. Sci, v.121, p.188-200, 2010.
- BRINSKO, S.P.; CROCKETT, E.C.; SQUIRES, E.L. Effect of centrifugation and partial removal of seminal plasma on equine spermatozoa motility after cooling and storage. Theriogenology, v.54, p.129-126, 2000.
- BRUEMMER, J.E.; REGER, H.; ZIBINSKI, G. et al Effect of storage at 5°C on the motility and cryopreservation of stallion epididymal spermatozoa. Theriogenology, v.58, p.5-7, 2002.
- DARENIUS, A. Experiences with chilled, transported equine semen. In: STALLION REPRODUCTION SYMPOSIUM, 1998, Montgomery. Proceedings.. Montgomery: Society for Theriogenology, American Association of Equine Practitioners, 1998. p.60-70.
- GARDE, J.; AGUADO, M.; PEREZ, S. et al Physiological characteristics of epididymal spermatozoa from postmortem rams. Theriogenology, v.41, p.2003, 1994.
- HARRISON, R.A.P.; VICKERS, S.E. Use of fluorescent probes to assess membrane integrity in mammalian spermatozoa. J. Reprod. Fertil, v.88, p.343-342, 1990.
- JASKO, D.J.; SQUIRES, E.L.; MORAN, D.M. et al Comparison of pregnancy rates utilizing fresh, cooled and frozen semen. In: INTERNATIONAL CONGRESS ANIMAL REPRODUCTION, 12., 1992, The Hague. Proceedings.. The Hague: ICAR; 1992. v.3, p.1439-1441.
- KARESKOSKI, A.M.; REILAS, T.; SANKARI, S. et al Composition of fractionated stallion ejaculates. Anim. Reprod. Sci, v.89, p.228-230, 2005.
- KARESKOSKI, A.M.; REILAS, T.; ANDERSSON, M. et al Motility and plasma membrane integrity of spermatozoa in fractionated stallion ejaculates after storage. Reprod. Domest. Anim, v.4, p.133-138, 2006.
- MONTEIRO, G.A.; PAPA, F.O.; ZAHN, F.S. et al Cryopreservation and fertility of ejaculated and epididymal stallion sperm. Anim. Reprod. Sci, v.127, p.197-201, 2011.
- MONTEIRO, G.A.; PAPA, F.O.; GUASTI, P.N. et al Fertilidade de espermatozoides recuperados da cauda do epidídimo de garanhões subférteis. Vet. Zootec, v.18, p.255-253, 2011.
- MANN, T.; LUTWAK-MANN, C. Male reproductive function and semen Berlin: Springer-Verlag, 1981.
- MOORE, H.D.M.; HIBBIT, K.C.S. The binding of labeled basic proteins by boar spermatozoa. J. Reprod. Fertil. Suppl, v.46, p.71-76, 1976.
- MOUSTAFA, A.R.; MEZAROS, I. Interrelationship between the total protein content of bovine seminal plasma and behavior of spermatozoa after freezing-and-thawing. Acta Vet.Acad.Sci.Hung., v.28, p.403-408, 1981.
- NISHIKAWA, Y. Studies on reproduction in horses: singularity and artificial control in reproductive phenomena. Japan: Japan Racing Association, 1959.
- NUNES, D.B.; ZUCCARI, C.E.S.N.; COSTA E SILVA, E.V. Fatores relacionados ao sucesso da inseminação artificial de éguas com sêmen refrigerado. Rev. Bras. Reprod. Anim, v.30, p.42-56, 2006.
- PADILLA, A.W.; FOOTE, R.H. Extender and centrifugation effects on the motility patterns of slow-cooled stallion spermatozoa. J. Anim. Sci, v.69, p.3308-3313, 1991.
- PAPA, F.O.; MELO, C.M.; FIORATTI, E.G. et al Freezing of stallion epididymal sperm. Anim. Reprod. Sci, v.107, p.293-291, 2008.
- PICKETT, B.W.; SULLIVAN, J.J.; BYERS, W.W. et al Effect of centrifugation and seminal plasma on motility and fertility of stallion and bull spermatozoa. Fertil. Steril, v.26, p.167-164, 1975.
- RIGBY, S.L.; BRINSKO, S.P.; COCHRAN, M. et alAdvances in cooled semen technologies: seminal plasma and semen extender. Anim. Reprod. Sci, v.68, p.171-180, 2001.
- SQUIRES, E.L.; PICKETT, B.W.; GRAHAM, J.K. et al Cooled and frozen stallion semen. Fort Collins: Animal Reproduction and Biotechnology Laboratory, 1999. p.1-38.
- TODD, P.; ARNS, M.J.; CHENOWETH, P. et al Influence of seminal plasma and processing on cold-stored stallion spermatozoa. Anim. Reprod. Sci, v.68, p.335-336, 2001.
Publication Dates
-
Publication in this collection
16 July 2013 -
Date of issue
June 2013
History
-
Received
25 Nov 2011 -
Accepted
04 Dec 2012