ABSTRACT
With the objective of detecting the presence of caprine lentivirus (CLV) in ewe milk and in ram semen, ten matrixes and four reproducers experimentally infected with CLV were used. Samples of ewe milk were collected during the four months of lactation, five collections per animal, totaling 50 samples. Regarding the rams, eight semen collections were made per animal, during one year of experimentation, totaling 32 samples. The milk and semen samples were submitted to DNA extraction and the nested polymerase chain reaction test (nPCR) to detect CLV proviral DNA. Eight (16%) of the milk samples were positive in nPCR originating from two ewes. Only one (3.12%) semen sample was positive. The amplification products were sequenced, and were confirmed to be a CLV genomic sequence. Thus, the presence of CLV proviral DNA in sheep milk and semen was demonstrated, confirming the feasibility of infection between species, and alerting to the risk of spreading infections.
Keywords:
lentiviruses; transmission; cross infection
RESUMO
Com o objetivo de detectar a presença do lentivírus caprino (LVC) no leite de ovelhas e no sêmen de carneiros, utilizaram-se 10 matrizes e quatro reprodutores infectados experimentalmente com o LVC. Foram coletadas amostras de leite das ovelhas durante os quatro meses de lactação, ocorrendo cinco coletas por animal, totalizando 50 amostras. Quanto aos carneiros, realizaram-se oito coletas de sêmen por animal, durante um ano de experimentação, totalizando 32 amostras. As amostras de leite e de sêmen foram submetidas à extração de DNA e à prova de reação em cadeia da polimerase do tipo nested (nPCR) visando à detecção de DNA proviral do LVC. Oito (16%) amostras de leite foram positivas na nPCR oriundas de duas ovelhas. Apenas uma (3,12%) amostra de sêmen apresentou positividade. Produtos da amplificação foram sequenciados, confirmando-se tratar de sequência genômica do LVC. Dessa forma, demonstrou-se a presença do DNA proviral do LVC em leite e sêmen de ovinos, confirmando a viabilidade da infecção entre espécies e, assim, alertando sobre o risco de que a infecção seja disseminada.
Palavras-chave:
lentiviroses; transmissão; infecção cruzada
INTRODUCTION
Caprine arthritis encephalitis (CAE) and ovine maedi-visna (MV) are diseases caused by small ruminant lentiviruses (SRLV), characterized by chronic evolution and progressive worsening until death (Blacklaws, 2012BLACKLAWS, B.A. Small ruminant lentiviruses: Immunopathogenesis of visna-maedi and caprine arthritis and encephalitis virus. Comp. Immunol. Microbiol. Infect. Dis., v.35, p.259-269, 2012.). Different clinical manifestations are known, the main ones being arthritis, pneumonia, encephalitis and mastitis, besides progressive weight loss (Pasick, 1998PASICK, J. Maedi-visna virus and caprine arthritis-encephalitis virus: distinct species or quasispecies and its implications for laboratory diagnosis. Can. J. Vet. Res., v.62, p.241-244, 1998.; Souza et al., 2015SOUZA, T.S.; PINHEIRO, R.R.; COSTA, J.N.; et al. Interspecific transmission of small ruminant lentiviruses from goats to sheep. Braz. J. Microbiol., v.46, p.867-874, 2015.).
The most important transmission routes are by ingesting contaminated milk and colostrum, and by contact with secretions containing the virus, such as those from the respiratory and reproductive tract. Thus, transmission may happen vertically, between the infected matrix and its offspring (Alvarez et al., 2005ÁLVAREZ, V.; ARRANZ, J.; DALTABUIT-TEST, M. et al. Relative contribution of colostrum from Maedi-Visna virus (MVV) infected ewes to MVV-seroprevalence in lambs. Res. Vet. Sci., v.78, p.237-243, 2005.); horizontally, by direct contact between infected and susceptible animals (Villoria et al., 2013VILLORIA, M.; LEGINAGOIKOA, I.; LUJÁN, L. et al. Detection of small ruminant lentivirus in environmental samples of air and water. Small Ruminant Res v.110, p.155-160, 2013.), and in iatrogenic way, with emphasis on artificial feeding and use of semen in reproduction techniques (Alvarez et al., 2006ÁLVAREZ, V.; ARRANZ, J.; DALTABUIT-TEST, M. et al. Relative contribution of colostrum from Maedi-Visna virus (MVV) infected ewes to MVV-seroprevalence in lambs. Res. Vet. Sci., v.78, p.237-243, 2005.; Andrioli et al., 2006ANDRIOLI, A.; GOUVEIA, A.M.G.; MARTINS, A.S. et al. Fatores de risco na transmissão do lentivírus caprino pelo sêmen. Pesqui. Agropecu. Bras, v.41, p.1313-1319, 2006.; Souza et al., 2013SOUZA, K.C.; PINHEIRO, R.R.; SANTOS, D.O. et al. Transmission of the caprine arthritis-encephalitis virus through artificial insemination. Small Ruminant Res , v.109, p.193-198, 2013. ).
