ABSTRACT
Glässer's disease is an emergent bacterial disease that affects swine husbandries worldwide causing important economic losses. The aetiological agent, Haemophilus parasuis, is currently divided in fifteen serovars but an increasing number of non-typeable serovars have been reported. Indirect hemagglutination (IHA) is indicated as a serotyping method for H. parasuis. In the present study, we describe an additional step that aims to work around a possible obstacle in the original protocol that may compromise the outcome of this assay. We observed that the choice of anticoagulant for blood collection influences and/or impairs spontaneous adsorption of H. parasuis antigens on sheep red blood cells (SRBCs). However, regardless of the anticoagulant used, chemical treatment of SRBCs with tannic acid induces a stable antigen adsorption (sensitization step). The addition of 1% BSA to SRBCs washing buffer and to antisera dilution augments IHA specificity. Tannic acid treated SRBCs combined with thermo-resistant H. parasuis antigens increases the assay resolution. Thus, our results demonstrate an improvement in the technique of H. parasuis serotyping that will prove valuable to understand Glässer's disease epidemiology and to better characterize serovars involved in outbreaks.
Keywords:
Haemophilus parasuis; diagnosis; tannic acid; serotyping; indirect hemagglutination
RESUMO
A Doença de Glässer é uma doença bacteriana emergente que afeta a produção de suínos em todo o mundo e causa importantes perdas econômicas. O agente etiológico, Haemophilus parasuis, é atualmente dividido em quinze sorovares; no entanto, um número crescente de cepas não tipificáveis tem sido relatado. O teste de hemaglutinação indireta (IHA) tem sido utilizado para a sorotipificação de H. parasuis. Neste estudo, descrevemos uma alteração no protocolo original de IHA e que supera uma limitação específica que pode comprometer o uso geral deste ensaio. Descobrimos que o tipo de anticoagulante utilizado para coletar os eritrócitos ovinos (SRBCs) pode comprometer a adsorção espontânea dos antígenos do H. parasuis. Por outro lado, o tratamento químico dos SRBCs com ácido tânico promove uma adsorção antigênica estável (passo de sensibilização) e independente do anticoagulante utilizado. O uso de 1% de BSA durante as lavagens dos SRBCs e na diluição dos antissoros incrementa a especificidade da IHA e, a combinação dos SRBCs tratados quimicamente com antígenos de H. parasuis termo-resistentes aumentam a resolução da IHA. Nossos resultados destacam uma melhoria na principal técnica de sorotipificação de H. parasuis, que auxiliará diretamente no entendimento da epidemiologia da Doença de Glässer e na caracterização dos sorovares envolvidos em surtos da doença.
Palavras-chave:
Haemophilus parasuis; diagnóstico; ácido tânico; sorotipificação; hemaglutinação indireta
INTRODUCTION
Haemophilus parasuis is a commensal gram-negative bacterium normally found in the upper respiratory tract of swine. Stress and viral or bacterial co-infections are important but not necessary stimuli for H. parasuis to trigger Glässer's disease (GD) (; ; ), a highly prevalent and ubiquitous infection of swine.
Currently, there are 15 known H. parasuis serovars (SV) that can be subdivided into highly virulent (SV 1, 5, 10, 12-14), moderately virulent (SV 2, 4 and 15), and weakly or non virulent (SV 3, 6-9 and 11) (). Reports of field isolates, non-typeable by serological methods currently used to classify H. parasuis, point out the antigenic diversity of this bacterium. Currently, the techniques used to serotype H. parasuis are gel immunodiffusion using heat-stable antigens () and indirect hemagglutination (IHA) (; ; ). Both assays are prone to subjective interpretation and depend on non-commercial antigens and, in the case of IHA, on the sensitization of sheep red blood cells (SRBCs) with H. parasuis antigens. Although reported as a natural phenomenon (), adsorption of H. parasuis onto commercially obtained or fresh SRBCs might be uneven or even fail (personal observation) compromising the assay reliability. Poor growth of some field isolates, scarce antigen production and a high degree of cross-reactivity contribute to hinder proper diagnosis and serovar allocation.
Although successfully used in previous work and by several laboratories, serotyping of H. parasuis by the classic IHA protocol (; ; ) proved to be troublesome. At our laboratory, SRBCs collected from several sheep breeds were not able to stably adsorb H. parasuis antigens. This observation raised the question as to whether a previous chemical treatment of the SRBCs could promote a more stable antigen binding capacity. Here we demonstrate that tannic acid treatment of SRBCs promotes solid adsorption of H. parasuis' antigens. Our findings might improve diagnosis and serotyping of H. parasuis and impact on vaccine design.
