ABSTRACT
A retrospective review of hematological reports of nine dogs detected with Hepatozoon canis infection by microscopic examination of blood smears in a laboratory in the municipality of Dourados, Mato Grosso do Sul, Brazil was conducted. This study aimed to evaluate the hematological profile of these infected dogs, in addition to the occurrence of coinfections with other agents that infect blood cells, since studies concerning canine hepatozoonosis in Brazil are scarce and there are some divergences regarding H. canis infection that still require a resolution. The nine cases of H. canis infection were identified among all dogs examined at the studied laboratory in 2009 and 2010, with an occurrence of 7/1,192 (0.59%; 95% CI 0.15 - 1.02%) positive dogs in the first year and 2/1,313 (0.15%; 95% CI 0.02 - 0.55%) cases in 2010. The analysis of the hematological reports showed an occurrence of coinfection between H. canis and other agents in two (2/9; 22.22%; 95% CI 2.81 - 60.00%) dogs, one with E. canis and another with Babesia spp. (1/9; 11.11%; 95% CI 0.28 - 48.24%). Only the blood test of one dog had no alterations, based on reference values. Anemia was the most frequent hematological alteration (6/9; 66.67%; 95% CI 29.93 - 92.51%). Although the occurrence of H. canis infection was low, significative hematological alterations were observed in most infected dogs. Coinfection with Babesia spp. and E. canis was detected in two dogs and the hematological alterations cannot be attributed exclusively to H. canis in these animals. Longitudinal studies would be of fundamental importance to determine the causality of these alterations. These results highlight the importance of differential diagnosis in dogs when there is clinical suspicion of infection by hemoparasites, since the hematological changes in dogs infected by H. canis are quite variable.
Keywords:
Hepatozoon canis; coinfection; anemia; erythrogram; leukogram
RESUMO
Realizou-se estudo retrospectivo de laudos hematológicos de nove cães detectados com infecção por Hepatozoon canis, por meio de exame microscópico de esfregaços sanguíneos, em um laboratório no município de Dourados, Mato Grosso do Sul, Brasil. Este estudo objetivou avaliar o perfil hematológico dos cães infectados, além da ocorrência de coinfecções com outros agentes que infectam células sanguíneas, tendo em vista que estudos a respeito da hepatozoonose canina no Brasil são escassos e que existem algumas divergências a respeito da infecção por H. canis que ainda requerem esclarecimento. Os nove casos de infecção por H. canis foram identificados dentre todos os cães examinados no laboratório estudado, em 2009 e 2010, com uma ocorrência de 7/1.192 (0,59%; IC 95% 0,15 - 1,02%) cães positivos no primeiro ano e de 2/1.313 (0,15%; IC 95% 0,02 - 0,55%) em 2010. A análise dos laudos hematológicos dos nove cães evidenciou a ocorrência de coinfecção entre H. canis e outros agentes em dois (2/9; 22,22%; IC 95% 2,81 - 60,00%) desses cães, um deles com E. canis e outro com Babesia spp. (1/9; 11,11%; IC 95% 0,28 - 48,24%). Apenas o exame sanguíneo de um cão não evidenciou alterações, com base nos valores de referência. Anemia foi a alteração hematológica mais frequentemente observada (6/9; 66,67%; IC 95% 29,93 - 92,51%). Embora a ocorrência de infecções por H. canis neste estudo tenha sido baixa, alterações hematológicas significativas foram observadas na maioria dos cães infectados. Coinfecções com Babesia spp. e E. canis foram observadas em dois cães, não sendo possível atribuir exclusivamente a H. canis as alterações hematológicas detectadas nesses animais. Estudos longitudinais seriam fundamentais para determinar a causalidade dessas alterações. Os resultados ressaltam a importância de realizar diagnóstico diferencial em cães quando há suspeita clínica de infecção por hemoparasitas, uma vez que as alterações hematológicas em cães infectados por H. canis são bastante variáveis.