The etiologic agents of CAE and MV were considered to be species-specific for many years. However, genomic analyzes of SRLV pointed to the occurrence of heterogeneous strains, evolved from viral prototypes of CAE and MV, able to infect both goats and sheep (Shah et al., 2004SHAH, C.A.; BÖNI, J.; HUDER, J.B. et al. Phylogenetic analysis and reclassification of caprine and ovine lentiviruses based on 104 new isolates: evidence for regular sheep-to-goat transmission and world-wide propagation through livestock trade. Virology , v.319, p.12-26, 2004.).
In this sense, the transmission of caprine lentivirus to sheep has already been proved (Souza et al., 2015SOUZA, T.S.; PINHEIRO, R.R.; COSTA, J.N.; et al. Interspecific transmission of small ruminant lentiviruses from goats to sheep. Braz. J. Microbiol., v.46, p.867-874, 2015.), but it is not yet clear whether animals infected with heterologous strains can transmit the infection. Thus, the objective of the present experiment was to detect the presence of caprine lentivirus proviral DNA in the milk and semen of experimentally infected sheep.
MATERIAL AND METHODS
The experiment was performed in the National Center of Goat and Sheep Research, belonging to the Brazilian Enterprize for Agricultural Research ("Empresa Brasileira de Pesquisa Agropecuária (Embrapa Caprinos e Ovinos),") in the municipality of Sobral (CE), Brazil, under aproval from the Committee of Ethics on Animal Use from the Vale de Acaraú State University ("Universidade Estadual do Vale do Acaraú") (number 001/2012).
In order to detect caprine lentivirus (CLV) in sheep milk and semen, two experimental groups of crossbred Morada and Santa Inês one year old animals were kept isolated in experimental stalls. The first group consisted of 10 ewes and the second of four rams. Both the ewes and rams were infected with CLV at birth, by feeding on colostrum and milk from infected goats. Infection by CLV was confirmed by nested polymerase chain reaction tests (nPCR) at seven days of life and the animals were clinically evaluated throughout the experiment.
In the group of ewes, milk samples were collected at 30, 60, 75, 90 and 120 days of lactation in sterile 15mL falcon vials after cleaning the teats using cheescloth and 70% alcohol. The ewes were milked delicately until 15mL milk was obtained from each teat.
The milk samples were centrifuged at 3,000g for 15 minutes and washed five times with phosphate-buffered saline solution (PBS) to obtain the cell pap, based on the methodology described by Sardi et al. (2012SARDI, S.I.; TORRES, J.A.; BRANDÃO, C.F.L. et al. Early detection of goats infected with lentivirus small ruminant virus by ELISA assay. Rev. Ciênc. Méd. Biol. v.11, p.35-40, 2012.). DNA was extracted according to Grimberg et al. (1989GRIMBERG, J.; NOWOSCHIK, S.; BELLUSCIO, L. et al. A simple and efficient non-organic procedure for the isolation of genomic DNA from blood. Nucleic Acids Res, v.17, p.83-90, 1989.), using Lithium chloride and proteinase K. After extraction, samples were stored at -20ºC, until the nPCR technique was performed.
In the group of rams, eight semen collections were made per animal, during one year of experimentation. Semen was collected using an artificial vagina, using an ewe in rut as a dummy.
For gel filtration in a Sephacryl S-40 column, 100µL of fresh semen was used, according to a method by Santurde et al. (1996SANTURDE, G.; SILVA, N.; VILLARES, R. et al. Rapid and high sensitivity test for direct detection of bovine herpesvirus-1 genome in clinical samples. Vet. Microbiol. v.49, p.81-92, 1996.), to remove impurites. Then, 3µL of each filtrate was transfered to steryle microtubes to extract the DNA, using 200µL Chelex 100 solution at 5%, 2µL proteinase K (10µg/µL) and 7µL Dithiothreitol at 1M, incubated in a water bath at 56°C, for 60 minutes. After this stage, samples were homogeneized for 10 seconds and heated in a boiling water bath (100°C) for eight minutes to inactivate proteinase K. Later the filtrate was centrifuged for three minutes at 13,000G and stored in a freezer at -20°C until the nPCR test was performed.