MATERIAL AND METHODS
H. parasuis reference strains for SV 1 to 15 (Nº4, SW140, SW114, SW124, Nagasaki, 131, 174, C5, D74, H555, H465, H425, 84-17975, 84-22113 and 84-15995, respectively) were used. Fifty clinical isolates of H. parasuis (isolated from 35 to 45 days old piglets showing fibrinous polyserositis) were molecularly serotyped () and also included in this study. These bacteria were cultivated on chocolate agar plates supplemented with 2.5 mg/ml glucose (Sigma) and 72 μg/ml nicotinamide adenine dinucleotide (NAD) (Sigma) for 24-36 h at 37°C and 5% CO2.
Hyperimmune antisera against all reference strains were produced in New Zealand rabbits (6-month old females), serologically negative for H. parasuis. The animals (n=15) were housed in individual cages and received feed and water ad libitum. The antisera were prepared as described by with some modifications. Whole bacteria were inactivated with 0.1% thimerosal (Sigma) () and adjusted to 1×1010 bacteria/ml using the FACS Verse(tm) Flow Cytometer equipped with a volumetric flow sensor (BD Biosciences). Then, they were mixed with Freund's complete adjuvant (Sigma; 1:1.2 ratio) and injected subcutaneously in rabbits. Booster immunization was administered 21 days later using whole bacteria adjuvanted with incomplete Freund's adjuvant (Sigma). Two weeks after, four intravenous injections (0.5 ml) using 108 inactivated bacteria were performed at 2-day intervals, followed one week later by four intravenous injections (1.0 ml) of live antigens. One week after the last injection, total sera was collected and stored at -80ºC. During the immunization process an indirect ELISA () was used to assess the kinetics of antibody response induced by each serovar (in duplicate). This experiment was conducted in accordance with the Ethical Committee for Animal Experimentation of UPF (protocol number 039/2012).
For antisera adsorption with heterologous reference strains, H. parasuis reference strains were grown on chocolate agar, inactivated with 0.1% thimerosal, washed and suspended in PBS (pH 7.2). Rabbit polyclonal antiserum to each SV was added to a mix of the 14 heterologous inactivated reference strains (containing 2×108 bacteria of each SV) and incubated for 2 h at 37°C, and then overnight at 4°C. Afterward, the antisera were heat-treated (56ºC/30min) to inactivate the complement system and adsorbed with 10% SRBC suspension (1 h at room temperature). The supernatant containing the non-adsorbed antibodies was aliquoted and stored at - 80ºC until use.
For surface antigen extraction, H. parasuis was grown as described above, harvested and suspended in 1mL 0.9% saline. Bacteria were incubated for 1h at 94°C, the suspension was then centrifuged for 10 min at 13,000 × g and the supernatant containing H. parasuis surface antigens was collected. In parallel, the antigens were prepared as described by .
SRBCs were collected from four sheep breeds (Île de France, Merino, Texel and Suffolk) using Vacutainer(r) collection tubes containing EDTA (BD). Alternatively, SRBCs were obtained using different anticoagulant such as lithium heparin, sodium citrate, acid citrate dextrose (ACD, 23 mmol/L citric acid, 45 mmol/L sodium citrate and 74mmol/L dextrose, pH 7.2), Alsever's solution (Sigma), or by mechanical defibrination. Total blood was centrifuged (for 10 min at 300 × g), SRBCs were harvested, washed 3x with saline and suspended to a final concentration of 3% in saline. Half of the SRBCs from each breed was incubated with tannic acid (0.05 (g/ml, Sigma) for 10 min at 37°C and then washed again 3x with saline. Tannic-acid treated and non-treated SRBCs were then incubated with H. parasuis surface antigen (3% SRBCs final concentration) for 90 min at 37°C with gently shaking. All sensitized SRBCs (SSRBCs) were then washed 3x with diluent buffer (saline containing 1% bovine serum albumin, BSA) and suspended in the same buffer to a 0.75% vol/vol.
Serotyping by altered indirect hemagglutination (IHA) was performed by two-fold serial dilutions of all antisera, from 1:10 to 1:20,480 in "U" botton 96-well plates. SSRBCs were added to a 1:1 vol/vol rate to antisera and plates were incubated for 2 h at 37°C. Non-sensitized SRBCs + dilution buffer or reference antiserum, and SSRBCs + dilution buffer were used as negative controls; SSRBCs + homologous antiserum were used as positive control. SSRBCs not treated with tannic acid + homologous antiserum was used as control of spontaneous antigen adsorption and hemagglutination. Antiserum titration was expressed as the reciprocal of the highest dilution giving a positive agglutination reaction. If the isolate was found to be positive to more than one antiserum, the serovar was attributed to the one exhibiting the highest titre, provided that there were at least two dilutions of difference in the titres. When this discrimination was not possible, the isolate was considered as non-typeable.
