ABSTRACT
Pemphigus foliaceus (PF) is the most common autoimmune skin disease in dogs. It is characterized by pustules, erosions, and crusts which occur due to the presence of autoantibodies that target intercellular adhesion. Histopathological examination is considered the gold standard pattern in the diagnosis, but may sometimes be inconclusive, especially when the characteristic findings are not identified. New diagnostic tests are continuously being developed and immunofluorescence assays, could be a valuable alternative diagnostic tool. This study aimed to evaluate the applicability of direct and indirect immunofluorescence (DIF and IIF) tests for the diagnosis of canine PF. Twenty eight dogs were divided into two groups: Group I with 14 dogs with PF and Group II (control) with 14 dogs with Superficial pyoderma (differential diagnoses of PF). All animals were submitted to skin biopsy to histopathological and DIF. Blood samples were collected to assess IIF. Comparing the DIF results against the histopathology test, there was an agreement of 75% (9/12) with a Kappa index of 0.77 (P<0.001). Considering IIF, the agreement was 100% (14/14), with a Kappa index of 1.0 (P<0.001). We conclude that DIF and IIF are highly effective and were useful and effective complementary examination tests for an improvement in the diagnosis of canine PF.
Keyword:
immunofluorescence; DIF; IIF; pemphigus foliaceus
RESUMO
O pênfigo foliáceo (PF) é considerado uma das doenças tegumentares autoimunes mais frequentes em cães. Clinicamente, caracteriza-se pela presença de pústulas, erosões e crostas. O exame histopatológico é considerado o teste diagnóstico de eleição, porém pode se mostrar inconclusivo, sobretudo quando os achados característicos da doença não são observados. Novas ferramentas diagnósticas têm sido desenvolvidas e os testes de imunofluorecência são uma valiosa alternativa. Este estudo teve como objetivo avaliar a aplicabilidade das reações de imunofluorescência direta (IFD) e indireta (IFI) para o diagnóstico do PF canino. Vinte e oito cães foram divididos em dois grupos: grupo I com 14 cães com PF e grupo II (controle) com 14 cães com piodermite superficial (um dos principais diagnósticos diferenciais do PF). Todos os animais foram submetidos à biópsia cutânea, seguida de exame histopatológico e IFD. Amostras de sangue foram coletadas para realização da IFI. Comparando-se os valores de IFD com o histopatológico, obtiveram-se valores de concordância de 75% (9/12), com índice Kappa de 0,77 (P<0,001). Já na IFI, a concordância foi de 100% (14/14), com índice Kappa de 1,0 (P<0,001). Concluiu-se, então, que a IFD e a IFI apresentaram excelentes resultados e podem ser consideradas novas alternativas diagnósticas do PF canino.
Palavras-chave:
imunofluorescência; IFD; IFI; pênfigo foliáceo
INTRODUCTION
Pemphigus foliaceus (PF) is an autoimmune skin disease which affects humans and animals and is characterized by the presence of immunoglobulin G (IgG) autoantibodies against epithelial targets located within the desmosomal core (Amagai, 2003AMAGAI, M. Desmoglein as a target in autoimmunity and infection. J. Am. Acad. Dermatol., v.48, p.244-252, 2003.). Several years ago, desmoglein-1 (DSG1), the major human PF (hPF) autoantigen, was identified as only a minor autoantigen in dogs with PF (Olivry et al., 2006OLIVRY, T.; LAVOY, A.; DUNSTON, S.M. et al. Desmoglein-1 is a minor autoantigen in dogs with pemphigus foliaceus. Vet. Immunol. Immunopathol.,v.110, p.245-55, 2006.), while desmocollin-1 represents a major canine PF autoantigen. The binding of these autoantibodies to desmocollin-1 results in the loss of cell-cell adhesion, which is referred to as acantholysis (Bizikova et. al., 2012BIZIKOVA, P.; DEAN, G.A.; HASHIMOTO, T. et al. Cloning and establishment of canine desmocollin-1 as a major autoantigen in canine pemphigus foliaceus. Vet. Immunol. Immunopathol., v.149, p.197-207, 2012.).