Palavras-chave:
Hepatozoon canis; coinfecção; anemia; eritrograma; leucograma
INTRODUCTION
Parasites detected in canine blood include protozoa of the genus Hepatozoon (Apicomplexa: Hepatozoidae), which contains hundreds of species, but two are infectious to dogs: H. canis and H. americanum. The latter is responsible for canine hepatozoonosis in North America, with characteristics that differ from those that occur in the rest of the world (Vincent-Johnson et al., 1997; Baneth et al., 2000BANETH, G.; BARTA, J.R.; SHKAP, V. et al. Genetic and antigenic evidence supports the separation of Hepatozoon canis and Hepatozoon americanum at the species level. J. Clin. Microbiol., v.38, p.1298-1301, 2000. ).
Dogs become infected with H. canis by ingesting a tick vector containing the parasite in its body cavity. Thus, the infection by the protozoan occurs in regions where its main vector and definitive host is found (Vincent-Johnson et al., 1997), the tick species Rhipicephalus sanguineus, also known as the brown dog tick (Nijhof et al., 2005). After ingestion, the parasite initiates merogony in various organs of the dog and gametogony in neutrophils and monocytes (Vincent-Johnson et al., 1997).
Studies concerning canine hepatozoonosis in Brazil are scarce. Divergences regarding pathogenicity, epidemiology and genetic characterization of H. canis infection still require resolution (O'Dwyer et al., 2006; Amoli et al., 2012AMOLI, A.R.; KHOSHNEGAH, J.; RAZMI, G. A preliminary parasitological survey of Hepatozoon spp. infection in dogs in Mashhad, Iran. Iran. J. Parasitol., v.7, p.99-103, 2012.). No single diagnostic method has been used as the sole means of confirming tick-borne diseases (Mylonakis et al., 2004MYLONAKIS, M.E.; KOUTINAS, A.F.; BANETH, G. et al. Mixed Ehrlichia canis, Hepatozoon canis, and presumptive Anaplasma phagocytophilum infection in a dog. Vet. Clin. Pathol., v.33, p.249-251, 2004.) and it is common to perform blood test when there is suspicion of infection by hemoparasites in dogs, to check and to determine what kind of alterations were induced.
In this way, this study aimed to assess the hematological alterations in nine cases of H. canis infection in dogs submitted to hematology and blood smear examination for screening of hemoparasites in the municipality of Dourados, Mato Grosso do Sul (MS).
MATERIALS AND METHODS
A longitudinal retrospective review of hematological reports of dogs was conducted with the approval of the institute's Research Ethics Committee (n.038/11, 13 may 2011), in order to verify the diagnosis of H. canis infection in dogs. The blood samples of these dogs were examined by the Clinical Pathology Laboratory of the Veterinary Hospital of Grande Dourados University Center (UNIGRAN), located in the municipality of Dourados (22o13'16"S; 54o48'20"W), MS, in central-western Brazil.
The blood samples were collected from January 2009 to December 2010, via venous puncture or skin prick of the ear tip to obtain one drop of capillary or peripheral blood (O'Dwyer et al., 2006).
Blood smears were prepared from venous or capillary blood, since microscopic examination of blood smears is the test of choice in routine clinical practice (O'Dwyer, 2011), which is a simple technique (Mercer and Craig, 1988MERCER, S.H.; CRAIG, T.M. Comparison of various staining procedures in the identification of Hepatozoon canis gamonts. Vet. Clin. Pathol., v.17, p.63-65, 1988.). The blood smears were stained by a quick panoptic method (Quick Panoptic test, Newprov(r), Brazil) and examined under an optical microscope (Eclipse E-200, Nikon(r), USA) through oil immersion objective (100x), to determine the presence of hemoparasites.
The slides were also used for leukocyte differential counts by visualizing 100 cells (Thrall, 2006THRALL, M.A. (Ed.). Hematologia e bioquímica clínica veterinária. São Paulo: Roca , 2006. 592p.). The total values of red blood cells (RBC), white blood cells (WBC) and hemoglobin were obtained using an automatic counter (Celm CC-530, Celm(r), Brazil), while mean packed cell volume (PCV) and quantification of total plasma protein (TPP) and fibrinogen were obtained using microhematocrit and refraction techniques (Thrall, 2006), respectively. The Wintrobe RBC indices were determined using the methodology described by Bush (2004BUSH, B.M. (Ed.). Interpretação de resultados laboratoriais para clínicos de pequenos animais. São Paulo: Roca, 2004. 384p.), through which the mean corpuscular hemoglobin concentration (MCHC) and mean corpuscular volume (MCV) were calculated. It is worth highlighting that only the hematological findings were evaluated in this study and the methods described above were performed in the laboratory routine. All data were tabulated in a spreadsheet with Excel(r) 2010 software (Microsoft, Redmond, WA).
