Abstracts
Stem cell therapy has shown promising results in tendinitis and osteoarthritis in equine medicine. The purpose of this work was to characterize the adipose-derived mesenchymal stem cells (AdMSCs) in horses through (1) the assessment of the capacity of progenitor cells to perform adipogenic, osteogenic and chondrogenic differentiation; and (2) flow cytometry analysis using the stemness related markers: CD44, CD90, CD105 and MHC Class II. Five mixed-breed horses, aged 2-4 years-old were used to collect adipose tissue from the base of the tail. After isolation and culture of AdMSCs, immunophenotypic characterization was performed through flow cytometry. There was a high expression of CD44, CD90 and CD105, and no expression of MHC Class II markers. The tri-lineage differentiation was confirmed by specific staining: adipogenic (Oil Red O), osteogenic (Alizarin Red), and chondrogenic (Alcian Blue). The equine AdMSCs are a promising type of adult progenitor cell for tissue engineering in veterinary medicine.
immunophenotypic characterization; differentiation; equine; adipose tissue; mesenchymal stem cell
O uso de células tronco tem demonstrado resultados promissores na terapia da tendinite e osteoartrite na medicina equina. O objetivo deste trabalho foi caracterizar as células tronco mesenquimais derivadas do tecido adiposo (AdCTMs) em cavalos através da (1) avaliação da capacidade das células progenitoras para realizar a diferenciação adipogênica, osteogênica e condrogênica; e (2) através da análise por citometria de fluxo, utilizando os marcadores stemness relacionados: CD44, CD90, CD105 e MHC de Classe II. Cinco cavalos sem raça definida, de 2 a 4 anos de idade foram utilizados para a coleta do tecido adiposo da base da cauda. Após o isolamento e cultivo das AdCTMs, a caracterização imunofenotípica foi realizada pela citometria de fluxo. Houve alta expressão dos marcadores CD44, CD90 e CD105, e não houve expressão do MHC Classe II. A diferenciação foi confirmada pela coloração específica: adipogênica (Oil Red O), osteogênico (Alizarin Red), e condrogênico (Alcian Blue). As AdCTMs são um tipo promissor de células progenitoras adulta para a engenharia tecidual na medicina veterinária.
caracterização imunofenotípica; diferenciação; equino; tecido adiposo; célula tronco mesenquimal
VETERINARY MEDICINE MEDICINA VETERINÁRIA
Characterization of mesenchymal stem cells derived from equine adipose tissue
Caracterização das células tronco mesenquimais derivadas do tecido adiposo equino
A.M. CarvalhoI; A.L.M. YamadaI; M.A. GolimII; L.E.C. ÁlvarezII; L.L. JorgeI; M.L. ConceiçãoI; E. DeffuneI; C.A. HussniI; A.L.G. AlvesI
IFaculdade de Medicina Veterinária e Zootecnia - Universidade Estadual Paulista - Botucatu, SP
IIFaculdade de Medicina de Botucatu - Universidade Estadual Paulista - Botucatu, SP
ABSTRACT
Stem cell therapy has shown promising results in tendinitis and osteoarthritis in equine medicine. The purpose of this work was to characterize the adipose-derived mesenchymal stem cells (AdMSCs) in horses through (1) the assessment of the capacity of progenitor cells to perform adipogenic, osteogenic and chondrogenic differentiation; and (2) flow cytometry analysis using the stemness related markers: CD44, CD90, CD105 and MHC Class II. Five mixed-breed horses, aged 2-4 years-old were used to collect adipose tissue from the base of the tail. After isolation and culture of AdMSCs, immunophenotypic characterization was performed through flow cytometry. There was a high expression of CD44, CD90 and CD105, and no expression of MHC Class II markers. The tri-lineage differentiation was confirmed by specific staining: adipogenic (Oil Red O), osteogenic (Alizarin Red), and chondrogenic (Alcian Blue). The equine AdMSCs are a promising type of adult progenitor cell for tissue engineering in veterinary medicine.