In dogs, PF represents the most common autoimmune skin disease, and is characterized by superficial pustules that predominantly affect the face, nasal planum, periocular skin and ears. The pattern is usually strikingly bilateral and symmetrical. When more generalized, lesions on the trunk accompany the classic involvement of the face and/or footpads. A remarkable finding of canine PF is the predilection for these sites (Ackerman, 1985ACKERMAN, L.J. Pemphigus and pemphigoid in domestic animals: an overview. Can. Vet. J., v.26, p.185-189.1985.; Olivry, 2006OLIVRY, T.; LAVOY, A.; DUNSTON, S.M. et al. Desmoglein-1 is a minor autoantigen in dogs with pemphigus foliaceus. Vet. Immunol. Immunopathol.,v.110, p.245-55, 2006.).
A diagnosis of canine PF is established based on the association of clinical signs, cytology and histopathology findings. However, the histopathological diagnosis may sometimes be inconclusive, and the diagnosis is no longer established (Kuhl et al., 1994KUHL, K.A.; SHOFER, F.S.; GOLDSCHMIDT, M.H. Comparative histopathology of pemphigus foliaceus and superficial folliculitis in the dog. Vet. Pathol., v.31, p.19-27, 1994.; Garrod and Chidgey, 2008GARROD, D.; CHIDGEY, M. Desmosome structure, composition and function. Biochim. Biophys. Acta.,v.1778, p.572-587, 2008.). In human medicine, the immunofluorescence techniques have emerged as useful methods for diagnosing autoimmune blistering conditions since the early 1960s and present excellent results (Beutner and Gordon, 1964BEUTNER, E.H.; JORDON, R.E. Demonstration of skin antibodies in sera of pemphigus vulgaris patients by indirect immunofluorescent staining. Proc. Soc. Exp. Biol. Med., v.117, p.505-510, 1964.; Brandsen et al., 1997BRANDSEN, R.; FRUSIC-ZLOTKIN, M.; LYUBIMOV, H. et al. Circulating pemphigus IgG in families of patients with pemphigus: comparison of indirect immunofluorescence, direct immunofluorescence, and immunoblotting. J. Am. Acad. Dermatol., v.36, p.44-52, 1997.). Currently, immunofluorescence tests are a useful tool for the laboratory diagnosis of autoimmune dermatosis in humans, since their clinical and histopathological findings may be inconclusive (Mutasim and Adams, 2001MUTASIM, D.F.; ADAMS, B.B. Immunofluorescence in dermatology. J. Am. Acad. Dermatol., v.45, p.803-822. 2001.; Aoki et al., 2004AOKI, V.; HUANG, M.H.; PERIGO, A.M. et al. Endemic pemphigus foliaceus (fogoselvagem) and pemphigus vulgaris: immunoglobulin G heterogeneity detected by indirect immunofluorescence. Rev. Hosp. Clin. Fac. Med. Sao Paulo., v.59, p.251-256, 2004.; Aoki et al., 2010).
There are two main subtypes of immunofluorescence tests: direct immunofluorescence (DIF), which is performed on perilesional skin or mucous membranes and detects tissue-bound autoantibodies; and indirect immunofluorescence (IIF), which quantifies a patient's autoantibodies circulating in the blood, utilizing canine footpad as substrates (Mutasim and Adams, 2001MUTASIM, D.F.; ADAMS, B.B. Immunofluorescence in dermatology. J. Am. Acad. Dermatol., v.45, p.803-822. 2001.; Aoki et al., 2010AOKI, V.; FUKUMORI, L.M.I.; FREITAS, E.L. et al. Imunofluorescência direta e indireta. An. Bras. Dermatol., v.85, p.490-500, 2010.).