DNA samples extracted from milk and semen were submited to the nPCR technique, according to Barlough et al. (1994BARLOUGH, J.; EAST, N.; ROWE, J.D. et al. Double-nested polymerase chain reaction for detection of caprine arthritis-encephalitis virus proviral DNA in blood, milk, and tissues of infected goats. J. Virol. Methods, v.50, p.101-113, 1994.), modified by Andrioli et al. (2006ANDRIOLI, A.; GOUVEIA, A.M.G.; MARTINS, A.S. et al. Fatores de risco na transmissão do lentivírus caprino pelo sêmen. Pesqui. Agropecu. Bras, v.41, p.1313-1319, 2006.). The reaction consisted of a total volume of 50µL, containing buffer (10mM tris-HCl, 50mM KCl and 1.5mM MgCl2), 100µM each dNTP, 20pmol each oligonucleotide primer, 2U Taq polymerase; 3µL sample in the first stage and 1µL of its product in the second stage, completed to the final volume with sterile water. In paralel with the tested samples, a negative (sterile water) and a positive sample (material extracted from cultured synovial membrane infected with lentivirus strain B1, circulating in the experimental flock of goats infected with lentivirus at Embrapa National Research Center for Goats and Sheep) were used as control.
Two pairs of oligonucleotide primers were used in the reaction, obtained from the sequence from the gag gene region, from the standard CAEV-Cork strain (Saltarelli et al., 1990SALTARELLI, M.; QUERAT, G.; KONINGS, D.A.M. et al. Nucleotide sequence and transcriptional analysis of molecular clones of CAEV which generate infectious virus. Virology , v.179, p.347-364, 1990.), namely P1 primers (5'-CAAGCAGCAGGAGGGAGAAGCTG-3') and P2 primers (5'-TCCTACCCCCATAATTTGATCCAC-3') used to obtain a target sequence of 297pb and P3 primers (5'-GTTCCAGC AACTGCAAACAGTAGCAATG-3') and P4 (5'ACCTTTCTGCTTCTTCATTTAATTTCCC-3'), to obtain a target fragment of 187pb (Rimstad et al., 1993RIMSTAD, E.; EAST, N.E.; TORTEN, M. et al. Delayed seroconversion following naturally acquired caprine arthritis-encephalitis virus infection in goats. Am. J. Vet. Res. v.54, p.1858-1862, 1993.).
Amplification reactions were performed in a thermocycler (Programmable Thermal Controller, PTC-100, MJ Research, Inc.), consisting of an inicial cycle at 94°C for five minutes; followed by 35 cycles of one minute at 94°C, one minute at 56°C and 45 seconds at 72°C; final extension at 72°C for seven minutes. The amplified samples and positive and negative controls, along with 100 bp DNA ladder(r) marker, were submited to agarose gel electrophoresis at 2% in TBE (Tris, borate and EDTA 0.1X), stained with ethidium bromide and visualized in an ultraviolet transilluminator (Andrioli et al., 2006ANDRIOLI, A.; GOUVEIA, A.M.G.; MARTINS, A.S. et al. Fatores de risco na transmissão do lentivírus caprino pelo sêmen. Pesqui. Agropecu. Bras, v.41, p.1313-1319, 2006.).
The samples positive in nPCR were sequenced on the Applied Biosystems(r) 3500 Genetic Analyzer platform. These sequencies were aligned using Clustal W (Thompson et al., 1994THOMPSON, J.D.; HIGGINS, D.G.; GIBSON, T.J. Clustal W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position specific gap penalties and weight matrix choice. Nucleic Acids Res v.22, p.4673-4680, 1994.), with the BioEdit Sequence Alignment Editor(r) program (Hall, 1999HALL, T.A. Bioedit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Ser., v.41, p.95-98, 1999.) and compared with sequencies from standard CAEV Cork and MVVK1514 strains, available in GenBank under numbers M33677 and M60610, respectivelly, along with the sequence obtained from the strain circulating in Embrapa National Research Center for Goats and Sheep (BR/CNPC-G1), classified as subtype B1.
RESULTS AND DISCUSSION
CLV proviral DNA was detected in the milk of ewes by the nPCR technique in eight out of the 50 analyzed samples (Tab. 1). These positive samples belonged to two out of the ten ewes used.
The capacity of nPCR for detecting proviral DNA in milk was verified in other studies (Gregory et al., 2011GREGORY, L.; LARA, M.C.C.S.H.; HASEGAWA, M.Y. et al. Detecção do vírus da artrite encefalite caprina em pulmão, glândula mamária, cérebro e líquido sinovial de cabras naturalmente infectadas pela técnica de nested-PCR. Med. Vet, v.5, p.7-11, 2011.; Sardi et al., 2012SARDI, S.I.; TORRES, J.A.; BRANDÃO, C.F.L. et al. Early detection of goats infected with lentivirus small ruminant virus by ELISA assay. Rev. Ciênc. Méd. Biol. v.11, p.35-40, 2012.); but they worked with goats and thus there was low detection in the face of the infection, which may be justified by the possible small number of copies of the viral genome present in the milk cells since the viral load varies (Barquero et al., 2013BARQUERO, N.; GOMEZ-LUCIA, E.; ARJONA, A. et al. Evolution of specific antibodies and proviral DNA in milk of small ruminants infected by small ruminant lentivirus. Viruses, v.5, p.2614-2623, 2013. ).