RESULTS AND DISCUSSION
IHA has been developed to be an accessible laboratory method for diagnosis and serotyping of H. parasuis clinical isolates (; ). This technique is based on the spontaneous adsorption of H. parasuis antigens on SRBCs which are then used as indicators of the antibody-antigen reaction (). Despite being described as a reliable and sensitive technique, the percentage of non-typeable clinical isolates varies widely, ranging from 7% () to 44% (). To simplify H. parasuis serotyping, described a molecular approach based on a multiplex PCR (mPCR) with high capacity to assign a specific serovar. However, this technique does not allow discriminating between SV 5 and 12, underlying the necessity to perform the IHA or another assay whenever the results are not conclusive.
Because commercial antigens are unavailable to be used in the SRBCs sensitization step, H. parasuis culture conditions as well as the protocols to obtain surface antigens might be directly related to the ability to successfully perform the IHA assay. In addition, there is no information about the commercially available SRBCs (if they have received or not previous chemical treatment) and this can also compromise the assay due to different absorption capacity for H. parasuis antigen. Although used by different groups (; ; ), we observed that non-treated SRBCs show different capacity to capture antigens from H. parasuis, which can compromise the results of this diagnosis technique.
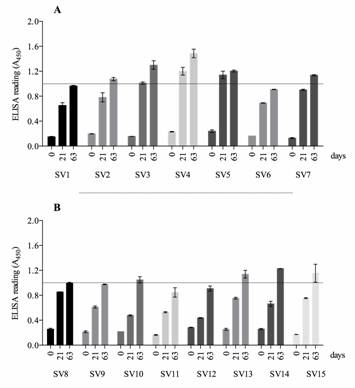
A panel of hyperimmune antisera to all reference serovars was produced in New Zealand rabbits and the antibody response was tested by ELISA using inactivated H. parasuis whole-cells as antigen (Figure 1). Rabbits seroconverted after the first immunization (day 21), with the highest antibody titres being reached for SV 4 and 5 (1.2 ± 0.1 and 1.14 ± 0.06 respectively, Figure 1A); while the rabbit immunized with SV 12 seroconverted only after the second antigen injection. At end of the hyperimmunization protocol (days 63) we have observed differences between the titres of antibodies from the 15 references serovars, indicating that some strains were less immunogenic (Figure 1A - B). Although previous studies reported the difficulty to produce good levels of antibodies against some reference serovars of H. parasuis (; ), in our study all serovars induced a satisfactory immune response. One aspect that needs to be considered between our and the previous studies is the molecule used for the chemical inactivation of the bacteria. All antisera produced by others, reported in the literature, used formaldehyde-treated whole-bacteria (solution between 0.3 to 0.5%) as antigen, while in our study we used thimerosal, an effective bacteriostatic molecule. It might be that thimerosal preserves additional epitopes in comparison with formalin and, consequently, overcomes the difficulty to produce antisera against the strain 174 (SV 7), as previously reported by , and .
Antisera production in New Zealand rabbits against H. parasuis reference strains. Kinetics of the antibody (IgG) response was analyzed by indirect ELISA during immunization against reference strains (SV 1 - 7 panel A, SV 8 - SV 15 panel B). Time points measured are plotted on the x axis and represent antibodies in the serum: before the first immunization (0), 21 days after the first injection (21) and 12 days after the last intravenous injection (63). Antisera were tested twice. Results are expressed as the mean ±SD.
Because we were unable to reproduce the classical IHA to titre the rabbit polyclonal antiserum (the results were not replicable), we hypothesized that the SRBCs present variable capacity to spontaneously adsorb H. parasuis antigen. Then, blood was collected from four sheep breeds (Île de France, Merino, Texel and Suffolk) and sensitized with H. parasuis antigen. When tested with the panel of polyclonal antibodies, none of the SRBCs spontaneously adsorbed H. parasuis antigens (data not shown). To discard the possibility that SRBCs surface molecules were altered by EDTA and became incapable of absorbing antigen, several anticoagulants were compared. SRBCs collected with lithium heparin, sodium citrate, ACD, Alsever solution and by mechanical defibrination process were also tested and all, with the exception of Alsever solution, failed to yield positive results on IHA, suggesting that the success of antigen adsorption onto SRBCs depends on the type of blood anticoagulant used.