Although these tests are widely used in human medicine, they are not yet a routine diagnostic examination in veterinary medicine and there is still a clear lack of studies on immunofluorescence reactions involving the autoimmune skin diseases. Immunofluorescence assays are capable of detecting antigen-antibody complexes which may be involved in the pathogenesis of auto-immune skin diseases in situ or in the circulation (Aoki et. al., 2010AOKI, V.; FUKUMORI, L.M.I.; FREITAS, E.L. et al. Imunofluorescência direta e indireta. An. Bras. Dermatol., v.85, p.490-500, 2010.). In São Paulo and in the rest of the country, unfortunately, immunological tests destined to diagnose autoimmune diseases in animal patients are rarely requested and performed, due to the scarce number of laboratories available that are prepared to execute them.
This study aimed to evaluate the applicability of direct immunofluorescence (DIF) and indirect immunofluorescence (IIF) tests for the diagnosis of canine pemphigus foliaceus.
MATERIALS AND METHODS
This study was performed according the Ethical Principles in Animal Research. Each owner signed a form that described the procedures to be performed and provided consent for the dog to be included in the study. The experimental protocol number 2930/2013 was approved by the Bioethics Committee of the School of Veterinary Medicine and Animal Science of University of São Paulo and involved 28 dogs with pustular skin diseases. Clinical and laboratorial examinations were performed on each dog. To assess the IIF, blood samples were collected and performed using the canine footpad as a substrate. A complete blood count (CBC) and serum biochemical profile (measurement of urea, creatinine, total protein, albumin, alanine transaminase and alkaline phosphatase activities) were also performed. Fragments of skin were extracted and submitted to histopathological examination and DIF.
The animals were allocated to one of the two groups described previously. Group I consisted of 14 dogs with clinical and histopathological findings consistent with the diagnosis of PF, while Group II consisted of 14 dogs with generalized pustules and crusted lesions with histopathological examination confirming them to be superficial pyoderma (SP). To compose the latter group, we selected animals with pyoderma because the lesions were very similar to those seen in dogs with PF and it is one of the main PF differential diagnoses (Kuhl et al., 1994KUHL, K.A.; SHOFER, F.S.; GOLDSCHMIDT, M.H. Comparative histopathology of pemphigus foliaceus and superficial folliculitis in the dog. Vet. Pathol., v.31, p.19-27, 1994.; Garrod and Chidgey, 2008GARROD, D.; CHIDGEY, M. Desmosome structure, composition and function. Biochim. Biophys. Acta.,v.1778, p.572-587, 2008.).
Disease severity was assessed every 15 or 30 days by a dermatologist at each serum collection time-point with a previously described scoring system: pemphigus foliaceus extent severity index-PEFESI. This scoring system consisted of the evaluation of the severity (0 none, 1 mild, 2 moderate and 3 severe) of the three cardinal lesions of PF (pustules, erosions and crusts) at 50 different areas covering the entire body surface, with the maximum PEFESI score theoretically achievable of 450 (3 x 3 x 50) (Olivry et al., 2003OLIVRY, T.; RIVIERRE, C.; MURPHY, K.M. Efficacy of cyclosporine for treatment induction of canine pemphigus foliaceus. Vet. Rec., v.152, p.53-54, 2003. )
Dogs with PF underwent therapy with prednisone (2-3mg/kg, PO once a day) and/or azathioprine (1.5-2.0mg/kg, PO once a day) for 30 days, at which point the medication dosages were reduced. In all Group I dogs, blood samples were taken during days 0 (pretreatment), 15, 30, 60 and 90 after institution of therapy. The clinical involvement degree was evaluated by PEFESI, over a period of 90 days. During treatment, animals were evaluated by physical exam to check their clinical improvement. A single blood sample was collected from Group II dogs. Hemolyzed and lipemic samples were discarded and new blood samples were collected under appropriate conditions. For all blood samples, serum was harvested and frozen at -20°C until assayed for IIF.
