Abstracts
The effects of different fasting periods on glycemia levels and on cardiorrespiratory parameters in tiletamine-zolazepam-anesthetized cats were evaluated. Twenty one animals were randomly assigned to three groups: 8 hours (G8), 12 hours (G12) or 18 hours (G18) of the preoperative fasting. The tiletamine-zolazepam (2 mg/kg) was administered intravenously. The heart rate (HR), respiratory rate (fR), rectal temperature (T R), glycemia (G), laboratorial glycemia (Glab), venous oxygen partial pressure (PvO2), venous carbon dioxide partial pressure (PvCO2), venous hemoglobin saturation (SvO2), pH, base deficit (BD), bicarbonate concentration (HCO3- ) and haematocrit were evaluated at 90 minutes after the last meal (T0), immediately before anesthesia (T1) and at ten (T2) and thirty (T3) minutes after tiletamine-zolezepam administration. The time between the administration of anesthetic and the cat's trial to elevate head (Th) and the interval between drug administration and aniamal's quadrupedal position (Tqp) were recorded. No differences among groups were recorded for glycemia, HR, PvO2, SvO2, pH, BD, HCO3-, Ht and Tqp. In G12 from T2, glycemia increased and from T1 PvCO2 decreased. At T1, PvO2 increased in all groups. In G8 and G12, from T1, DB and HCO3- decreased. In G12 and G18, from T2, Ht decreased. In G12, the Th mean was higher than G8. In conclusion, in tiletamine-zolazepam-anesthetized cats, the different preoperative fasting did not influence glycemia, blood-gas and cardiorrespiratory parameters. Additionally, there was no relationship between glycemia and anesthesia recovery.
cats; dissociative anesthesia; glycemia; monitoring; recovery
Avaliaram-se os efeitos de diferentes períodos de jejum sobre os níveis glicêmicos e os parâmetros cardiorrespiratórios em gatos anestesiados com tiletamina-zolazepam. Vinte um animais foram distribuídos aleatoriamente em três grupos diferenciados entre si pelo período de jejum: oito horas (G8), doze horas (G12) e dezoito horas (G18). A tiletamina-zolazepam (2mg/kg) foi administrada por via intravenosa. A frequência cardíaca (FC), frequência respiratória (fR), temperatura retal (TR), glicemia, glicemia laboratorial (Glab), pressões parciais de oxigênio (PvO2) e dióxido de carbono (PvCO2) no sangue venoso, saturação de hemoglobina do sangue venosos (SvO2), pH, déficit de base (DB), bicarbonato (HCO3-) e hematócrito (Ht) foram mensurados 90 minutos após a última refeição (T0), imediatamente antes da anestesia (T1) e 10 (T2) e 30 (T3) minutos após a administração do anestésico. Foram avaliados os períodos entre a administração do anestésico e a tentativa dos gatos de levantar a cabeça (Th) e o intervalo de tempo entre a administração do anestésico e o posicionamento quadrupedal (Tpq) do animal. Diferenças entre os grupos não foram registradas para glicemia, HR, PvO2, SvO2, pH, BD, HCO3-, Ht e Tpq. No G12, a partir de T1 e T2, a PvCO2diminuiu e a glicemia aumentou, respectivamente. No G8 e G12, a partir de T1, DB e HCO3-diminuíram. No G12 e no G18, a partir de T2, Ht diminui. O Th no G12 foi maior que no G8. Conclui-se que, em gatos anestesiados com tiletamina-zolazepam, os diferentes períodos de jejum não influenciaram na glicemia, nos parâmetros hemagosométricos e cardiorrespiratórios. Adicionalmente, não há correlação entre a glicemia e a recuperação anestésica.