Thus, we hypothesized that a chemical modification of unknown molecules present on SRBCs surface would allow H. parasuis antigen adsorption. In fact, after treating SRBCs (collected by most of the methods described here) from all breeds with tannic acid, we used them for IHA showing that this treatment increases the antigen adsorption stability by modifying some unknown molecules present on SRBC surface. Tannic acid has been used to treat SRBCs in IHA tests to detect antibodies against other pathogens such as Mycobacterium tuberculosis (Boyden, 1950BOYDEN, S. V. Haemagglutinins from Mycobacterium tuberculosis. Nature, v. 165, p. 765, 1950.) or Borrelia burgdorferi (). Because bacterial lipopolysaccharides are naturally adsorbed onto the surface of SRBCs, while some protein antigens or small peptides require tannic acid pre-conditioning, we hypothesize that H. parasuis antigens that bind to SRBCs are also of a protein nature, or at least part of the antigen is made up of peptides. In contrast, it has been previously reported that H. parasuis antigens are of a lipopolysaccharide nature with intrinsic capacity to bind on SRBCs surface (Tadjine et al., 2004).
To verify whether the thermic treatment could modify the antigens used in the IHA assay, we compared the protocols described by and by . Our results demonstrate that both methods can be used for H. parasuis antigen production. However, to some serovars, the thermic treatment increased the title of hemmaglutination reaction (average of 3 serial dilution) when compared with the method described by (Table 1). Another important difference between these two techniques was the time required to produce the antigens and, consequently, to reach the results. The thermic procedure can provide the final result in one day, an advantage for clinical veterinarians.
Results of rabbit antibodies titration by altered IHA using two types of antigens (thermic and osoluble antigen) from the 15 reference strains of H. parasuis. The hyperimmune antisera were produced against whole-bacteria inactivated with thimerosal. Table layout obtained from Turni and Blackall (2005)
Regardless of the chemical nature of the antigens, it would be interesting to identify the molecules adsorbed to SRBCs that are unique to each serovar and use them to develop less laborious serological assays that could be widely used to characterize geographical distribution and improve serovar allocation of yet non-typeable isolates. In addition, we found that the use of 1% BSA, instead of rabbit serum, avoided nonspecific reactions of rabbit serum with H. parasuis antigens so that cross-reactions among reference serovars were not detected.
Using the tannic-acid treated SRBCs, we titrated specific antibodies in the panel of hyperimmune sera. The titres of hemagglutinating antibodies were between 1,280 to 20,480, the highest titre being against SV 9 (20,480), SV 2, 6, 12 (all with 10,240) (Table 1). Because H. parasuis shares immunogenic and conserved epitopes that might interfere with IHA, cross-reactivity was inhibited by adsorbing each serum to a panel of the heterologous SV. Consequently, the serum titre after adsorption against homologous bacteria decreased in some serovars, especially in SV1, SV4 and SV5. However, all antisera titration enabled to perform IHA with high sensitivity (Table 1).
The altered indirect hemagglutination (aIHA) was evaluated using 50 clinical samples that were previously typed by mPCR as SV 1 (n=7), SV 2 (n=2), SV 4 (n=12), SV 5 or SV 12 (n=17), SV 14 (n=6) and non-typeable (n=6). The mPCR assay allowed us to assign a serovar to 88% of the samples (44/50). Using the aIHA with thermic antigen extraction, it was possible to assign the same serovar to the clinical isolates with the exception of some samples belonging to the SV 4, SV 12 and SV 14, for which two, one and three isolates respectively were assigned by aIHA as non-typeable. Amongst the isolates typed as SV 5 or 12 by mPCR we were able by aIHA to identify 10 isolates as SV 5, 6 isolates as SV 12 and 1 isolate was non-typeable. The 6 strains that were non-typeable by mPCR were also non-typeable by aIHA. Thus, using aIHA we properly typed 38 out of the 50 clinical samples (76%) compared to 88% of typing with mPCR. Although less sensitive than mPCR, aIHA is a powerfull and cheaper assay that can be easily applied to characterize clinical isolate and to discriminate between most H. parasuis clinical isolates.
CONCLUSION
We demonstrated that the chemical treatment of SRBCs increases the adsorption stability of H. parasuis antigen onto SRBCs by modification of yet unknown molecules. The modification proposed in this study represents a major step toward understanding the biochemical nature of H. parasuis antigens related to serotyping. The combination of chemical preparation of SRBCs with antigen obtained by thermic treatment showed the best hemagglutination resolution, which points to the use of aIHA to conduct H. parasuis serotyping independently, or as a complement to mPCR. Finally, all the comparisons between methods proposed in this study can be used as a support to develop reagents and tools for diagnosis of GD.