Biopsies for histopathology and direct immunofluorescence were obtained from dogs with PF and SP. In all dogs, at least two skin fragments were obtained, one from skin lesions and the other from the perilesional region, regardless of their location. One of the skin biopsies was fixed in 10% formalin, embedded in paraffin and submitted for routine histological technique using hematoxylin-eosin. The other biopsy sample was moistened with 0.9% saline solution, taken for immediate cryopreservation in tissue freezing medium (Leica manufacturer) and stored at -20ºC for DIF.
The skin fragment was cut on a cryostat at -20ºC. After cryosection, four 4µm thick cuts were placed on albuminized slides. The slides were placed in a humidity chamber at room temperature, and the conjugates (anti-dog immunoglobulins produced in immunized animals and labeled with fluorescein isothiocyanate) were placed on the cuts. The conjugates were diluted in TBS pH 7.5 (tris-buffered saline - calcium acetate buffer) containing Evans blue dye (Interlabmanufacturer). Anti-dog IgA, (1:40 dilution, Bethyl Laboratories), anti-dog IgM (1:40 dilution, Bethyl Laboratories), anti-dog IgG (1:20 dilution, SIGMA) and anti-dog C3 (1:40 dilution, Bethyl Laboratories) were used. A conjugate was used for each slide. After 30min of incubation, the slides were washed in TBS pH 7.5 for three periods of 10min each. Buffered glycerin (pH 9/0.5M) and a glass coverslip were used to mount the slides. The slides were read with an epiluminescence microscope (model HBO 50, Zeiss, Oberkochen, Germany).
For this method, the footpad was chosen as a substrate. Punch biopsy samples from the footpad were collected post-mortem from dogs that were euthanized for diseases other than immune-mediated ones. Following sampling, specimens were embedded in cryopreservation tissue freezing medium (Leica manufacturer) and stored at -20ºC until cryomicrotomy. Four µm sections were cut on a cryostat and stored at -20°C until use. Four µm cryostat sections of the footpad were incubated for 30min with sera dilutions starting at 1:10. The slides were washed in Tris-buffered saline (TBS) once (20min) and then covered with fluorescein isothiocyanate-conjugated (FITC) goat anti-dog IgG at a dilution of 1:25 (Fluorescein-Labeled Antidog to Dog IgG (H+L), Kirkegaard & Perry Laboratories, Inc. USA) for 30min. After three additional 10min washes (TBS), the slides were mounted in buffered glycerol and examined under an epiluminescent microscope (model HBO 50, Zeiss, Oberkochen, Germany).
The software used for statistical analysis was the SPSS version 16.0 for Windows (Chicago, IL., USA). The IIF titers and PEFESI were analyzed with Spearman’s correlation test. Fisher's exact test was performed to compare the sensitivity and specificity of DIF and IIF compared to histopathology. The Kappa index (k) was used to express the degree of agreement of DIF and IIF results compared with histopathology taking into consideration values between "negative 1" (complete disagreement) and "positive 1" (total agreement). For the results obtained, we considered a p-value of < 5% to be statistically significant.