In this experiment, proviral DNA detection by nPCR was 16% and this may be due to the low viral load. This fact is associated to the variability in the milk cellular composition, mainly due to monocytes that shelter proviral DNA, that may be caused by several factors, influencing the diagnostic capacity of nPCR for CLV in milk (Ravazzolo et al., 2006RAVAZZOLO, A.P.; NENCI, C.; VOGT, H.R. et al. Viral load, organ distribution, histopathological lesions and cytokine mRNA expression in goats infected with a molecular clone of the caprine arthritis encephalitis virus. Virology , v.350, p.116-127, 2006. ; Gomes et al., 2010GOMES, V.; AMATO, A.L.; PONTE, G.C.T.G. et al. Contagem automática e microscópica direta das células somáticas do leite de ovelhas da raça lacaune, utilizando como corantes o Rosenfeld e verde de metil e Pironina-Y. Ciênc. Anim. Bras, v.11, p.162-167, 2010.; Barbosa et al., 2012BARBOSA, D.A.; BLAGITZ, M.G.; BATISTA, C.F. et al. Contagem automática e microscópica direta de células somáticas do leite de ovelhas Santa Inês, utilizando como corantes o Broadhurst-palley e a hematoxilina-eosina. Ciênc. Anim., v.22, p.17-23, 2012. ; Blacklaws, 2012BLACKLAWS, B.A. Small ruminant lentiviruses: Immunopathogenesis of visna-maedi and caprine arthritis and encephalitis virus. Comp. Immunol. Microbiol. Infect. Dis., v.35, p.259-269, 2012.).
The detection of CLV proviral DNA in ewe milk proved the presence of infected cells, but not necessarily the presence of the viral particle in the milk, suggesting the mammary gland as a potential route for eliminating CLV (Ravazzolo et al., 2006RAVAZZOLO, A.P.; NENCI, C.; VOGT, H.R. et al. Viral load, organ distribution, histopathological lesions and cytokine mRNA expression in goats infected with a molecular clone of the caprine arthritis encephalitis virus. Virology , v.350, p.116-127, 2006. ; Gregory et al., 2009GREGORY, L.; LARA, M.C.C.S.H.; VILLALOBOS, E.M.C. et al. Detecção do vírus da atrite encefalite caprina em amostras de leite de cabras pela reação em cadeia da polimerase (PCR) e Nested-PCR. ARS Vet, v.25, p.142-146, 2009. ).
It has already been proved that cells carrying proviral DNA are able to transmit the virus (Herrmann-Hoesing et al., 2007HERRMANN-HOESING, L.M.; PALMER, G.H.; KNOWLES, D.P. Evidence of proviral clearance following postpartum transmission of an ovine lentivirus. Virology, v.362, p.226-234, 2007.). Thus, the milk of ewes infected with CLV proviral DNA must be considered among the risk factors for controlling the disease.
It is noteworthy that there was no clinical manifestation of the disease in the ewes, the udders remained healthy throughout the experimental period, discarding the possibility of indurative mastitis, which is in agreement with the low viral replication in the mammary gland, which reduced the occurrence of injuries (Gregory et al., 2009GREGORY, L.; LARA, M.C.C.S.H.; VILLALOBOS, E.M.C. et al. Detecção do vírus da atrite encefalite caprina em amostras de leite de cabras pela reação em cadeia da polimerase (PCR) e Nested-PCR. ARS Vet, v.25, p.142-146, 2009. ). Clinically healthy animals may have a lower viral load (Ravazzolo et al., 2006RAVAZZOLO, A.P.; NENCI, C.; VOGT, H.R. et al. Viral load, organ distribution, histopathological lesions and cytokine mRNA expression in goats infected with a molecular clone of the caprine arthritis encephalitis virus. Virology , v.350, p.116-127, 2006. ), and this possibly justifies the discreet detection of positivity in nPCR.
The presence of caprine lentivirus proviral DNA was also observed in a sample of sheep semen, from the 32 evaluated sheep (Tab. 2).