The most frequent hematological changes in infected dogs were described according to reference values described by Rizzi et al. (2010RIZZI, T.E.; MEINKOTH, J.H.; CLINKENBEARD, K.D. Normal hematology of the dog. In: WEISS, D.J.; WARDROP, K.J. (Eds.). Schalm's veterinary hematology. 6.ed. Ames: Wiley-blackwell, 2010. p.799-810.), used by the laboratory where the blood test were performed. The percentages described and their 95% confidence intervals (95% CI) were calculated using the Stata version 9.2 software (Stata Corp., Texas, USA), considering a significance level (α) of 5% (Triola, 2005TRIOLA, M.F. (Ed.). Introdução à estatística. 9.ed. Rio de Janeiro: LTC, 2005. 682p.).
RESULTS
The nine cases of canine infection by H. canis were detected by direct observation in blood smears among 2,505 (9/2,505; 0.36%; 95% CI 0.12 - 0.59%) dogs examined from January 2009 to December 2010.
In these years, the parasite H. canis was detected less frequently in comparison to other two agents of blood infection in dogs: Babesia spp. and Ehrlichia canis. In 2009, 7/1,192 (0.59%; 95% CI 0.15 - 1.02%; average = 0.58 cases/month) dogs were positive for H. canis, which represents 7.61% (7/92; 95% CI 2.19 - 13.03%) of dogs diagnosed in this year with infection by at least one of these three agents (Babesia spp., Ehrlichia canis and/or H. canis). Also in 2009, 66/1,192 (5.54%; 95% CI 4.24 - 6.83%; average = 5.5 cases/month) dogs were detected with E. canis infection and 19/1,192 (1.59%; 95% CI 0.88 - 2.30%; average = 1.58 cases/month) with Babesia spp.
In 2010, only 2/1,313 dogs (0.15%; 95% CI 0.02 - 0.55%; average = 0.17 cases/month) resulted positive to H. canis, accounted as 2.86% (2/70; 95% CI 0.35 - 9.94%) of positive dogs for at least one of the three agents of blood infection cited. Babesia spp. was the most frequent hemoparasite in 2010, with 39/1,313 (2.97%; 95% CI 2.05 - 3.89%; average = 3.25 cases/month) infected dogs, while 29/1,313 (2.21%; 95% CI 1.41 - 3.00%; average = 2.41 cases/month) dogs were infected by E. canis.
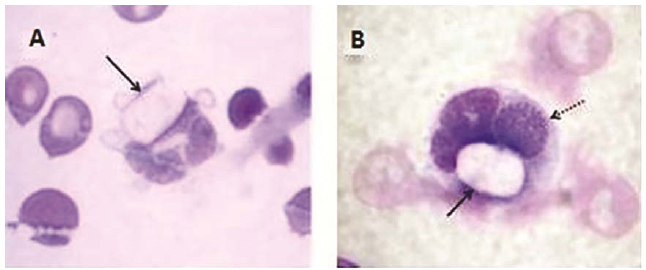
Coinfection was registered in 2/9 (22.22%; 95% CI 2.81 - 60.00%) dogs infected with H. canis, one of them with Babesia spp. (dog #5) (1/9; 11.11%; 95% CI 0.28 - 48.24%) and another one with E. canis (dog number two), which could be visualized in the same host cell (Figure 1). The hematological results of the nine infected dogs are described in detail in tables 1 and 2 (Table 1 and 2).
Blood smears prepared from venous or capillary blood. Gamonts of H. canis in neutrophils from dogs (A, B). Note the coinfection by H. canis (solid arrow) and E. canis (dotted arrow) in B. (Quick Panoptic, 100X).
Seven of the nine infected dogs (77.78%; 95% CI 40.00 - 97.18%) presented alterations in the erythrogram (Table 1) and 8/9 (88.89%; 95% CI 51.75 - 99.72%) in the leukogram (Tab. 2 ). Only one dog (#9) showed no alterations in the blood test.