anestesia dissociativa; gatos; glicemia; monitoramento; recuperação
VETERINARY MEDICINE MEDICINA VETERINÁRIA
Different fasting periods in tiletamine-zolezepam-anethetized cats: Glycemia, recovery, blood-gas and cardiorrespiratory parameters
Diferentes períodos de jejum em gatos anestesiados com tiletamina-zolazepam: Glicemia, recuperação, parâmetros hematológicos e cardiorrespiratórios
A.P. GeringI; N. NunesII, * * Autor para correspondência ( corresponding author) E-mail: geringbr@yahoo.com.br ; M.C.C. OliveiraI; M. HorrI; P.C.F. LopesI; A.A. TormenaIV
IAluna de pós-graduação - Faculdade de Ciências Agrárias e Veterinárias (FCAV-Unesp) - Jaboticabal, SP
IIFaculdade de Ciências Agrárias e Veterinárias (FCAV-Unesp) - Jaboticabal, SP
IIIAluna de graduação - Faculdade de Ciências Agrárias e Veterinárias (FCAV-Unesp) - Jaboticabal, SP
ABSTRACT
The effects of different fasting periods on glycemia levels and on cardiorrespiratory parameters in tiletamine-zolazepam-anesthetized cats were evaluated. Twenty one animals were randomly assigned to three groups: 8 hours (G8), 12 hours (G12) or 18 hours (G18) of the preoperative fasting. The tiletamine-zolazepam (2 mg/kg) was administered intravenously. The heart rate (HR), respiratory rate (fR), rectal temperature (TR), glycemia (G), laboratorial glycemia (Glab), venous oxygen partial pressure (PvO2), venous carbon dioxide partial pressure (PvCO2), venous hemoglobin saturation (SvO2), pH, base deficit (BD), bicarbonate concentration (HCO3 - ) and haematocrit were evaluated at 90 minutes after the last meal (T0), immediately before anesthesia (T1) and at ten (T2) and thirty (T3) minutes after tiletamine-zolezepam administration. The time between the administration of anesthetic and the cat's trial to elevate head (Th) and the interval between drug administration and aniamal's quadrupedal position (Tqp) were recorded. No differences among groups were recorded for glycemia, HR, PvO2, SvO2, pH, BD, HCO3-, Ht and Tqp. In G12 from T2, glycemia increased and from T1 PvCO2 decreased. At T1, PvO2 increased in all groups. In G8 and G12, from T1, DB and HCO3- decreased. In G12 and G18, from T2, Ht decreased. In G12, the Th mean was higher than G8. In conclusion, in tiletamine-zolazepam-anesthetized cats, the different preoperative fasting did not influence glycemia, blood-gas and cardiorrespiratory parameters. Additionally, there was no relationship between glycemia and anesthesia recovery.
Keywords: cats, dissociative anesthesia, glycemia, monitoring, recovery
RESUMO
Avaliaram-se os efeitos de diferentes períodos de jejum sobre os níveis glicêmicos e os parâmetros cardiorrespiratórios em gatos anestesiados com tiletamina-zolazepam. Vinte um animais foram distribuídos aleatoriamente em três grupos diferenciados entre si pelo período de jejum: oito horas (G8), doze horas (G12) e dezoito horas (G18). A tiletamina-zolazepam (2mg/kg) foi administrada por via intravenosa. A frequência cardíaca (FC), frequência respiratória (fR), temperatura retal (TR), glicemia, glicemia laboratorial (Glab), pressões parciais de oxigênio (PvO2) e dióxido de carbono (PvCO2) no sangue venoso, saturação de hemoglobina do sangue venosos (SvO2), pH, déficit de base (DB), bicarbonato (HCO3-) e hematócrito (Ht) foram mensurados 90 minutos após a última refeição (T0), imediatamente antes da anestesia (T1) e 10 (T2) e 30 (T3) minutos após a administração do anestésico. Foram avaliados os períodos entre a administração do anestésico e a tentativa dos gatos de levantar a cabeça (Th) e o intervalo de tempo entre a administração do anestésico e o posicionamento quadrupedal (Tpq) do animal. Diferenças entre os grupos não foram registradas para glicemia, HR, PvO2, SvO2, pH, BD, HCO3-, Ht e Tpq. No G12, a partir de T1 e T2, a PvCO2diminuiu e a glicemia aumentou, respectivamente. No G8 e G12, a partir de T1, DB e HCO3-diminuíram. No G12 e no G18, a partir de T2, Ht diminui. O Th no G12 foi maior que no G8. Conclui-se que, em gatos anestesiados com tiletamina-zolazepam, os diferentes períodos de jejum não influenciaram na glicemia, nos parâmetros hemagosométricos e cardiorrespiratórios. Adicionalmente, não há correlação entre a glicemia e a recuperação anestésica.
Palavras-chave: anestesia dissociativa, gatos, glicemia, monitoramento, recuperação
INTRODUCTION
Too often operations are undertaken with inadequate patient preparation (Muir, 2007). Fasting reduces the chance of vomiting in the perioperative period and the associated concern of aspiration (Dyson, 1994). Besides, the distended stomach may interfere with the free movement of the diaphragm muscle and hinder breathing. The increase of intestinal volume may impair breathing, because it can promote a cranial motion of visceral mass compressing the diaphragm (Ambrósio, 2002).