The presence of CLV proviral DNA has already been detected in naturally and experimentally infected male goats. Paula et al. (2009PAULA, N.R.O.; ANDRIOLI, A.; CARDOSO, J.F.S. et al. Profile of the Caprine arthritis-encephalitis virus (CAEV) in blood, semen from bucks naturally and experimentally infected in the semi-arid region of Brazil. Small Ruminant Res , v.85, p.27-33, 2009.) verified intermittence in positivity to nPCR when conducting studies with goats infected with the same strain as used in the presentexperiment.
The physical examination of the ewes and rams did not indicate any clinical alteration of infection by lentivirus. This absence of symptoms has been reported in other studies (Cavalcante et al., 2013CAVALCANTE, F.R.A., ANDRIOLI, A.; PINHEIRO, R.R. et al. Detecção do vírus da Artrite Encefalite Caprina por nested PCR e nested RT-PCR em ovócitos e fluido uterino. Arq. Inst. Biol., v.80, p.381-386, 2013.; Souza et al., 2015SOUZA, T.S.; PINHEIRO, R.R.; COSTA, J.N.; et al. Interspecific transmission of small ruminant lentiviruses from goats to sheep. Braz. J. Microbiol., v.46, p.867-874, 2015.), and may be associated with factors such as the pathogenicity of the viral strain for sheep, since it was circulating only in goats and, demonstrating symptoms in them. Possibly it did not establish a natural mechanism of adaptation to the ovine species, corroborating with the absence of clinical manifestation (Gregory et al., 2011GREGORY, L.; LARA, M.C.C.S.H.; HASEGAWA, M.Y. et al. Detecção do vírus da artrite encefalite caprina em pulmão, glândula mamária, cérebro e líquido sinovial de cabras naturalmente infectadas pela técnica de nested-PCR. Med. Vet, v.5, p.7-11, 2011.; Rachid et al., 2013RACHID, A.; CROISÉ, B.; RUSSO, P. et al. Diverse host-virus interactions following caprine arthritis-encephalitis virus infection in sheep and goats. J. Gen. Virol, v.94, p.634-642, 2013.).
It is important to highlight that some viral quasispecies are proven to be more adapted to goats and others to sheep, and that the species interaction is different, which must be considered when interpreting results (Shah et al., 2004SHAH, C.A.; BÖNI, J.; HUDER, J.B. et al. Phylogenetic analysis and reclassification of caprine and ovine lentiviruses based on 104 new isolates: evidence for regular sheep-to-goat transmission and world-wide propagation through livestock trade. Virology , v.319, p.12-26, 2004.; Souza et al., 2015SOUZA, T.S.; PINHEIRO, R.R.; COSTA, J.N.; et al. Interspecific transmission of small ruminant lentiviruses from goats to sheep. Braz. J. Microbiol., v.46, p.867-874, 2015.; Rachid et al., 2013RACHID, A.; CROISÉ, B.; RUSSO, P. et al. Diverse host-virus interactions following caprine arthritis-encephalitis virus infection in sheep and goats. J. Gen. Virol, v.94, p.634-642, 2013.).
It is also important to highlight the possibility of the virus entering a state of quiescence, when its genetic material remains integrated to the nuclear DNA, but without being activated, or in an intracellular environment, but not integrated (Cavalcante et al., 2013CAVALCANTE, F.R.A., ANDRIOLI, A.; PINHEIRO, R.R. et al. Detecção do vírus da Artrite Encefalite Caprina por nested PCR e nested RT-PCR em ovócitos e fluido uterino. Arq. Inst. Biol., v.80, p.381-386, 2013.), justifying the absence of replication that would stimulate the clinical manifestation.
Regarding the discreet detection of positivity in nPCR, this may be due to the low viral replication in those infection sites, and therefore the capacity to detect genetic material in the tissues (Barquero et al., 2013BARQUERO, N.; GOMEZ-LUCIA, E.; ARJONA, A. et al. Evolution of specific antibodies and proviral DNA in milk of small ruminants infected by small ruminant lentivirus. Viruses, v.5, p.2614-2623, 2013. ), although breast is a preferred site for lentivirus replication in small ruminants (Ravazzolo et al., 2006RAVAZZOLO, A.P.; NENCI, C.; VOGT, H.R. et al. Viral load, organ distribution, histopathological lesions and cytokine mRNA expression in goats infected with a molecular clone of the caprine arthritis encephalitis virus. Virology , v.350, p.116-127, 2006. ; Gregory et al., 2011GREGORY, L.; LARA, M.C.C.S.H.; HASEGAWA, M.Y. et al. Detecção do vírus da artrite encefalite caprina em pulmão, glândula mamária, cérebro e líquido sinovial de cabras naturalmente infectadas pela técnica de nested-PCR. Med. Vet, v.5, p.7-11, 2011.).