Keywords: immunophenotypic characterization, differentiation, equine, adipose tissue, mesenchymal stem cell
RESUMO
O uso de células tronco tem demonstrado resultados promissores na terapia da tendinite e osteoartrite na medicina equina. O objetivo deste trabalho foi caracterizar as células tronco mesenquimais derivadas do tecido adiposo (AdCTMs) em cavalos através da (1) avaliação da capacidade das células progenitoras para realizar a diferenciação adipogênica, osteogênica e condrogênica; e (2) através da análise por citometria de fluxo, utilizando os marcadores stemness relacionados: CD44, CD90, CD105 e MHC de Classe II. Cinco cavalos sem raça definida, de 2 a 4 anos de idade foram utilizados para a coleta do tecido adiposo da base da cauda. Após o isolamento e cultivo das AdCTMs, a caracterização imunofenotípica foi realizada pela citometria de fluxo. Houve alta expressão dos marcadores CD44, CD90 e CD105, e não houve expressão do MHC Classe II. A diferenciação foi confirmada pela coloração específica: adipogênica (Oil Red O), osteogênico (Alizarin Red), e condrogênico (Alcian Blue). As AdCTMs são um tipo promissor de células progenitoras adulta para a engenharia tecidual na medicina veterinária.
Palavras-chave: caracterização imunofenotípica, diferenciação, equino, tecido adiposo, célula tronco mesenquimal
INTRODUCTION
The clinical use of cellular therapy and tissue engineering in veterinary medicine is developing rapidly. The relative abundance and easy access to adipose tissue has raised great interest among researchers using these cells as a source of mesenchymal stem cells (MSCs) in equine medicine. There are positive results related to the use of adipose-derived mesenchymal stem cells (AdMSCs) in disorders such as tendinitis (Del Bue et al., 2008; De Mattos Carvalho et al., 2011).
Traditionally, the process of isolating the nucleated cell fraction from the adipose tissue is based on collagenase digestion, followed by a series of centrifugation steps to isolate the specific cells. This cell fraction is referred to as stromal vascular fraction (SVF). The SVF is a heterogeneous cell population including endothelial and epithelial cells, fibroblasts, mastocytes, preadipocytes, and MSCs. (Zuk et al., 2001). The amount of MSCs within the SVF seems to be directly related to the amount of adipose tissue collected. Nevertheless, there is a considerable variation from one animal to another (Vidal and Lopez, 2011). The separation of equine AdMSCs from the SVF is based on the principle of adhesion, which allows isolation and characterization of a homogeneous cell population for further use in cell therapy (Pascucci et al., 2011).
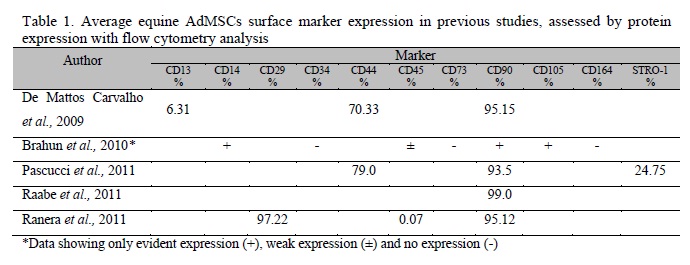
Several studies reported the characterization of AdMSCs in horses, where the most common processes are osteogenic, adipogenic and chondrogenic (tri-lineage) differentiation of progenitor cells (Vidal et al., 2007, 2008; Kisiday et al., 2008; Mambelli et al., 2009; Colleoni et al., 2009; Toupadakis et al., 2010; Ahern et al., 2011; Schwarz et al., 2011a,b). Only five recent studies have reported the analyses of surface antigens of AdMSCs by flow cytometry (de Mattos Carvalho et al., 2009; Braun et al., 2010; Pascucci et al., 2011; Raabe et al., 2011; Ranera et al., 2011). The results from these studies are demonstrated in Table 1.
Assessments of the results from previous studies have shown CD90 as the only marker used in every experiment, disregarding the use of other markers. It was also demonstrated that CD90 is expressed in over 90% of equine AdMSCs. However, despite these advantages, there is no species-specific CD90 antibody for use in horses. Moreover, CD90 is also expressed in fibroblasts (Sorrel and Caplan, 2009), which are morphologically similar to stem cells maintained in culture. Unlike antigen CD34, which is used as a positive marker for the immunoselection of hematopoietic stem cells (Krause et al., 1996), a unique antibody for MSCs characterization has not yet been identified. Thus, multiple markers have to be used together for flow cytometry and tri-lineage differentiation in order to characterize equine AdMSCs. However, identifying one or more surface markers that positively identify MSCs has proven to be more challenging for two main reasons: (1) there are clear interspecies differences in cell surface marker expression; and (2) the immunophenotype of putative MSCs changes with time in culture (Stewart and Stewart, 2011).