The most frequent alteration observed in these dogs was anemia, diagnosed in 6/9 infected dogs (66.67%; 95% CI 29.93 - 92.51%), out of which 3/6 (50.0%; 95% CI 11.81 - 88.19%) were normocytic normochromic (dogs #1, #5, and #7), 1/6 (16.67%; 95% CI 0.42 - 64.12%) was macrocytic hypochromic (dog #2), and also 1/6 was microcytic (dog #6).
In the leukogram, leukocytosis was diagnosed in 1/9 dog (11.11%; 95% CI 0.29 - 48.25%) and 2/9 (22.22%; 95% CI 2.81 - 60.01%) had leukopenia. Neutrophil count (band and segmented) were within reference values in 3/9 (33.33%; 95% CI 7.48 - 70.07%) and neutrophil left shift was observed in 4/9 dogs (44.44%; 95% CI 13.70 - 78.80%). Moderate regenerative shift occurred in 2/9 dogs (22.22%; 95% CI 2.81 - 60.01%) (dogs #2 and #4), severe degenerative shift in 1/9 (11.11%; 95% CI 0.29 - 48.25%) (dog #1) and discrete degenerative shift also in 1/9 (dog #6).
Eosinopenia was observed in 4/9 dogs (44.44%; 95% CI 13.70 - 78.80%) and eosinophilia in 2/9 (22.22%; 95% CI 2.81 - 60.01%). No changes were observed in lymphocyte levels of 6/9 dogs (66.67%; 95% CI 29.93 - 92.51%), or monocytes of 5/9 dogs (55.56%; 95% CI 21.20 - 86.30%).
Regarding the levels of plasma proteins, 4/9 dogs (44.44%; 95% CI 13.70 - 78.80%) presented increased levels, but no alterations were observed in 5/9 dogs (55.56%; 95% CI 21.20 - 86.30%). Low and increased levels of plasma fibrinogen were observed in 3/9 dogs (33.33%; 95% CI 7.48 - 70.07%).
DISCUSSION
In this study, low occurrence of H. canis infection in dogs was observed, mainly when compared to other agents, i.e., E. canis and Babesia spp., in the years in which H. canis was diagnosed. Therefore, it is noteworthy that this data could be related to the diagnostic method.
The direct method of blood smear examination shows notably low sensitivity for detecting H. canis, especially in dogs at an early stage of the infection, chronic aparasitemic infection or low parasitemia (Baneth et al., 1998BANETH, G.; SHKAP, V.; SAMISH, M. et al. Antibody response to Hepatozoon canis in experimentally infected dogs. Vet. Parasitol., v.74, p.299-305, 1998.). Infected dogs usually have very low parasitaemia, which occurs in no more than 3% of the neutrophils or monocytes (O'Dwyer et al., 2001). This rate can reach 100% in severe infections, though no such reports have been registered in Brazil (O'Dwyer, 2011). Because this is a retrospective study of hematological reports, it was not possible to perform additional tests.
Similar to this study, low percentage of infection by H. canis has been reported by other authors using the same parasitological method in Brazil. The occurrence of coinfection with H. canis and Babesia spp. or E. canis was reported in some of these studies and it can be attributed to the common tick vector, R. sanguineus (Chhabra et al., 2013CHHABRA, S.; UPPAL, S.K.; SINGLA, L.D. Retrospective study of clinical and hematological aspects associated with dogs naturally infected by Hepatozoon canis in Ludhiana, Punjab, India. Asian. Pac. J. Trop. Biomed., v.3, p.483-86, 2013.).
In Campo Grande, also in MS State, Salgado et al. (2007SALGADO, F.P.; HONER, M.R.; ISHIKAWA, M.M. et al. Hemoparasitos e carrapatos em cães procedentes do Centro de controle de Zoonoses de Campo Grande, estado de Mato Grosso do Sul, Brasil. Rev. Bras. Med. Vet., v.29, p.113-116, 2007.) determined prevalence for H. canis of 2.40% in 167 dogs, but all of them were coinfected with E. canis, in contrast to the results obtained in this study, in which only one dog presented coinfection with this agent and another one with Babesia spp. However, similar to this, O'Dwyer et al. (2006) investigated H. canis in 222 dogs in São Paulo, São Paulo State (SP), and observed 5.86% (13/222) positive results, five of them coinfected with Babesia spp. and two with E. canis.