It is also important to consider that regarding the detection of genetic material, small ruminant lentivirus have the characteristic named intermittence, alternating between positivity and negativity, depending on the presence of infected cells in the evaluated sample (Alvarez et al., 2005ÁLVAREZ, V.; ARRANZ, J.; DALTABUIT-TEST, M. et al. Relative contribution of colostrum from Maedi-Visna virus (MVV) infected ewes to MVV-seroprevalence in lambs. Res. Vet. Sci., v.78, p.237-243, 2005.; Paula et al., 2009PAULA, N.R.O.; ANDRIOLI, A.; CARDOSO, J.F.S. et al. Profile of the Caprine arthritis-encephalitis virus (CAEV) in blood, semen from bucks naturally and experimentally infected in the semi-arid region of Brazil. Small Ruminant Res , v.85, p.27-33, 2009.).
Regarding the genetic sequences obtained by sequencing samples positive in nPCR (Figure 1), it was verified that they are sequences of the caprine lentivirus gag gene, with some changes occurring in nucleotides in the studied fragment (Feitosa et al., 2010FEITOSA, A.L.V.L.; TEIXEIRA, M.F.S.; PINHEIRO, R.R. et al. Phylogenetic analysis of small ruminant lentiviruses from northern Brazil. Small Ruminant Res, v.94, p.205-209, 2010.; Souza et al., 2015SOUZA, T.S.; PINHEIRO, R.R.; COSTA, J.N.; et al. Interspecific transmission of small ruminant lentiviruses from goats to sheep. Braz. J. Microbiol., v.46, p.867-874, 2015.).
Partial sequences of the small ruminant lentivirus gag gene: results obtained from nested PCR in milk from ewes and semen from rams infected with caprine lentivirus.
Regarding the homology of the sequences, it was verified that sequences of milk from ewes 04 and 07 had 98% and 97% homology with CLV Cork, and 90% and 88% with strain MVV K1514, respectively. The sequence obtained from semen demonstrated 100% homology with CLV Cork and 90% with MVV K1514. This may be explained by the selection imposed by the milk and semen compartmentalization over the caprine lentivirus (CLV) population. The compartmentalization of small ruminant lentivirus (SRLV) quasispecies is determined as the genetic distinction of SRLV isolates in tissues from different animals, corresponding to viral sub-populations in the same individual (Ramirez et al., 2012RAMÍREZ, H.; REINA, R.; BERTOLOTTI, L. et al. Study of compartmentalization in the visna clinical form of small ruminant lentivirus infection in sheep. BMC Vet. Res v.8, p.1-12, 2012.).
Comparison of fragments obtained provides important information regarding the modified nucleic sites, and these alterations may be associated with mutations that would lead to the adaptation of the viral strain to the host. However, this experiment was not enough to attest a mutation linked to adaptation. It is emphasized that those modifications may influence the process of viral replication (Barquero et al., 2013BARQUERO, N.; GOMEZ-LUCIA, E.; ARJONA, A. et al. Evolution of specific antibodies and proviral DNA in milk of small ruminants infected by small ruminant lentivirus. Viruses, v.5, p.2614-2623, 2013. ).
Thus, there are several verified characteristics influencing lentivirus transmission, since the dissemination of the disease is not species-specific, and the viral quasispecies are closely linked to the evasion mechanisms of the immune response, resulting from viral RNA transcription errors by reverse transcriptase, which may be correlated with the nucleic variations observed in the present study (Pasick et al., 1998PASICK, J. Maedi-visna virus and caprine arthritis-encephalitis virus: distinct species or quasispecies and its implications for laboratory diagnosis. Can. J. Vet. Res., v.62, p.241-244, 1998.; Reina et al., 2006REINA, R.; MORA, M.I.; GLARIA, I. et al. Molecular characterization and phylogenetic study of Maedi visna and caprine arthritis encephalitis viral sequences in sheep and goats from Spain. Virus Res., v.121, p.189-198, 2006. ).
CONCLUSIONS
Caprine lentivirus proviral DNA was detected in ewemilk and in ram-semen, in experimentally infected animals, thus suggesting the technical feasibility of infection passing from goats to sheep, despite the absence of clinical signs. The small changes observed on the genetic sequences obtained suggest that the viral strain may adapt to the new species because of the possibility of errors in gene transcription. The possibility of infection re-transmission highlights the attention that should be given to control measures of the disease in small ruminants.
ACKNOWLEDGEMENTS
The authors thank the Foundation for Research Support of the State of Bahia (Fabesp) for fostering the project, the National Council of Scientific and Technological Development (CNPq) for funding the research. They also thank the Coordination of Higher Education Personnel Improvement (Capes) for the scholarship and Embrapa - National Research Center for Goats and Sheep for supporting the execution of the experiment.