De Schauwer and collaborators (2011) proposed a standard protocol to ensure the correct characterization of equine MSCs. The isolated cells must be plastic-adherent under standard culture conditions. The tri-lineage differentiation should be confirmed using standard in vitro tissue culture-differentiating conditions. Furthermore, equine MSCs should express CD29, CD44 and CD90 and not express CD14, CD79 and MHC-II.
Based on this new panel, the aim of the present work was to characterize the AdMSCs in horses through (1) the assessment of the capacity of progenitor cells to perform adipogenic, osteogenic and chondrogenic differentiation; and (2) through flow cytometry analysis using the stemness related markers: CD44, CD90, CD105 and MHC Class II.
MATERIALS AND METHODS
The experimental protocol was approved by the Committee of Ethics and Animal Welfare of the School of Veterinary Medicine and Animal Science, São Paulo State University, Brazil, and was performed under international guidelines for the care and use of experimental animals. Five horses (1 male and 4 female) of undefined breed, aging between 2 and 4 years were used.
For adipose tissue collection the horses were sedated with xylazine (0.7mg/kg, i.v.) followed by s.c. injection of 2% lidocaine chloride with epinephrine using an inverted L-block. An incision of approximately 8cm in length was performed parallel to and approximately 15cm below the spinal column. Approximately 2mL of adipose tissue was collected and stored in a sterile 50mL conic tube containing RPMI-1640 medium (Sigma Chemical Co., St. Louis, MO, USA), the skin was sutured with nylon using a simple separated pattern.
To perform the isolation of stromal vascular fraction cells, the adipose tissue was submitted to successive washes with PBS followed by mechanical separation using a no. 15 scalpel blade and the digestive action of 0.02% of type I collagenase (Gibco, Grand Island, NY, USA) in RPMI-1640 medium, in a humidified heater at 37°C, under 5% CO2 for 12 h. The resulting solution was neutralized with Knockout DMEM (Dulbecco's modified Eagle's medium, Gibco, Grand Island, NY, USA) containing 10% FBS, followed by centrifugation with a relative centrifugal force (RCF) of 260 x g for 10min and the supernatant was aspirated and added to PBS and homogenized for future centrifugation.
The SVF was suspended on two 70cm2 cell culture plates and maintained in a heater at 37ºC and 5.0% CO2, using the Knockout DMEM medium with 10% FBS. The medium was changed every 2 days until a minimum confluence of 70% of the plate occurred. Then, trypsinization was conducted. Fifty microliters were collected and transferred to a hemolysis tube for cell count. The cells were quantified using hemocytometer to evaluate cellular viability by the Trypan Blue (Gibco, Grand Island, NY, USA) exclusion test. The remainder was transferred in equal volumes to two 75cm2 culture plates that were further incubated at 37ºC and 5.0% CO2. Cell culture was maintained up to the third passage using samples from two horses and up to the second passage for the remaining horses, after which the cell count and MSCs characterization were conducted.
The progenitor cells were differentiated toward adipogenic, osteogenic, and chondrogenic lineages to demonstrate their multipotentiality, in agreement with the stated requirements to ensure characterization of equine MSCs (De Schauwer et al., 2011). All of the differentiations were performed in triplicate, and a sample was kept in basal medium during 14 days (adipogenic and osteogenic), and 21 days (chondrogenic) to ensure the non-differentiation of control cells.
For adipogenic differentiation, cells in passage two were plated at 20,000 cells/cm2 in 24-well plates and cultured under adipogenic conditions for 14 days. The medium (STEMPRO®, Gibco, Grand Island, NY, USA) was changed every 3 days, the cells were fixed in 10% formaldehyde solution for 10 minutes, rinsed with PBS and stained with Oil Red O (Gibco, Grand Island, NY, USA).