RESULTS
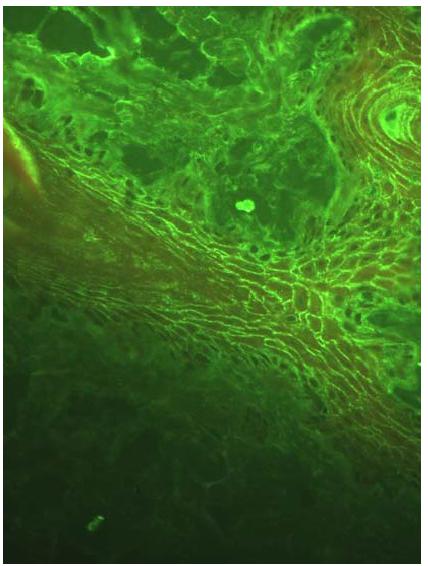
All dogs from Group II (control/SP) were negative on DIF to all antibodies tested (IgA, IgG, IgM and C3). Unfortunately, it was not possible to apply the DIF technique in two of the PF dogs because appropriate skin biopsies were not collected. In Group I, positive DIF was established in nine (75%) of the twelve dogs tested. A positive reaction was characterized by homogeneous fluorescent green intercellular fluorescence with different intensities with IgG antibody (Figure 1). Only one animal showed C3 intercellular deposition fluorescence concomitant with IgG deposition. In three dogs with PF, it was possible to demonstrate the deposition of IgM immune complexes and C3 complement factor in the basal zone membrane (BZM), also associated with the intercellular fluorescence observed with IgG. All dogs were negative for IgA.
Moderate homogenous fluorescence intensity, noted in intraepithelial and intercellular regions, representing the presence of anti-IgG antibodies in a canine patient with PF
Indirect immunofluorescence was positive in all 14 animals (100%) with PF with titers ranging from 40 to 640. An intraepithelial intercellular fluorescence was considered a positive pattern (Figure 2). In animals of group II this intercellular pattern was not noted and therefore no false-positive results were seen.
Intense fluorescence shown in intraepithelial and intercellular regions, representing the IIF reaction in a canine patient with PF.
In this study, a total of 12 skin biopsies from dogs with PF confirmed by histopathology were tested by DIF in order to compare assay sensitivity and specificity. The overall sensitivity of DIF was 75%, with a specificity of 100%. Moreover, there was a high correlation between DIF and histopathology (Kappa index of 0.772, P<0.001). In addition, serum samples from Group I dogs were tested by IIF and the sensitivity and specificity were both 100% with a high correlation between IIF and histopathology (Kappa index of 1.0, P<0.001), (Table 1).
Five of the 14 dogs with PF had periods of clinical worsening. In two of those dogs, there was an increase in IIF titers associated with a higher PEFESI. In the other three dogs, IIF titers did not raise with increasing PEFESI. In four dogs from Group I, the IIF titers remained positive even with the clinical improvement evaluated and characterized by the PEFESI. IIF titers decreased in association with clinical improvement in the other ten dogs with PF.
There was a weak positive correlation between PEFESI and IIF titers (r=0.470 and P<0.0001) (Figure 3).
DISCUSSION
In human medicine, immunofluorescence assays are widely used for the diagnosis of auto-immune blistering diseases (Aoki et al., 2010AOKI, V.; FUKUMORI, L.M.I.; FREITAS, E.L. et al. Imunofluorescência direta e indireta. An. Bras. Dermatol., v.85, p.490-500, 2010.). It is a valuable and useful tool, especially when the histopathological examination (gold standard) is inconclusive. In some cases, the histopathological characteristic findings of PF cannot be found, and the diagnosis can be easily confused, which culminates in the wrong choice of therapy and consequently worsens the condition.
The deposition of immunoreagents in the intercellular space frequently occurs in the dermatopathies of the pemphigus group and it is very important to identify the class of deposited immunoglobulins, their preferential binding at different levels of the epidermis as well as any deposition at other sites. These aspects of the immunofluorescence can be evidenced in 100% of the human patients with the active disease, when appropriate collection of cutaneous fragment (Rani and Hussain, 2003RANI, Z.; HUSSAIN, I. Review article Immunofluorescence diseases in immunobullous. J. Pakis. Assoc. Dermatol., v.13, p.76-88, 2003. ; Aoki et al., 2010AOKI, V.; FUKUMORI, L.M.I.; FREITAS, E.L. et al. Imunofluorescência direta e indireta. An. Bras. Dermatol., v.85, p.490-500, 2010.).