Mundim et al. ( 2008MUNDIM, A.V.; MORAIS, I.A.; TAVARES, M. et al. Clinical and hematological signs associated with dogs naturally infected by Hepatozoon sp. and with other hematozoa: a retrospective study in Uberlandia, Minas Gerais, Brazil. Vet. Parasitol., v.153, p.3-8, 2008.) reported 115 cases of natural infection by H. canis in dogs from the State of Minas Gerais and 26 (22.61%) coinfection events; 20 (17.39%) coinfected with Ehrlichia spp., two (1.74%) with Babesia spp. and four (3.48%) with both agents. According to Baneth et al. (2015BANETH, G.; HARRUS, S.; GAL, A.; AROCH, I. Canine vector-borne coinfections: Ehrlichia canis and Hepatozoon canis in the same host monocytes. Vet. Parasitol., v.208, p.30-34, 2015. ), coinfection of the same host cell suggests that infection with one pathogen may permit or enhance invasion or prolonged cellular survival of the other. They observed the same host monocytes infected with H. canis and E. canis, as detected in one dog in this study (Figure 1-B), and they confirmed it by molecular characterization of the infecting agents and quantitative assessment of coinfected cells.
Variable occurrence rates of Hepatozoon infection in dogs have also been reported in other countries. In Iran, Amoli et al. ( 2012AMOLI, A.R.; KHOSHNEGAH, J.; RAZMI, G. A preliminary parasitological survey of Hepatozoon spp. infection in dogs in Mashhad, Iran. Iran. J. Parasitol., v.7, p.99-103, 2012.) detected Hepatozoon spp. using examination by blood smears in 2/51 (3.92%) stray dogs and 2/203 (0.98%) client-owned dogs. El-Dakhly et al. (2013) detected the parasite in 45 (23.56%) naturally infected dogs from Japanese islands and peninsulas by Giemsa-stained blood smears and 81 (41.33%) by Polymerase Chain Reaction (PCR), suggesting molecular techniques as a more sensitive diagnostic method than blood smear. On other hand, Maia et al. (2015MAIA, C.; ALMEIDA, B.; COIMBRA, M. et al. Bacterial and protozoal agents of canine vector-borne diseases in the blood of domestic and stray dogs from southern Portugal. Parasitol. Vectors, v.8, p.138, 2015. Available in: <http://www.parasitesandvectors.com/content/pdf/s13071-015-0759-8.pdf>. Accessed in: October 2, 2015.
http://www.parasitesandvectors.com/conte...
) detected low occurrence (31/101, 3.07%) of H. canis infection in dogs from Portugal by PCR, as well as Aktas et al. (2015AKTAS, M.; OZUBEK, S.; ALTAY, K. et al. A molecular and parasitological survey of Hepatozoon canis in domestic dogs in Turkey. Vet. Parasitol., v.209, p.264-267, 2015. ) in Turkey (3/285, 1%). In Israel, Baneth et al. (2015BANETH, G.; HARRUS, S.; GAL, A.; AROCH, I. Canine vector-borne coinfections: Ehrlichia canis and Hepatozoon canis in the same host monocytes. Vet. Parasitol., v.208, p.30-34, 2015. ) observed a low range of infected monocytes and neutrophils (0.45-3.78%), however, 50% of all monocytes were coinfected with E. canis morulae.
Although few cases of H. canis infection have been diagnosed in this study, significant and variable hematological changes were observed in infected dogs. Anemia is a common finding in canine hepatozoonosis and is occasionally severe (Baneth et al., 2003BANETH, G.; MATHEW, J.S.; SHKAP, V. et al. Canine hepatozoonosis: two disease syndromes caused by separate Hepatozoon spp. Trends Parasitol., v.19, p.27-31, 2003. ). Chhabra et al. observed that anemia was a hematological alteration in most dogs infected by H. canis (73.53%) and they have associated it with the chronicity of infection. Most cases of anemia in canine hepatozoonosis are often characterized as normocytic, normochromic and non-regenerative (Mundim et al., 2008MUNDIM, A.V.; MORAIS, I.A.; TAVARES, M. et al. Clinical and hematological signs associated with dogs naturally infected by Hepatozoon sp. and with other hematozoa: a retrospective study in Uberlandia, Minas Gerais, Brazil. Vet. Parasitol., v.153, p.3-8, 2008.; Baneth and Weigler, 1997); even though the condition can be regenerative (Baneth and Weigler, 1997; Baneth et al., 2003).