Thus, with most types of anesthesia, it is best to have patients off feed for 12h before the procedure. It should be recalled that some species are adversely affected by fasting (Muir, 2007). According to Dyson (1994), whenever possible, cats should have food withheld for 8-12 hours prior to anesthesia. While for Bednarski (2007), healthy dogs and cats should be fasted at least 6 hours prior to being anesthetized. A much shorter period of starvation of 1-2h is recommended for young cats, below 3 weeks of age (Dyson, 1994), or dogs and cats below 8 weeks old and those weighing less than 2kg (Bednarski, 2007). For cats, water is usually freely available before anaesthesia (Dyson, 1994).
During fasting, in healthy animal the body compensates for this calorie deficit in the short term by first utilizing hepatic glycogen and then by mobilizing amino acids from the muscle. Glycogen stores are rapidly depleted, particularly in carnivores such as cats (Freeman and Chan, 2006). However, prolonged fasting may also produce dehydration, hypovolemia and hypoglycemia (Green et al., 1996).
In halothane-anesthetized dogs submitted to fasting of 12, 18 and 24 hours, Guimarães et al. (2007) concluded that with 12 hours of preoperative fasting, animals showed a higher rise in glycemia levels in recovery anesthesia, but solid fasting of the 18 hours is recommended to ensure a complete absence of the solid food content in the stomach. Additionally, these authors affirmed that during anesthesia the cardiorrespiratory parameters were not influenced by preoperative fasting periods.
Conversely, in dogs maintained in different pre-operative fasting periods (6 to 8 hours, 12 to 14 hours, more than 16 hours) and anesthetized with halothane, no differences were observed among the three starvation times considering the glycemia value before and after anesthesia (Nogueira et al., 2003).
However, there was no information on the correlation of the different fasting periods with plasma glycemia in cats. Therefore, this study was designed to assess the effects of three different fasting times on glycemia levels, cardiorrespiratory parameters and recovery in tiletamine-zolezepam-anesthetized cats.
MATERIALS AND METHODS
This study was approved by the Institutional Ethics and Animal Welfare Committee with protocol nº 023593/11.
Twenty one mature mongrel cats, nine males and twelve females were enrolled in the study. All animals were determined healthy based on clinical and laboratorial evaluation. Blood, urine, electrocardiogram and thoracic radiography exams were all done. They were provided with water and regular cat food and kept in individual cages at the Veterinary Teaching Hospital.
The cats were randomly assigned to one of three groups: 8 hours (G8, n=7, 4.3±0.5kg), 12 hours (G12, n=7, 4.1±0.4kg) or 18 hours (G18, n=7, 4.2±0.7kg) of preoperative fasting. Before anesthesia, all animals were submitted to radiography exams to detect the presence or absence of food in the stomach and intestine.
The catheter was placed in the right and left cephalic vein to administer anesthesia and to obtain venous blood in order to evaluate the amount of haematocrit (Ht) (Abovet da ABX, abc Pach LMGE Montpellier, São Paulo, Brazil), venous carbon dioxide partial pressure (PvCO2), venous oxygen partial pressure (PvO2), venous hemoglobin saturation (SvO2) and pH. The blood gas and pH measurements were corrected for rectal temperature (TR °C) measured with a digital thermometer. The blood gas analyzer (Roche Omni C blood gas analyzer; Roche Diagnostics, Mannheim, Germany) calculated base deficit (BD) and bicarbonate concentration (HCO3 - ). The 0.3mL of venous blood was collected in a syringe (1 mL) with heparin.
In each cat capillary samples were collected from the inner pinna and glycemia (G) was measured from the same drop of blood with the Optium Xceed® (Optium Xceed, Abbot Laboratories Limited, England, United Kingdom) meters at random order. At the same time, a peripheral blood sample was collected and 1 glycemia level (Glab) was measured with the reference method (Labquest, Labtest diagnóstica S/A, Lagoa Santa, Minas Gerais, Brasil).
Then, tiletamine-zolezepam (Zoletil 50, Virbac do Brasil Indústria e Comércio Ltda, São Paulo, São Paulo, Brazil) was administered at 2mg/kg intravenously. The endotracheal intubation was performed and cats were breathing room air. The animals were positioned on right lateral recumbency until they assumed a quadrupedal position.