Comparing the DIF results against the histopathology test (gold standard), there was an agreement of 75% (9/12) with a Kappa index of 0.77 (P<0.001) which means a degree of substantial arrangement between the two methods. A similar percentage was also observed by other authors who obtained positivity’s ranging from 66 to 80% (Olivry, 2006OLIVRY, T.; LAVOY, A.; DUNSTON, S.M. et al. Desmoglein-1 is a minor autoantigen in dogs with pemphigus foliaceus. Vet. Immunol. Immunopathol.,v.110, p.245-55, 2006.). This positivity can vary depending on the biopsied skin used for DIF. Some of the collected skin fragments were friable and difficult to cut in a cryostat and part of them were obtained from perilesional regions on the abdomen where the skin is usually thinner. This fact may lead to an increased difficulty in detecting immune-complexes in canine patients. Thus, proper collection of biopsy samples is very important for optimal DIF examination.
A deposition of IgM immune complexes and complement factor C3 in the basal membrane area was also observed in this study via DIF in three cases of dogs affected by PF. The deposition of immune complexes in the basement membrane zone (BMZ), apart from the deposition of IgG in the intercellular space, was observed in human patients with endemic PF (Reis et al., 1991REIS, V.M.S.; CUCÊ, L.C.; RIVITTI, E.A. Anatomopatologia e imunfluorescência direta e indireta das lesões de pênfigo foliáceo endêmico resistentes à corticoterapia. Rev. Inst. Med. Trop. São Paulo., v.33, p.97-103, 1991.).
In human patients, the discontinuous deposition of immune complexes in the BMZ is less specific and can be observed in distinct diseases, such as actinic keratoses, polymorphic rash, and porphyria, and in normal skin exposed to sunlight ((Mehta et al., 2010MEHTA, V.; SARDA, A.; BALACHANDRAN, C. Lupus band test. Indian J. Dermatol. Venereol. Leprol., v.76, p.298-300, 2010.). Linear or focal deposits of IgM and C3 can occur in the BMZ due to a wide variety of inflammatory diseases, and very often when harvested from fragments of the face or other places exposed to solar radiation (Dahl, 1983DAHL, M.V. Usefulness of direct immunofluorescence in patients with lupus erythematosus. Arch. Dermatol., v.119, p.1010-1017, 1983.). We believe that these animals with PF had immune complexes deposited on the BMZ secondary to sunlight exposure of abdominal skin.
Comparing the IIF results against the histopathology test (gold standard) there was an agreement of 100% (14/14) with a Kappa index of 1.0 (P<0.001), showing perfect arrangement between the IIF and the histopathology. There were no IIF false positive reactions in dogs with SP and the results vary according to the substrate used, with 65% positivity when employing the bovine esophagus (Iwasaki et al., 1996IWASAKI, T.; SHIMIZU, M.; OBATA, H. et al. Effect of substrate on indirect immunofluorescence test for canine pemphigus foliaceus. Vet. Pathol., v.33, p.332-336, 1996.) and up to 84% with the canine footpad (Lennon et al., 2006LENNON, E.M.; BRUIN, A.; MEULEMEESTER, J. et al. Immunological heterogeneity of canine pemphigus foliaceus: I-variability of indirect immunofluorescence patterns. Vet. Dermatol., v.17, p.216, 2006. (Abstract).).
As already observed in other studies, animals with PF presented titers ranging from 40 to 10,240 (Nishifuji et al., 2005); in our study, titers ranged from 40 to 640. In four out of the 14 dogs with PF the IIF titers remained positive even with the clinical improvement evaluated and characterized by PEFESI. The perpetuation of the IgG titers in dogs in full remission of PF suggests the involvement of the IgG subclass IgG1, which may be present in patients with controlled endemic pemphigus foliaceus or individuals at the pre-clinical stage (Aoki et al., 2015AOKI, V.; RIVITTI, E. A.; DIAZ, L.A. Update on fogoselvagem, an endemic form of pemphigus foliaceus. J. Dermatol., v.42, p.18-26, 2015.). We postulated that this may also occur in dogs with PF, but unfortunately, in the present study, the subclasses of IgG were not differentiated in order to prove this association in dogs.