Gondim et al. (1998GONDIM, L.F.; KOHAYAGAWA, A.; ALENCAR, N.X. et al. Canine hepatozoonosis in Brazil: description of eight naturally occurring cases. Vet. Parasitol., v.74, p.319-323, 1998.) reported anemia in 7/8 (87.50%) dogs infected with H. canis in Botucatu, SP, Brazil, between October 1993 and April 1994. Similarly, in our study, 6/9 (66.67%) infected dogs presented anemia and in 33.33% of them the anemia was normocytic normochromic. Also in Brazil, Mundim et al. (2008MUNDIM, A.V.; MORAIS, I.A.; TAVARES, M. et al. Clinical and hematological signs associated with dogs naturally infected by Hepatozoon sp. and with other hematozoa: a retrospective study in Uberlandia, Minas Gerais, Brazil. Vet. Parasitol., v.153, p.3-8, 2008.) diagnosed normocytic normochromic anemia in 70.43% dogs infected with H. canis.
In contrast, the WBC counts in the majority of dogs infected by H. canis decrease under the reference values, which differs from the results for American canine hepatozoonosis, in which leukocytosis is often severe (Vincent-Johnson et al., 1997). In agreement, the results of leukograms in this study showed counts within the reference values in most dogs, the same number that had anemia (6/9; 66.67%).
Neutrophilia and leukocytosis can be detected in H. canis infection and according to Mundim et al. (2008MUNDIM, A.V.; MORAIS, I.A.; TAVARES, M. et al. Clinical and hematological signs associated with dogs naturally infected by Hepatozoon sp. and with other hematozoa: a retrospective study in Uberlandia, Minas Gerais, Brazil. Vet. Parasitol., v.153, p.3-8, 2008.) it may be due to inflammatory response to tissue invasion and multiplication by H. canis, which can be exacerbated by secondary bacterial infections concomitant with other hematozoa. The authors reported leukocytosis in 39.13% of dogs infected with H. canis in the State of Minas Gerais and neutrophilia in 48.70%. In our study, neutrophilic leukocytosis was observed only in one (11.11%) dog (#8). Furthermore, the authors also observed that neutrophil left shift was a common alteration in the infected dogs and 40.87% of them have eosinopenia, which is in agreement with this study, in which these two alterations were observed in 44.44% of infected dogs.
Increased levels of plasma proteins were detected in four infected dogs, probably due to hyperglobulinemia. This is a frequent finding, caused by the stimulation of the humoral response induced by H. canis, which can be confirmed by the observation of the increase in the amount of plasma proteins, or by electrophoresis of serum proteins showing increased α and β globulin fractions (Vincent-Johnson et al., 1997; Aguiar et al., 2004AGUIAR, D.M.; RIBEIRO, M.G.; SILVA, W.B. et al. Hepatozoonose canina: achados clínico-epidemiológicos em três casos. Arq. Bras. Med. Vet. Zootec., v.56, p.411-413, 2004.).
Additionally, other studies in Brazil describe variable hematological changes in dogs infected by H. canis. Melo Junior et al. (2008) reported a dog naturally infected with H. canis that presented regenerative anemia, leukocytosis with marked neutrophil left shift, monocytosis and thrombocytopenia. Aguiar et al. (2004AGUIAR, D.M.; RIBEIRO, M.G.; SILVA, W.B. et al. Hepatozoonose canina: achados clínico-epidemiológicos em três casos. Arq. Bras. Med. Vet. Zootec., v.56, p.411-413, 2004.) also described regenerative anemia, leukocytosis with neutrophilia and monocytosis in hematological exams of three naturally infected dogs, together with lymphopenia and hypergammaglobulinemia in two of these dogs. Three-out-of-eight cases of canine hepatozoonosis diagnosed by Gondim et al. (1998GONDIM, L.F.; KOHAYAGAWA, A.; ALENCAR, N.X. et al. Canine hepatozoonosis in Brazil: description of eight naturally occurring cases. Vet. Parasitol., v.74, p.319-323, 1998.) had lymphopenia and four had monocytosis. Similarly, lymphopenia and monocytosis were diagnosed in three infected dogs in this study.