REFERENCES
- ÁLVAREZ, V.; ARRANZ, J.; DALTABUIT-TEST, M. et al. Relative contribution of colostrum from Maedi-Visna virus (MVV) infected ewes to MVV-seroprevalence in lambs. Res. Vet. Sci, v.78, p.237-243, 2005.
- ÁLVAREZ, V.; DALTABUIT-TEST, M.; ARRANZ, J. et al. PCR detection of colostrum-associated Maedi-Visna virus (MVV) infection and relationship with ELISA-antibody status in lambs. Res. Vet. Sci , v.80, p.226-234, 2006.
- ANDRIOLI, A.; GOUVEIA, A.M.G.; MARTINS, A.S. et al. Fatores de risco na transmissão do lentivírus caprino pelo sêmen. Pesqui. Agropecu. Bras, v.41, p.1313-1319, 2006.
- BARBOSA, D.A.; BLAGITZ, M.G.; BATISTA, C.F. et al. Contagem automática e microscópica direta de células somáticas do leite de ovelhas Santa Inês, utilizando como corantes o Broadhurst-palley e a hematoxilina-eosina. Ciênc. Anim, v.22, p.17-23, 2012.
- BARLOUGH, J.; EAST, N.; ROWE, J.D. et al. Double-nested polymerase chain reaction for detection of caprine arthritis-encephalitis virus proviral DNA in blood, milk, and tissues of infected goats. J. Virol. Methods, v.50, p.101-113, 1994.
- BARQUERO, N.; GOMEZ-LUCIA, E.; ARJONA, A. et al. Evolution of specific antibodies and proviral DNA in milk of small ruminants infected by small ruminant lentivirus. Viruses, v.5, p.2614-2623, 2013.
- BLACKLAWS, B.A. Small ruminant lentiviruses: Immunopathogenesis of visna-maedi and caprine arthritis and encephalitis virus. Comp. Immunol. Microbiol. Infect. Dis., v.35, p.259-269, 2012.
- CAVALCANTE, F.R.A., ANDRIOLI, A.; PINHEIRO, R.R. et al. Detecção do vírus da Artrite Encefalite Caprina por nested PCR e nested RT-PCR em ovócitos e fluido uterino. Arq. Inst. Biol., v.80, p.381-386, 2013.
- FEITOSA, A.L.V.L.; TEIXEIRA, M.F.S.; PINHEIRO, R.R. et al. Phylogenetic analysis of small ruminant lentiviruses from northern Brazil. Small Ruminant Res, v.94, p.205-209, 2010.
- GOMES, V.; AMATO, A.L.; PONTE, G.C.T.G. et al. Contagem automática e microscópica direta das células somáticas do leite de ovelhas da raça lacaune, utilizando como corantes o Rosenfeld e verde de metil e Pironina-Y. Ciênc. Anim Bras, v.11, p.162-167, 2010.
- GREGORY, L.; LARA, M.C.C.S.H.; HASEGAWA, M.Y. et al. Detecção do vírus da artrite encefalite caprina em pulmão, glândula mamária, cérebro e líquido sinovial de cabras naturalmente infectadas pela técnica de nested-PCR. Med. Vet, v.5, p.7-11, 2011.
- GREGORY, L.; LARA, M.C.C.S.H.; VILLALOBOS, E.M.C. et al. Detecção do vírus da atrite encefalite caprina em amostras de leite de cabras pela reação em cadeia da polimerase (PCR) e Nested-PCR. ARS Vet, v.25, p.142-146, 2009.
- GRIMBERG, J.; NOWOSCHIK, S.; BELLUSCIO, L. et al. A simple and efficient non-organic procedure for the isolation of genomic DNA from blood. Nucleic Acids Res, v.17, p.83-90, 1989.
- HALL, T.A. Bioedit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Ser., v.41, p.95-98, 1999.
- HERRMANN-HOESING, L.M.; PALMER, G.H.; KNOWLES, D.P. Evidence of proviral clearance following postpartum transmission of an ovine lentivirus. Virology, v.362, p.226-234, 2007.
- PASICK, J. Maedi-visna virus and caprine arthritis-encephalitis virus: distinct species or quasispecies and its implications for laboratory diagnosis. Can. J. Vet. Res, v.62, p.241-244, 1998.
- PAULA, N.R.O.; ANDRIOLI, A.; CARDOSO, J.F.S. et al. Profile of the Caprine arthritis-encephalitis virus (CAEV) in blood, semen from bucks naturally and experimentally infected in the semi-arid region of Brazil. Small Ruminant Res , v.85, p.27-33, 2009.
- RACHID, A.; CROISÉ, B.; RUSSO, P. et al. Diverse host-virus interactions following caprine arthritis-encephalitis virus infection in sheep and goats. J. Gen. Virol, v.94, p.634-642, 2013.