Heart rate (HR) in beats minute-1 was measured using a stethoscope (Estetoscópio Littmann Pediátrico, 3M do Brasil, Sumaré, São Paulo, Brazil) positioned between the 3rd and 5th left intercostal space. Respiratory rate (fR) was measured using a stethoscope positioned on the chest. The observations of beats and breaths were performed during 60 seconds and by the same person in all animals.
The time of anesthesia recovery was observed. The time between the administration of anesthetic and cat trial to elevate head (Th) and the interval between drug administration and animal's quadrupedal position (Tqp) were recorded.
The first measurement (T0) was taken 90 minutes after each cat's last meal. The second measurement was done immediately before anesthesia (T1) and additional recordings were performed at ten (T2) and thirty (T3) minutes after tiletamine-zolezepam administration.
Parametric data were subjected to one-way analysis of variance (ANOVA) to determine the difference among the different time points of the same group. Two-way ANOVA was used between groups. Bonferroni test was used for post-hoc multiple comparisons at a level of 0.05. Analyses were performed using Prism 5 for Windows (Prism 5, GraphPad Software Inc, California, United States).
RESULTS
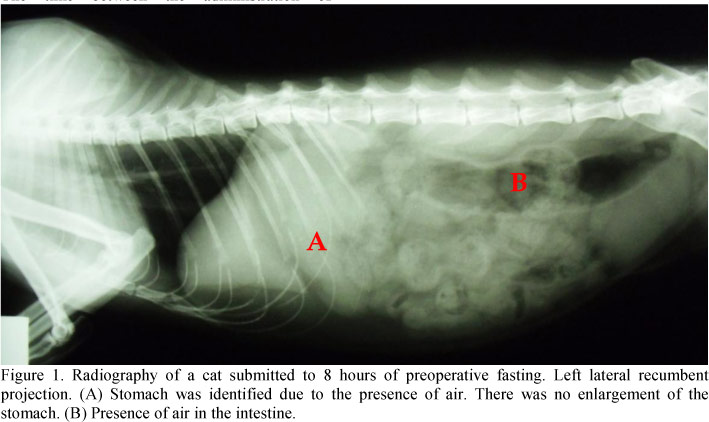
In all radiographies of all groups, food was absent in the stomach and intestine (Figure 1). For G and Glab no differences among groups were observed. In G12, the G means at T0 and T1 were lower than values at T2 and T3. In the same group, Glab at T2 was higher than at T1 and T0, which was lower than at T3 (Table 1).
Means with different capital letters within each column and with different lower case letters within each row differed significantly. (p<0.05). HR: heart rate, fR: breath rate, TR: rectal temperature, PvO2: venous oxygen partial pressure, PvCO2: venous carbon dioxide partial pressure, SvO2: venous hemoglobin saturation, BD: base deficit, HCO3 - : bicarbonate concentration, Ht: haematocrit.
For HR, no differences among groups or times were observed. At T1, the fR values in G8 and G12 were lower than in G18, which was higher than in G12 at T3. In G18, fR at T0 was lower than at T1 (Table 1). In G12, at T1, TR was lower than G8 and G18. Only in G12, from T0 TR decreased.
In all groups, PvO2 at T0 was lower than at T1, while in G12 PvCO2 decreased from T0 (Table 1). At T1, PvCO2 in G8 was higher than in G12. No differences were registered among groups or among times for SvO2 and DB. The bicarbonate values decreased from T0 in G8 and G12, but in G18 no differences were observed. For pH, no differences among groups were observed. In G12, pH at T1 was higher than at T0 and T2.
For Ht, in G18, the mean at T1 was higher than at T2 and T3. In G12, it was higher at T1 than at T2 and T3, which was lower than at T0 (Table 1). In G12, the Th mean was higher than G8 (Table 2).
DISCUSSION
An adequate level of blood glucose is important for cerebral metabolism. Hypoglycemia might occur during general anesthesia but is most common as a nonspecific hormonal response to the stress of anesthesia and operation (Haskins, 2007). However, in anesthetized animals, there may be no outward sign (Pascoe, 2006). According to Kaneko et al. (1997), for cats, the normal glucose values are between 73 and 134mg/dL (4.10 and 7.44 mmol/L). Thus, in this study, at T0 and T1, the glycemia levels were lower than reference values, except for G in G12. This hypoglycemia can be associated with a long preoperative fast (Green et al., 1996) used 157 animals in this study.