It should be noted that all hematological changes described in this study are due to natural H. canis infection. One infected dog was coinfected by E. canis and another by Babesia spp., and both presented abnormalities in the hematological results that cannot be attributed exclusively to H. canis infection (O'Dwyer, 2011). One infected dog had no alterations, but the hematological disorders of the other six dogs can be attributed to H. canis, since they were not diagnosed with additional pathology at the time of hematology.
However, it is notable that the hematologic alterations detected in these dogs infected by H. canis were quite variable, as the literature describes. Similarly, the pathogenicity of the parasite needs to be further elucidated and the clinical signs produced in the infection are also variable. Therefore, veterinarians should make the differential diagnosis in dogs, to avoid the underdiagnosis of H. canis.
CONCLUSIONS
Significant hematological alterations were detected in dogs infected by H. canis in this study. Although anemia was detected in most infected dogs, the hematological changes were quite variable, which highlight the importance of differential diagnosis in dogs, for H. canis and other agents that infect canine blood cells. Longitudinal studies would be of fundamental importance to determine the causality of these alterations.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the direction, trainees and staff of the Clinical Pathology Laboratory of the Veterinary Hospital of the Grande Dourados University Center (UNIGRAN).
REFERENCES
- AGUIAR, D.M.; RIBEIRO, M.G.; SILVA, W.B. et al. Hepatozoonose canina: achados clínico-epidemiológicos em três casos. Arq. Bras. Med. Vet. Zootec., v.56, p.411-413, 2004.
- AKTAS, M.; OZUBEK, S.; ALTAY, K. et al. A molecular and parasitological survey of Hepatozoon canis in domestic dogs in Turkey. Vet. Parasitol., v.209, p.264-267, 2015.
- AMOLI, A.R.; KHOSHNEGAH, J.; RAZMI, G. A preliminary parasitological survey of Hepatozoon spp. infection in dogs in Mashhad, Iran. Iran. J. Parasitol., v.7, p.99-103, 2012.
- BANETH, G.; BARTA, J.R.; SHKAP, V. et al. Genetic and antigenic evidence supports the separation of Hepatozoon canis and Hepatozoon americanum at the species level. J. Clin. Microbiol., v.38, p.1298-1301, 2000.
- BANETH, G.; HARRUS, S.; GAL, A.; AROCH, I. Canine vector-borne coinfections: Ehrlichia canis and Hepatozoon canis in the same host monocytes. Vet. Parasitol., v.208, p.30-34, 2015.
- BANETH, G.; MATHEW, J.S.; SHKAP, V. et al. Canine hepatozoonosis: two disease syndromes caused by separate Hepatozoon spp. Trends Parasitol., v.19, p.27-31, 2003.
- BANETH, G.; SHKAP, V.; SAMISH, M. et al. Antibody response to Hepatozoon canis in experimentally infected dogs. Vet. Parasitol., v.74, p.299-305, 1998.
- BANETH, G.; WEIGLER, B. Retrospective case-control study of hepatozoonosis in dogs in Israel. J. Vet. Intern. Med., v.11, p.365-70, 1997.
- BUSH, B.M. (Ed.). Interpretação de resultados laboratoriais para clínicos de pequenos animais. São Paulo: Roca, 2004. 384p.
- CHHABRA, S.; UPPAL, S.K.; SINGLA, L.D. Retrospective study of clinical and hematological aspects associated with dogs naturally infected by Hepatozoon canis in Ludhiana, Punjab, India. Asian. Pac. J. Trop. Biomed., v.3, p.483-86, 2013.
- EL-DAKHLY, K.M.; GOTO, M.; NOISHIKI, K. et al. Prevalence and diversity of Hepatozoon canis in naturally infected dogs in Japanese, Islands and Peninsulas. Parasitol. Res., v.112, p.3267-3274, 2013.