ACKNOWLEDGEMENTS
This work was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, grant 485807/2013-0). M.M. was recipient of a postdoctoral fellowship from the Coordenação de Aperfeiçoamento de Pessoal de Ensino Superior (CAPES). M.S.L. was supported by a Master fellowships from the Fundação Universidade de Passo Fundo.
REFERENCES
- BOYDEN, S. V. Haemagglutinins from Mycobacterium tuberculosis. Nature, v. 165, p. 765, 1950.
- BROCKMEIER, S. L.; LOVING, C. L.; MULLINS, M. A. et al Virulence, transmission, and heterologous protection of four isolates of Haemophilus parasuis. Clin Vaccine Immunol, v. 20, p. 1466-72, 2013.
- DEL RIO, M. L.; GUTIERREZ, C. B.; RODRIGUEZ FERRI, E. F. Value of indirect hemagglutination and coagglutination tests for serotyping Haemophilus parasuis. J Clin Microbiol, v. 41, p. 880-2, 2003.
- FRANDOLOSO, R., MARTINEZ, S., RODRIGUEZ-FERRI, E. F., GARCIA-IGLESIAS, M. J., PEREZ-MARTINEZ, C., MARTINEZ-FERNANDEZ, B., GUTIERREZ-MARTIN, C. B. Development and characterization of protective Haemophilus parasuis subunit vaccines based on native proteins with affinity to porcine transferrin and comparison with other subunit and commercial vaccines. Clin Vaccine Immunol, v. 18, p. 50-8, 2011.
- HOWELL, K. J.; PETERS, S. E.; WANG, J. et al Development of a Multiplex PCR Assay for Rapid Molecular Serotyping of Haemophilus parasuis. J Clin Microbiol, v. 53, p. 3812-21, 2015.
- KIELSTEIN, P.; RAPP-GABRIELSON, V. J. Designation of 15 serovars of Haemophilus parasuis on the basis of immunodiffusion using heat-stable antigen extracts. J Clin Microbiol, v. 30, p. 862-5, 1992.
- KIM, J.; CHUNG, H. K.; JUNG, T. et al Postweaning multisystemic wasting syndrome of pigs in Korea: prevalence, microscopic lesions and coexisting microorganisms. J Vet Med Sci, v. 64, p. 57-62, 2002.
- MARTIN DE LA FUENTE, A. J.; RODRIGUEZ-FERRI, E. F.; FRANDOLOSO, R. et al Systemic antibody response in colostrum-deprived pigs experimentally infected with Haemophilus parasuis. Res Vet Sci, v. 86, p. 248-53, 2009.
- MITTAL, K. R.; HIGGINS, R.; LARIVIERE, S. Determination of antigenic specificity and relationship among Haemophilus pleuropneumoniae serotypes by an indirect hemagglutination test. J Clin Microbiol, v. 17, p. 787-90, 1983.
- MOROZUMI, T.; NICOLET, J. Some antigenic properties of Haemophilus parasuis and a proposal for serological classification. J Clin Microbiol, v. 23, p. 1022-5, 1986.
- PAVIA, C. S.; WORMSER, G. P.; BITTKER, S. et al An indirect hemagglutination antibody test to detect antibodies to Borrelia burgdorferi in patients with Lyme disease. J Microbiol Methods, v. 40, p. 163-73, 2000.
- RAFIEE, M.; BLACKALL, P. J. Establishment, validation and use of the Kielstein-Rapp-Gabrielson serotyping scheme for Haemophilus parasuis. Aust Vet J, v. 78, p. 172-4, 2000.
- RAPP-GABRIELSON, V. J.; GABRIELSON, D. A. Prevalence of Haemophilus parasuis serovars among isolates from swine. Am J Vet Res, v. 53, p. 659-64, 1992.
- TADJINE, M.; MITTAL, K. R.; BOURDON, S. et al Development of a new serological test for serotyping Haemophilus parasuis isolates and determination of their prevalence in North America. J Clin Microbiol, v. 42, p. 839-40, 2004.
- TURNI, C.; BLACKALL, P. J. Comparison of the indirect haemagglutination and gel diffusion test for serotyping Haemophilus parasuis. Vet Microbiol, v. 106, p. 145-51, 2005.
Publication Dates
-
Publication in this collection
Feb 2017
History
-
Received
26 July 2016 -
Accepted
27 July 2016