To perform the osteogenic characterization of AdMSCs, the progenitor cells at passage 3 were plated at 20,000 cells/cm2 in 24-well plates and cultured ender osteogenic conditions for 14 days, the medium (STEMPRO®, Gibco, Grand Island, NY, USA) was changed every 3 days, the cells were fixed in 10% formaldehyde solution for 10 minutes, rinsed with sterile water and stained with Alizarin Red (Gibco, Grand Island, NY, USA).
Chondrogenesis was induced in micromass pellet cultures prepared with 1×106 cells placed in a 15mL polypropylene conical tube. The pellet was cultured at 37°C with 5% CO2 in 2mL of chondrogenic medium (STEMPRO®, Gibco, Grand Island, NY, USA), the medium was changed every 3 days. Following a 3-week incubation period, the pellet was fixed in 10% formaldehyde solution for 24 hours at room temperature, embedded in paraffin and cut into 5μm sections, and was stained with haematoxylin for general histology and Alcian blue dye to detect sulfated proteoglycans.
The selection of the antibodies was partly based on the state of the art knowledge in equine MSCs research. Thus, equine progenitor cells should express CD29, CD44, and CD90 and not express CD14, CD79, and MHC-II (De Schauwer et al., 2011). Flow cytometry was performed with a FACSCalibur (BD, San Jose, CA, USA), from the first to the third passage using the mouse anti-rat CD90-FITC monoclonal antibody (mAb) (Caltag Laboratories, Burlingame, CA, USA) and mouse anti-human CD105-FITC mAb (AbD Serotec, Kidlington, Oxford, UK) to evaluate interspecies expression; and the specific mAb mouse anti-horse CD44 (AbD Serotec, Kidlington, Oxford, UK) and mouse anti-horse MHC Class II (AbD Serotec, Kidlington, Oxford, UK), which were marked with secondary mAb goat anti-mouse IgG-FITC (Molecular Probes, Eugene, OR, USA) (Figure 1).
RESULTS
Adipose tissue was successfully collected and a mean of 2g of material was obtained followed by isolation of AdMSCs. Regarding the animals, skin incisions healed without complications. Colonies of fibroblast-like cells were observed in all of the cultures the day following isolation and plating. Cell culture was maintained up to the third passage using samples from two horses and up to the second passage for the remaining horses.
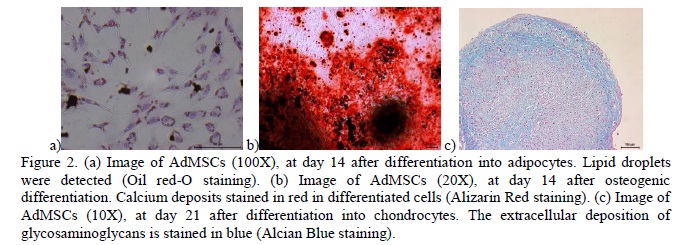
The ability for tri-lineage differentiation of AdMSCs was confirmed using the human commercial differentiation media (Gibco, Grand Island, NY, USA) (Figure 2). Oil red O-stained lipid droplets were observed inside the cells under adipogenic induction. The osteogenic differentiation was confirmed with Alizarin Red staining on differentiated cells, and where the calcium deposits formed during osteogenic differentiation the stains were done in red. Control cultures did not exhibit differentiation. Chondrogenic potential was evaluated using the pellet culture system. Cells undergoing chondrogenic differentiation appeared blue when stained with Alcian blue, due to the sulfated proteoglycans produced in the extracellular matrix during cartilage differentiation.
Flow cytometry analysis revealed the expression of CD90 and CD105 in all the passages tested (1st to 3rd), determining interspecies cross-reaction between rat and horse, and between human and horse, respectively. CD44 also reacted with MSCs in all the passages. As expected, there was no reaction of the MHC Class II marker on MSCs (Table 2).
DISCUSSION
The techniques to retrieve SVF from adipose tissue followed by culture for expansion and isolation of MSCs in our study are similar to the techniques described before (Vidal et al., 2007). Our results confirmed the adherence of MSCs in culture in <48 hours (Schäffler and Büchler, 2007).