A blood glucose concentration below 60 mg/dL (3.33 mmol/L) should be treated with 2.5% to 5.0% glucose infusion, according to Haskins (2007). However, in this study, during anesthesia the monitoring of glycemia levels was performed with Optium Xceed ® meters that showed glucose values above 60 mg/dL, thus, the treatment was not instituted in these first times.
In dogs submitted to a preoperative fast of 8, 12 or 24 hours and anesthetized with halothane, hypoglycemia was not observed during anesthesia (Guimarães et al., 2007). This difference can be attributed to the species studied, because fasting or food deprivation has not been associated with lipid accumulation in obese human beings or dogs, suggesting a peculiarity of the feline species (De Bruijne, 1979).
From T2, glycemia levels were higher than 73mg/dL (4.10mmol/L) in all groups and in G12, G and Glab increased significantly from T2 (Table 1). The level of glucose increase has been described in dogs anaesthetized with enflurane (Naziroğlu and Gunay, 1999) and in gazelles anaesthetized with tiletamine - zolazepam - xylazine (Yaralioglu-Gurgoze et al., 2005). Acute hyperglycaemia has been reported in cattle given xylazine, reflecting the inhibition of insulin release from the pancreatic β cells by the α 2-adrenaergic receptors (Hsu and Hummel, 1981; Lemke, 2007).
Hyperglycemia is characterized by blood glucose concentration >200mg/dL (11.23 mmol/L) and can occur with a typical anesthetic-surgical experience (Haskins, 2007). In this study, hyperglycemia was not observed, but an increase in glycemia levels was registered. If the anesthesia time was longer there might be a hyperglycemia recording. Hyperglycemia is a ubiquitous phenomenon in the perioperative period, linked to the preoperative metabolic state of the patient, neuroendocrine stress response, acute perioperative insulin resistance, as its intraoperative management (Akhtar et al., 2010). However, this event has not been investigated (Haskins, 2007).
For cats, normal HR values are between 120 and 240 beats/minute (Tilley and Burtnick, 1999). Thus, the means observed in this study were within of this interval (Table 1) and no differences were observed among groups and among times. Thus, the preoperative fasting did not influence HR.
In unanesthetized cats fR is around 22 breaths/minute and when anesthetized it is around 30 breaths/ minute (McDonell and Kerr, 2007). In this study, in G8 and G12 the means were close to these values. However, in G18, fR increased at T1 and T3. Since different animals were used each groups, we believe that this event can be attributed to stress, ecause T1 was the measurement immediately before anesthesia, and T3 was thirty 192 minutes after tiletamine-zolezepam administration, the time that cats started to awake. However, the difference among groups was observed at T1 and T3, we did not attribute it to different fasting periods. Guimarães (2006), in halothane-anesthetized dogs submitted to three different fasting periods (12, 18 and 24 hours), did not register fR differences among groups.
Rectal temperature was stable in G8 and G18 (Table 1), but in G12, at T1 and T3, a TR decrease was observed. We attributed it to individual differences because Guimarães (2006) did not observe differences among groups for TR. Besides, we expected an increase of the rectal temperature, because of animal stress due to the procedure.
According to Haskins (2004), a normal PvO2 value is between 40 and 50mm Hg in healthy dogs breathing room air. The PvO2 values registered in this study were within this interval (Table 1). Dias et al. (2009), in propofol-anesthetized dogs with high intracranial pressure, observed PvO2 means above 40 mm Hg, and they attributed it to the use of FiO2 levels of 1.0 and 0.6, differing from this study in that the animals breathed room air.
At T1 PvO2 increased when compared to T0 in all groups. We attribute this event to the administration of dissociative anesthetic. P O2 increases when cardiac output (CO) increases (Morgan et al. 2005), which was not evaluated in this study. However, it is known that dissociative drugs cause myocardial stimulation, which is associated with increased cardiac work and myocardial oxygen consumption. In healthy animals, increases in myocardial oxygen supply usually result from increased CO (Lin, 2007). Thus, we believe that the PvO2 increase was due to a CO increase.
O2 increases when cardiac output (CO) increases (Morgan et al. 2005), which was not evaluated in this study. However, it is known that dissociative drugs cause myocardial stimulation, which is associated with increased cardiac work and myocardial oxygen consumption. In healthy animals, increases in myocardial oxygen supply usually result from increased CO (Lin, 2007). Thus, we believe that the PvO2 increase was due to a CO increase.
A normal SvO2 is reported as 75% (37), with a variation from 60% to 80% (Zaja, 2007). Thus, it can be assumed that both groups had good tissue oxygenation. Additionally, different fasting periods did not change PvO2 and SvO2.