Five of the 14 dogs with PF had periods of clinical worsening. In three dogs, IIF titers were not elevated with increasing PEFESI. Studies with human PF analyzing serial titers concluded that pemphigus antibody titers did not always correlate well with disease severity. Moreover, it is difficult to identify a relationship between antibody levels and disease severity in humans who take any systemic therapy because therapy improves disease earlier than antibody titers decrease. In this way, because antibody titers correspond to the later acquired immune response, they may not raise as fast as clinical signs during relapses.
In contrast, in this study, we also observed that IIF titers decreased dramatically after the introduction of immunosuppressive therapy in the majority of dogs with PF, as seen in other papers (Nishifuji et al., 2005; Aksu et al., 2010AKSU, D.; PEKSARI, Y., ARICA, I.E., GURGEY E. Assessing the autoantibody levels in relation to disease severity and therapy response in pemphigus patients. Indian J. Dermatol., v.55, p.342-347, 2010.). A parallel relationship between IIF titers and disease activity was seen in two of the 14 dogs with PF. Those two dogs had periods of clinical worsening, and an increase in IIF titers associated with a higher PEFESI was observed. A weak correlation was also observed in this study between IIF titers and disease activity. Thus, IIF can also be used as a way to monitor disease; however, studies with a greater number of dogs and longer periods of observation should be conducted to better understand the actual fluctuation in antibody titers of animals throughout therapy.
CONCLUSION
With the results obtained, we conclude that positivity to the DIF and IIF assays for canine pemphigus foliaceus was established by the presence of a homogeneous fluorescent green intercellular fluorescence with different intensities with IgG antibody. The substantial degree of agreement and reliability shown between DIF and IIF with histopathology indicate that both assays are useful and effective complementary examination tests for an improvement of the diagnosis of canine PF. A weak correlation was observed in this study between IIF titers and PF disease activity. Additional studies are necessary to determine whether or not IIF titers can be used to monitor the disease, help to identify relapses and guide the decision of immunosuppressive therapy for PF canine cases.
ACKNOWLEDGEMENTS
The authors are grateful to Alexandre M. Périgo and Lígia M.I. Fukumori for all the support with the immunofluorescence tests. We also thank Prof. Dr. Denise S. Schwartz for conducting the statistical work and Prof. Dr. Mitika K. Hagiwara for the support in structuring the work.
REFERENCES
- ACKERMAN, L.J. Pemphigus and pemphigoid in domestic animals: an overview. Can. Vet. J., v.26, p.185-189.1985.
- AKSU, D.; PEKSARI, Y., ARICA, I.E., GURGEY E. Assessing the autoantibody levels in relation to disease severity and therapy response in pemphigus patients. Indian J. Dermatol., v.55, p.342-347, 2010.
- AMAGAI, M. Desmoglein as a target in autoimmunity and infection. J. Am. Acad. Dermatol., v.48, p.244-252, 2003.
- AOKI, V.; FUKUMORI, L.M.I.; FREITAS, E.L. et al. Imunofluorescência direta e indireta. An. Bras. Dermatol., v.85, p.490-500, 2010.
- AOKI, V.; HUANG, M.H.; PERIGO, A.M. et al. Endemic pemphigus foliaceus (fogoselvagem) and pemphigus vulgaris: immunoglobulin G heterogeneity detected by indirect immunofluorescence. Rev. Hosp. Clin. Fac. Med. Sao Paulo., v.59, p.251-256, 2004.
- AOKI, V.; RIVITTI, E. A.; DIAZ, L.A. Update on fogoselvagem, an endemic form of pemphigus foliaceus. J. Dermatol., v.42, p.18-26, 2015.