The potential for differentiation within several mesenchymal cell lineages is an integral component of the MSCs phenotype. Tri-lineage differentiation capacity is possible because of the requirements for osteogenic, chondrogenic, and adipogenic differentiation as well as the appropriate phenotype-specific assays for these lineages which are well characterized in vitro (Stewart and Stewart, 2011).
The unequivocal immunophenotyping of equine MSCs is hampered by the lack of a specific marker and the limited availability of monoclonal anti-horse antibodies. To date, commercial antibodies recognizing equine epitopes are only available for CD13, CD44 and MHC-II (De Schauwer et al., 2011). Thus, we made the immunophenotypic characterization of isolated cells using the species-specific markers CD44 and MHC Class II as well as markers from other species, such as CD90, CD105. We did not use the marker horse anti-mouse CD13, because of its lack of expression in equine MSCs in previous studies (de Mattos Carvalho et al., 2009; Radcliffe et al., 2010).
The data from our experiment demonstrates compatibility with the results from other studies (Table 1), confirming the requirements proposed by De Schauwer and colleagues (2011) for the appropriate characterization of MSCs from horses. However, despite our results and the results from others (Braun et al., 2010; Pascucci et al., 2011; Raabe et al., 2011; Ranera et al., 2011), comprehensive research has to be conducted, regarding new markers to improve this protocol for characterization of equine MSCs. It is also important to determine whether any given immunophenotype is clinically superior to unsorted or alternatively sorted cell populations (Stewart and Stewart, 2011).
CONCLUSIONS
The present study showed the tri-lineage differentiation and the immunophenotypic characterization of the surface of equine AdMSCs using the CD44, CD90, CD105 and MHC Class II markers classifying these cells as a promising type of progenitor cell that can be used in equine cellular therapy. These findings will also optimize AdMSCs phenotype definition in further studies related to the characterization of these cells.
ACKNOWLEDGEMENT
This work was supported by grants from the São Paulo Research Foundation (FAPESP) process 2009/10670-8 and 2010/03567-3.
Recebido em 26 de março de 2012
Aceito em 6 de maio de 2013
E-mail: armandodvm@gmail.com
- AHERN, B.J.; SCHAER, T.P.; TERKHORN, S.P. et al Evaluation of equine peripheral blood apheresis product, bone marrow, and adipose tissue as sources of mesenchymal stem cells and their differentation potential. Am. J. Vet. Res, v.72, p.127-133, 2011.
- BRAUN, J.; HACK, A.; WEIS-KLEMM, M. et al Evaluation of the osteogenic and chondrogenic differentiation capacities of equine adipose tissuederived mesenchymal stem cells. Am. J. Vet. Res, v.71, p.1228-1236, 2010.
- COLLEONI, S.; BOTTANI, E.; TESSARO, I. et al Isolation, growth and differentiation of equine mesenchymal stem cells: effect of donor, source, amount of tissue and supplementation with basic fibroblast growth factor. Vet. Res. Commun., v.33, p.811-821, 2009.
- DEL BUE, M.; RICCÒ, S.; RAMONI, R. et al Equine adipose-tissue derived mesenchymal stem cells and platelet concentrates: their association in vitro and in vivo. Vet. Res. Commun, v.32, p.51-55, 2008.
- DE MATTOS CARVALHO, A.; ALVES, A.L.G.; GOLIM, M.A. et al Isolation and immunophenotypic characterization of mesenchymal stem cells derived from equine species adipose tissue. Vet. Immunol. Immunopathol, v.132, p.303-306, 2009.
- DE MATTOS CARVALHO, A.; ALVES, A.L.G.; OLIVEIRA, P.G.G. et al Use of adipose tissue-derived mesenchymal stem cells for experimental tendinitis therapy in equines. J. Equine Vet. Sci, v.31, p.26-34, 2011.
- DE SCHAUWER, C.; MEYER, E.; VAN DE WALLE, G.R. et al Markers of stemness in equine mesenchymal stem cells: a plea for uniformity. Theriogenology, v.75, p.1431-1443, 2011.
- KISIDAY, J.D.; KOPESKY, P.W.; EVANS, C.H. et al Evaluation of adult equine bone marrow and adipose-derived progenitor cell chondrogenesis in hydrogel cultures. J. Orthop. Res, v.26, p.322-331, 2008.