According to King and Hendricks (1997), the PvCO2 is usually 6 to 7mm Hg higher than the PaCO2, whereas Haskins (2004) considers the normal difference to be 3 to 6mm Hg. In G8 and G18, PvCO2 was maintained stable during the entire procedure, but in G12, PvCO2 at T0 was higher than in other times (Table 1). Additionally, at T1, G8 was higher than G12.
Almost 75% of the carbon dioxide produced by tissue metabolism is normally carried to the lungs in the form of bicarbonate (Haskins, 2004). The dissolved carbon dioxide in the blood reacts with water to form carbonic acid. Inside the red blood cells there is a protein enzyme called carbonic anhydrase, which catalyzes the reaction between carbon dioxide and water and accelerates this reaction (Guyton and Hall, 2006). The carbonic acid formed in the red cells (H2CO3) dissociates into hydrogen and bicarbonate ions (H+ and HCO3 - ). Most of the hydrogen ions then combine with the hemoglobin in the red blood cells, because the hemoglobin protein is a powerful acid-base buffer. In turn, many of the bicarbonate ions diffuse from the red cells into the plasma, while chloride ions diffuse into the red cells to take their place (Haskins, 2004; Guyton and Hall, 2006). This is made possible by the presence of a special bicarbonate-chloride carrier protein in the red cell membrane that shuttles these two ions in opposite directions at high speeds (Guyton and Hall, 2006). Thus, the means of bicarbonate registered in this study can be justified by differences observed for PvCO2. In G12, at T0 bicarbonate was higher than in other times. At T1, HCO3- mean in G8 was higher than in G12, but without statistical significance (Table 1).
Additionally, in G12, PvO2 at T0 was lower than in other times, and this event contributed to PvCO2 decrease (T1 to T3), because of the Haldane effect, which results from the simple fact that the combination of oxygen with hemoglobin in the lungs causes the hemoglobin to become a stronger acid. This displaces carbon dioxide from the blood and into the alveoli in two ways: the more highly acidic hemoglobin has less tendency to combine with carbon dioxide to form carbaminohemoglobin and also causes it to release an excess of hydrogen ions, and these bind with bicarbonate ions to form carbonic acid; which then dissociates into water and carbon dioxide, and the carbon dioxide is released from the blood into the alveoli and, finally, into the air (Guyton and Hall, 2006).
For cats, the normal pH venous value is 7.300±0.087, for BD it is -5.7±4.6 mmol/L, and for bicarbonate it is 19.4±4.0 mmol/L (Trim, 1994). Thus, means registered in this study were within normal intervals (Table1). It was known that acute increases in PaCO2 promote an increase in intracellular CO2 levels, which shifts the reaction CO2 + H2O ↔ H2CO3↔ HCO3- + H+ to the right (Johnson and Morais 2005). However, in this study, the observed PvCO2 was stable in G8 and G12, while pH and HCO3- decreased, but without clinical relevance. In G18, PvCO2 was stable during the entire procedure and it allowed for pH, BD and bicarbonate not to change.
The normal interval for Ht species is 26.1 to 46.7% (Ford and Mazzaferro, 2007), thus the values recorded in this study were within this interval. However, in G12 and G18, Ht decreased at T2 and T3 when compared to T1. This event can be attributed to anesthesia, because these procedures can modulate the red blood cell patrimony and affect intra and postoperative blood loss (Borghi et al., 2005). Besides, the drugs used in this procedure can change the antioxidant enzyme activities (Godin and Garnett, 1994). In tiletamina-zolazepam - anethetized sheep (7.5 mg/kg intramusculary) Ht decreased, and authors concluded that this drug can impair the enzymatic and non-enzymatic antioxidant defense potential in the blood plasma (Ceylan et al., 2007). Thus, the preoperative fasting did not influence Ht.
In G8 Th was lower than in G12, but no differences were observed between these groups for glycemia. This difference can be attributed to individual variability. Therefore, in halothane-anesthetized dogs maintained in different pre-operative fast times, the relationship between anesthesia recovery and glycemia level was not observed (Nogueira et al., 2003), corroborating this study.
In conclusion, in tiletamine-zolazepam-anesthetized cats, the different preoperative fasting did not influence glycemia and cardiorrespiratory parameters. Additionally, there was no relationship between glycemia and anesthesia recovery.