- GONDIM, L.F.; KOHAYAGAWA, A.; ALENCAR, N.X. et al. Canine hepatozoonosis in Brazil: description of eight naturally occurring cases. Vet. Parasitol., v.74, p.319-323, 1998.
- MAIA, C.; ALMEIDA, B.; COIMBRA, M. et al. Bacterial and protozoal agents of canine vector-borne diseases in the blood of domestic and stray dogs from southern Portugal. Parasitol. Vectors, v.8, p.138, 2015. Available in: <http://www.parasitesandvectors.com/content/pdf/s13071-015-0759-8.pdf>. Accessed in: October 2, 2015.
» http://www.parasitesandvectors.com/content/pdf/s13071-015-0759-8.pdf - MELO JUNIOR, O.A.; MIRANDA, F.J.B.; ALMEIDA, J. et al. Hepatozoonose canina em Campos dos Goytacazes, RJ. Arq. Cienc. Vet. Zool., v.11, p.73-75, 2008.
- MERCER, S.H.; CRAIG, T.M. Comparison of various staining procedures in the identification of Hepatozoon canis gamonts. Vet. Clin. Pathol., v.17, p.63-65, 1988.
- MUNDIM, A.V.; MORAIS, I.A.; TAVARES, M. et al. Clinical and hematological signs associated with dogs naturally infected by Hepatozoon sp. and with other hematozoa: a retrospective study in Uberlandia, Minas Gerais, Brazil. Vet. Parasitol., v.153, p.3-8, 2008.
- MYLONAKIS, M.E.; KOUTINAS, A.F.; BANETH, G. et al. Mixed Ehrlichia canis, Hepatozoon canis, and presumptive Anaplasma phagocytophilum infection in a dog. Vet. Clin. Pathol., v.33, p.249-251, 2004.
- NIJHOF, A.M.; GUGLIELMONE, A.A.; HORAK, I.G. TicksBase. v.5.6, 2015. Available in: <http://www.catalogueoflife.org/annual-checklist/2011/details/database/id/30>. Accessed in: February 28, 2015.
» http://www.catalogueoflife.org/annual-checklist/2011/details/database/id/30 - O'DWYER, L.H. Brazilian canine hepatozoonosis. Rev. Bras. Parasitol. Vet., v.20, p.181-193, 2011.
- O'DWYER, L.H.; MASSARD, C.L.; SOUZA, J.C.P. Hepatozoon canis infection associated with dog ticks of rural areas of Rio de Janeiro State, Brazil. Vet. Parasitol., v.94, p.143-50, 2001.
- O'DWYER, L.H.; SAITO, M.E.; HASEGAWA, M.Y.; KOHAYAGAWA, A. Prevalence, hematology and serum biochemistry in stray dogs naturally infected by Hepatozoon canis in São Paulo. Arq. Bras. Med. Vet. Zootec., v.58, p.688-690, 2006.
- RIZZI, T.E.; MEINKOTH, J.H.; CLINKENBEARD, K.D. Normal hematology of the dog. In: WEISS, D.J.; WARDROP, K.J. (Eds.). Schalm's veterinary hematology. 6.ed. Ames: Wiley-blackwell, 2010. p.799-810.
- SALGADO, F.P.; HONER, M.R.; ISHIKAWA, M.M. et al. Hemoparasitos e carrapatos em cães procedentes do Centro de controle de Zoonoses de Campo Grande, estado de Mato Grosso do Sul, Brasil. Rev. Bras. Med. Vet., v.29, p.113-116, 2007.
- THRALL, M.A. (Ed.). Hematologia e bioquímica clínica veterinária. São Paulo: Roca , 2006. 592p.
- TRIOLA, M.F. (Ed.). Introdução à estatística. 9.ed. Rio de Janeiro: LTC, 2005. 682p.
- VINCENT-JOHNSON, N.; MACINTYRE, D.K.; BANETH, G. Canine hepatozoonosis: pathophysiology, diagnosis, and treatment. Compend. Cont. Educ. Pract. Vet., v.19, p.51-65, 1997.
Publication Dates
-
Publication in this collection
Sep-Oct 2016
History
-
Received
08 Sept 2015 -
Accepted
2016