- KRAUSE, D.S.; FACKLER, M.J.; CIVIN, C.I. et al CD34: Structure, biology and clinical utility. Blood, v.87, p.1-13, 1996.
- MAMBELLI, L.I.; SANTOS, E.J.C.; FRAZÃO, P.J.R. et al Characterization of equine adipose tissue-derived progenitor cells before and after cryopreservation. Tissue Eng. Part C: Methods, v.15, p.87-94, 2009.
- PASCUCCI, L.; CURINA, G.; MERCATI, F. et al Flow cytometric characterization of culture expanded multipotent mesenchymal stromal cells (MSCs) from horse adipose tissue: Towards the definition of minimal stemness criteria. Vet. Immunol. Immunopathol, v.144, p.499-506, 2011.
- RAABE, O.; SHELL, K.; WÜRTZ, A. et al Further insights into the characterization of equine adipose tissue-derived mesenchymal stem cells. Vet. Res. Commun, v.35, p.355-365, 2011.
- RADCLIFFE, C.H.; FLAMINIO, M.J.B.F.; FORTIER, L.A. Temporal analysis of equine bone marrow aspirate during establishment of putative mesenchymal progenitor cell populations. Stem Cells Dev, v.19, p.269-81, 2010.
- RANERA, B.; LYAHYAI, J.; ROMERO, A. et al Immunophenotype and gene expression profiles of cell surface markers of mesenchymal stem cells derived from equine bone marrow and adipose tissue. Vet. Immunol. Immunopathol, v.144, p.147-154, 2011.
- SCHÄFFLER, A.; BÜCHLER, C. Concise Review: Adipose Tissue-Derived Stromal Cells-Basic and Clinical Implications for Novel Cell-Based Therapies. Stem Cells, v.25, p.818-827, 2007.
- SCHWARZ, C.; LEICHT, U.; DROSSE, I. et al Characterization of adipose-derived equine and canine mesenchymal stem cells after incubation in agarose-hydrogel. Vet. Res. Commun., v.35, p.487-499, 2011a.
- SCHWARZ, C.; LEICHT, U.; ROTHE, C. et al Effects of different media on proliferation and differentiation capacity of canine, equine and porcine adipose derived stem cells. Res. Vet. Sci, v.93, p.457-462, 2011b.
- SORREL, J.M.; CAPLAN, A.I. Chapter 4 Fibroblasts A diverse population at the center of it All. Int. Rev. Cell Molecular Biol., v.276, p.161- 214, 2009.
- STEWART, M.C.; STEWART, A.A. Mesenchymal stem cells: characteristics, sources, and mechanisms of action. Vet. Clin. North Am. Equine Pract, v.27, p.243-261, 2011.
- TOUPADAKIS, C.A.; WONG, A.; GENETOS, D.C. et al Comparison of the osteogenic potential of equine mesenchymal stem cells from bone marrow, adipose tissue, umbilical cord blood, and umbilical cord tissue. Am. J. Vet. Res., v.71, p.1237-1245, 2010.
- VIDAL, M.A.; KILROY, G.E.; LOPEZ, M.J. et al Characterization of equine adipose tissue-derived stromal cells: adipogenic and osteogenic capacity and comparison with bone marrow-derived mesenchymal stromal cells. Vet. Surgery, v.36, p.613-622, 2007.
- VIDAL, M.A.; LOPEZ, M.J. Adipogenic differentiation of adult equine mesenchymal stromal cells. Meth. Mol. Biology, v.702, p.61-75, 2011.
- VIDAL, M.A.; ROBINSON, S.O.; LOPEZ, M.J. Comparison of chondrogenic potential in equine mesenchymal stromal cells derived from adipose tissue and bone marrow. Vet. Surgery, v.37, p.713-724, 2008.
- ZUK, P.A.; ZHU, M.; MIZUNO, H. Multi-lineage cells from human adipose tissue: implication for cell-based therapies. Tissue Engineering, v.7, p.211, 2001.
Publication Dates
-
Publication in this collection
03 Sept 2013 -
Date of issue
Aug 2013
History
-
Received
26 Mar 2012 -
Accepted
06 May 2013