ACKNOWLEDGEMENTS
The authors would like to thank the Fundação de Amparo à Pesquisa do Estado de São Paulo - FAPESP, for the financial support and scholarship.
Recebido em 3 de julho de 2012
Aceito em 7 de maio de 2013
- AKHTAR, S.; BARASH, P.G; INZUCCHI, S.E. Scientific principles and clinical implications of perioperative glucose regulation and control. Anesth. Analg., v.110, p.478-497, 2010.
- AMBRÓSIO, A.M. Anestesia e sistema digestório. In: FANTONI, D.T.; CORTOPASSI, S.R. (Eds) Anestesia em cães e gatos. São Paulo: Roca, 2002. cap.26, p.260 - 270.
- BEDNARSKI, R.M. Dogs and cats. In: TRANQUILLI, W.J.; THURMON, J.C.; GRIMM, K.A. (Eds). Lumb & Jones' Veterinary anesthesia and analgesia. 4.ed. Oxford: Blackwell Publising, 2007. p.705-715.
- BORGHI, B.; CASATI, A; IUORIO, S. Effect of different anesthesia techniques on red blood cell endogenous recovery in hip arthroplasty. J. Clin. Anesth., v.17, p.96-101, 2005.
- CEYLAN, C.; AYDILEK, N.; IPEK, H. Effects of tiletamine-zolazepam anaesthesia on plasma antioxidative status and some haematological parameters in sheep. Acta Vet. Hung., v.55, p.191-197, 2007.
- DE BRUIJNE, J.J. Biochemical observations during total star vation in dogs. Int. J. Obes., v.3, p.239-247, 1979.
- DIAS, L.G.G.G.; NUNES, N.; LOPES, P.C.F. et al The effects of 2 levels of the inspired oxygen fraction on blood gasvariables in propofol-anesthetized dogs with high intracranial pressure. Can. J. Vet. Res., v.73, p.111-116, 2009.
- DYSON, D.H. Pre-operative assessment. In: HALL, L.W.; TAYLOR, P.M. (Eds). Anaesthesia of the cat London: W.B. Saunders, 1994. p.105-110.
- FORD, R.B.; MAZZAFERRO, E.M. Manual de procedimentos veterinários e tratamento emergencial 8.ed. São Paulo: Roca, 2007. 747p.
- FREEMAN, L.M.; CHAN, D.L. Total parenteral nutrition. In: DiBARTOLA, S.P. (Ed). Fluid, electrolyte, and acid-base disorders in small animal practice. 3.ed. St Louis: Elsevier, 2006. p.584-601.
- GODIN, D.V.; GARNETT, M.E. Effects of various anesthetic regimens on tissue antioxidant enzyme activities. Res. Commun. Chem. Pathol. Pharmacol., v.83, p.93.101, 1994.
- GREEN, C.R.; PANDIT, S.K.; SCHORD, M.A. Preoperative fasting time: is the traditional policy changing? Results of a national survey. Anesth. Analg, v.83, p.123-128, 1996.
- GUIMARÃES, S.M Comparação entre diferentes períodos de jejum em cães submetidos à anestesia geral inalatória: aspectos clínicos, bioquímicos e eletrolíticos 2006, 144f. Dissertação (Mestrado em Medicina Veterinária). Faculdade de Medicina Veterinária e Zootecnia, Universidade Estadual Paulista, Botucatu, SP.
- GUIMARÃES, S.M.; OLIVA, V.N.L.S; MAIA, C.A.A. et al Correlação de diferentes períodos de jejum com níveis séricos de cortisol, glicemia plasmática, estado clínico e equilíbrio ácido-base em cães submetidos à anestesia geral inalatória. Braz. J. Vet. Res. Anim. Sci., v.44, p.96-102, 2007.
- GUYTON, A.C.; HALL, J.E. Transporte de oxigênio e dióxido de carbono no sangue e nos líquidos teciduais. In: GUYTON, A.C.; HALL, J.E. (Eds). Tratado de fisiologia médica. 11.ed. Rio de Janeiro: Elsevier, 2006. p.502-513.
- HASKINS, S.C. Interpretation of blood gas measurements. In: KING, L.G. (Ed) Textbook of respiratory disease in dogs and cats 1.ed. Philadelphia: Saunders, 2004. p.181-192.
- HASKINS, S.C. Monitoring anesthetizes patients. In: TRANQUILLI, W.J.; THURMON, J.C.; GRIMM, K.A. (Eds). Lumb & Jones' Veterinary anesthesia and analgesia. 4.ed. Oxford: Blackwell Publising, 2007. p.533-558.