- RAMÍREZ, H.; REINA, R.; BERTOLOTTI, L. et al. Study of compartmentalization in the visna clinical form of small ruminant lentivirus infection in sheep. BMC Vet. Res v.8, p.1-12, 2012.
- RAVAZZOLO, A.P.; NENCI, C.; VOGT, H.R. et al. Viral load, organ distribution, histopathological lesions and cytokine mRNA expression in goats infected with a molecular clone of the caprine arthritis encephalitis virus. Virology , v.350, p.116-127, 2006.
- REINA, R.; MORA, M.I.; GLARIA, I. et al. Molecular characterization and phylogenetic study of Maedi visna and caprine arthritis encephalitis viral sequences in sheep and goats from Spain. Virus Res., v.121, p.189-198, 2006.
- RIMSTAD, E.; EAST, N.E.; TORTEN, M. et al. Delayed seroconversion following naturally acquired caprine arthritis-encephalitis virus infection in goats. Am. J. Vet. Res. v.54, p.1858-1862, 1993.
- SALTARELLI, M.; QUERAT, G.; KONINGS, D.A.M. et al. Nucleotide sequence and transcriptional analysis of molecular clones of CAEV which generate infectious virus. Virology , v.179, p.347-364, 1990.
- SANTURDE, G.; SILVA, N.; VILLARES, R. et al. Rapid and high sensitivity test for direct detection of bovine herpesvirus-1 genome in clinical samples. Vet. Microbiol. v.49, p.81-92, 1996.
- SARDI, S.I.; TORRES, J.A.; BRANDÃO, C.F.L. et al. Early detection of goats infected with lentivirus small ruminant virus by ELISA assay. Rev. Ciênc. Méd. Biol. v.11, p.35-40, 2012.
- SHAH, C.A.; BÖNI, J.; HUDER, J.B. et al. Phylogenetic analysis and reclassification of caprine and ovine lentiviruses based on 104 new isolates: evidence for regular sheep-to-goat transmission and world-wide propagation through livestock trade. Virology , v.319, p.12-26, 2004.
- SOUZA, K.C.; PINHEIRO, R.R.; SANTOS, D.O. et al. Transmission of the caprine arthritis-encephalitis virus through artificial insemination. Small Ruminant Res , v.109, p.193-198, 2013.
- SOUZA, T.S.; PINHEIRO, R.R.; COSTA, J.N.; et al. Interspecific transmission of small ruminant lentiviruses from goats to sheep. Braz. J. Microbiol., v.46, p.867-874, 2015.
- THOMPSON, J.D.; HIGGINS, D.G.; GIBSON, T.J. Clustal W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position specific gap penalties and weight matrix choice. Nucleic Acids Res v.22, p.4673-4680, 1994.
- VILLORIA, M.; LEGINAGOIKOA, I.; LUJÁN, L. et al. Detection of small ruminant lentivirus in environmental samples of air and water. Small Ruminant Res v.110, p.155-160, 2013.
Publication Dates
-
Publication in this collection
Mar-Apr 2017
History
-
Received
12 May 2016 -
Accepted
28 Sept 2016


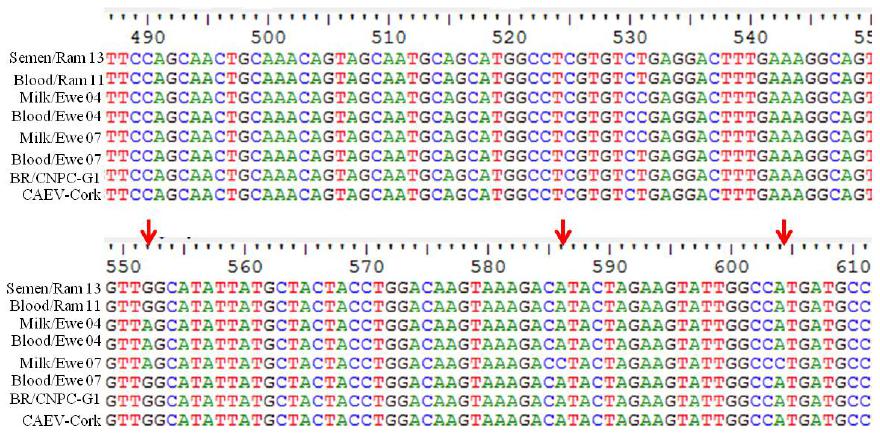 *Red arrow indicates change of nucleotid in relation to the genomic sequence of the standard CAEV Corkstrain.
*Red arrow indicates change of nucleotid in relation to the genomic sequence of the standard CAEV Corkstrain.