- BEUTNER, E.H.; JORDON, R.E. Demonstration of skin antibodies in sera of pemphigus vulgaris patients by indirect immunofluorescent staining. Proc. Soc. Exp. Biol. Med., v.117, p.505-510, 1964.
- BIZIKOVA, P.; DEAN, G.A.; HASHIMOTO, T. et al. Cloning and establishment of canine desmocollin-1 as a major autoantigen in canine pemphigus foliaceus. Vet. Immunol. Immunopathol., v.149, p.197-207, 2012.
- BRANDSEN, R.; FRUSIC-ZLOTKIN, M.; LYUBIMOV, H. et al. Circulating pemphigus IgG in families of patients with pemphigus: comparison of indirect immunofluorescence, direct immunofluorescence, and immunoblotting. J. Am. Acad. Dermatol., v.36, p.44-52, 1997.
- DAHL, M.V. Usefulness of direct immunofluorescence in patients with lupus erythematosus. Arch. Dermatol., v.119, p.1010-1017, 1983.
- GARROD, D.; CHIDGEY, M. Desmosome structure, composition and function. Biochim. Biophys. Acta.,v.1778, p.572-587, 2008.
- IWASAKI, T.; SHIMIZU, M.; OBATA, H. et al. Effect of substrate on indirect immunofluorescence test for canine pemphigus foliaceus. Vet. Pathol., v.33, p.332-336, 1996.
- KUHL, K.A.; SHOFER, F.S.; GOLDSCHMIDT, M.H. Comparative histopathology of pemphigus foliaceus and superficial folliculitis in the dog. Vet. Pathol., v.31, p.19-27, 1994.
- LENNON, E.M.; BRUIN, A.; MEULEMEESTER, J. et al. Immunological heterogeneity of canine pemphigus foliaceus: I-variability of indirect immunofluorescence patterns. Vet. Dermatol., v.17, p.216, 2006. (Abstract).
- MEHTA, V.; SARDA, A.; BALACHANDRAN, C. Lupus band test. Indian J. Dermatol. Venereol. Leprol., v.76, p.298-300, 2010.
- MUTASIM, D.F.; ADAMS, B.B. Immunofluorescence in dermatology. J. Am. Acad. Dermatol., v.45, p.803-822. 2001.
- NIISHIFUJI, K.; YOSHIDA-YAMAKITA, K.; IWASAKI, T. A canine pemphigus foliaceus case showing parallel relationship of disease activity and titer of serum anti-keratinocyte cell surface antibodies. J. Vet. Med. Sci., v.67, p.943-945, 2005.
- OLIVRY, T. A review of autoimmune skin diseases in domestic animals: I - superficial pemphigus. Vet. Dermatol., v.17, p.291-305, 2006.
- OLIVRY, T.; LAVOY, A.; DUNSTON, S.M. et al. Desmoglein-1 is a minor autoantigen in dogs with pemphigus foliaceus. Vet. Immunol. Immunopathol.,v.110, p.245-55, 2006.
- OLIVRY, T.; RIVIERRE, C.; MURPHY, K.M. Efficacy of cyclosporine for treatment induction of canine pemphigus foliaceus. Vet. Rec., v.152, p.53-54, 2003.
- RANI, Z.; HUSSAIN, I. Review article Immunofluorescence diseases in immunobullous. J. Pakis. Assoc. Dermatol., v.13, p.76-88, 2003.
- REIS, V.M.S.; CUCÊ, L.C.; RIVITTI, E.A. Anatomopatologia e imunfluorescência direta e indireta das lesões de pênfigo foliáceo endêmico resistentes à corticoterapia. Rev. Inst. Med. Trop. São Paulo., v.33, p.97-103, 1991.
Publication Dates
-
Publication in this collection
May-Jun 2018
History
-
Received
17 Apr 2017 -
Accepted
04 Aug 2017