- HSU, W.H.; HUMMEL, S.K. Xylazine-induced hyperglycemia in cattle: a possible involvement of a2-adrenergic receptors regulating insulin release. Endocrinology, v.109, p.825-829, 1981.
- JOHNSON, R.A.; MORAIS, H.A. Respiratory acid-base disorders. In: DIBARTOLA, S.P. (Ed) Fluid, electrolyte and acid-base disorders in small animal practice. 3.ed. Philadelphia: Saunders Elsevier, 2005. p.283-296.
- KANEKO, J.J. Carbohydrate metabolism and its diseases. In: KANEKO, J.J.; HARVEY, J.W.; BRUSS, M.L. (Eds). Clinical biochemistry of domestic animals. 5.ed. San Diego: Academic Press, 1997. p.45-81.
- KING, L.G.; HENDRICKS, J.C. Testes clínicos da função pulmonar. In: ETTINGER, S.J. (Ed). Tratado de medicina interna veterinária. 1.ed. São Paulo: Manole, 1997. p.1041-1063.
- LEMKE, K.A. Anticholinergics and sedatives. In: TRANQUILLI, W.J.; THURMON, J.C.; GRIMM, K.A. (Eds). Lumb & Jones' Veterinary anesthesia and analgesia. 4.ed. Oxford: Blackwell Publising, 2007. p.203-239.
- LIN, H. Dissociative anesthetics. In: TRANQUILLI, W.J.; THURMON, J.C.; GRIMM, K.A. (Eds). Lumb & Jones' Veterinary anesthesia and analgesia. 4.ed. Oxford: Blackwell Publising, 2007. p.301-353.
- McDONELL, W.N.; KERR, C.L. Respiratory system. In: TRANQUILLI, W.J.; THURMON, J.C.; GRIMM, K.A. (Eds). Lumb & Jones' Veterinary anesthesia and analgesia. 4.ed. Oxford: Blackwell Publising, 2007. p.117-151.
- MORGAN JR, G.E.; MIKHAIL, M.S.; MURRAY, M.J. Respiratory physiology: The effects of anesthesia. In: MORGAN JR, G.E.; MIKHAIL, M.S.; MURRAY, M.J. (Eds). Clinical Anesthesiology. 4.ed. New York: Lange Medical books/McGraw-Hill, 2005. p.537-570.
- MUIR, W.W. Considerations for general anesthesia. In: TRANQUILLI, W.J.; THURMON, J.C.; GRIMM, K.A. (Eds). Lumb & Jones' Veterinary anesthesia and analgesia. 4.ed. Oxford: Blackwell Publising, 2007. p.7-30.
- NAZIROGLU, M.; GUNAY, C. The levels of some antioxidant vitamins, glutathione peroxidase and lipoperoxidase during the anesthesia of dogs. Cell Biochem. Funct., v.17, p.207-212, 1999.
- NOGUEIRA, L.C.; CORTOPASSI, S.R.G.; INTELIZANO, T.R. et al Efeitos do jejum alimentar pré-cirúrgico sobre a glicemia e o período de recuperação anestésica em cães. Braz. J. Vet. Res. Anim. Sci., v.40, p.20-25, 2003.
- PASCOE, P.J. Perioperative management of fluid therapy. In: DIBARTOLA, S.P. (Ed). Fluid, electrolyte, and acid-base disorders in small animal practice. 3.ed. St Louis: Elsevier, 2006. p.377-391.
- TILLEY, L.P.; BURTINICK, N.L. Eletrocardiography for the small animal practitioner 1.ed. Wyoming :Teton New Media, 1999. 106p.
- TRIM, C.M. Monitoring the anesthetized cat. In: HALL, L.W.; TAYLOR, P.M. (Eds). Anaesthesia of the cat London: W.B. Saunders, 1994. p.194-223.
- YARALIOGLU-GURGOZE, S.; SINDAK, N.; SAHIN, T. et al Levels of glutathione peroxidase, lipoperoxidase and some biochemical and haematological parameters in gazelles anaesthetised with a tiletamin-zolazepam-xylazine combination. Vet. J., v.169. p.126-128, 2005.
- ZAJA, J. Venous oximetry. Signa Vitae, v.2, p.6-10, 2007.
Publication Dates
-
Publication in this collection
07 Jan 2014 -
Date of issue
Dec 2013
History
-
Received
03 July 2012 -
Accepted
07 May 2013